In Vitro Killing of Canine Urinary Tract Infection Pathogens by Ampicillin, Cephalexin, Marbofloxacin, Pradofloxacin, and Trimethoprim/Sulfamethoxazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Compounds
2.3. MIC Testing
2.4. MPC Testing
2.5. Kill Experiments
2.6. Statistical Analysis
3. Results
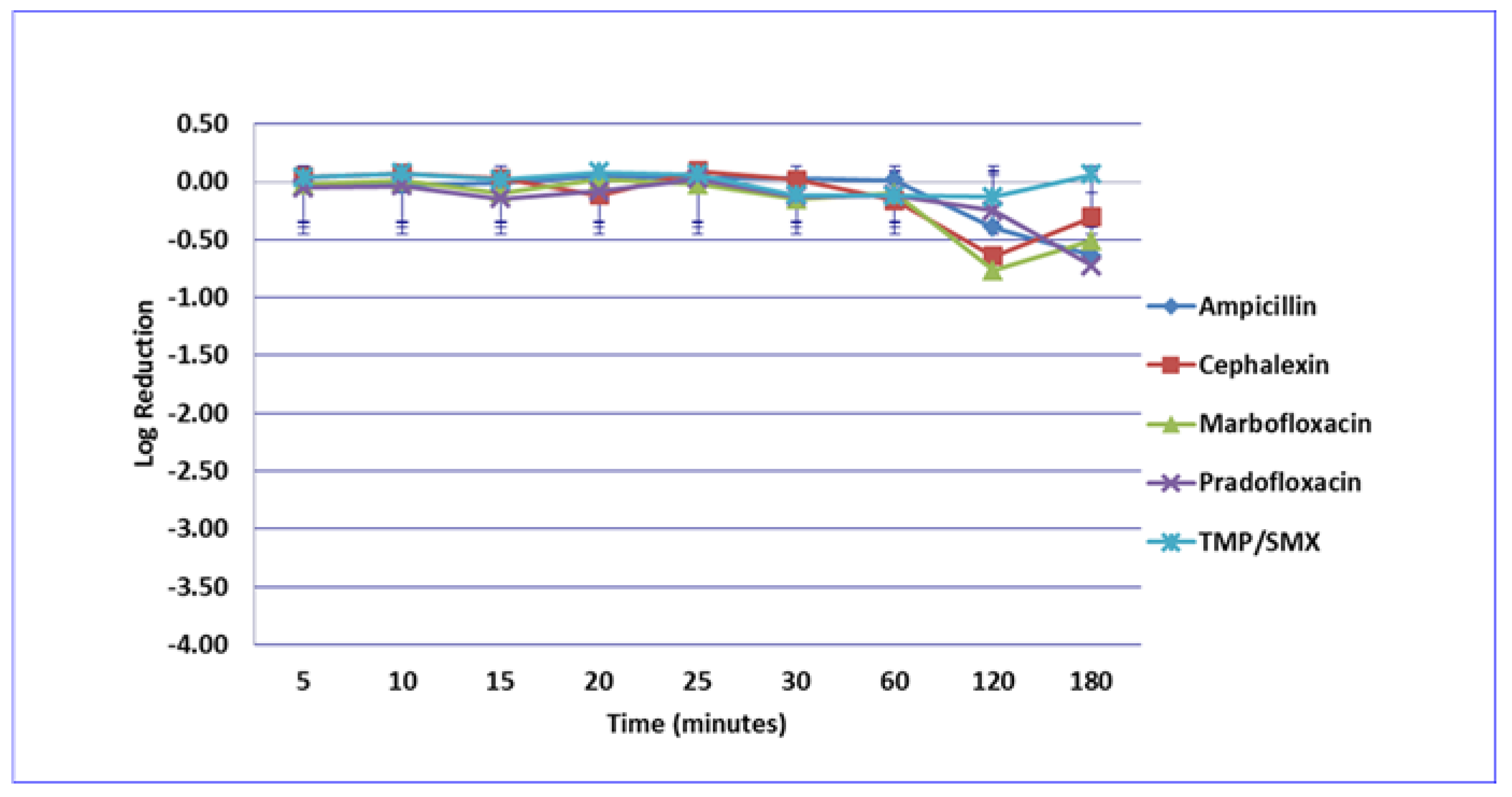
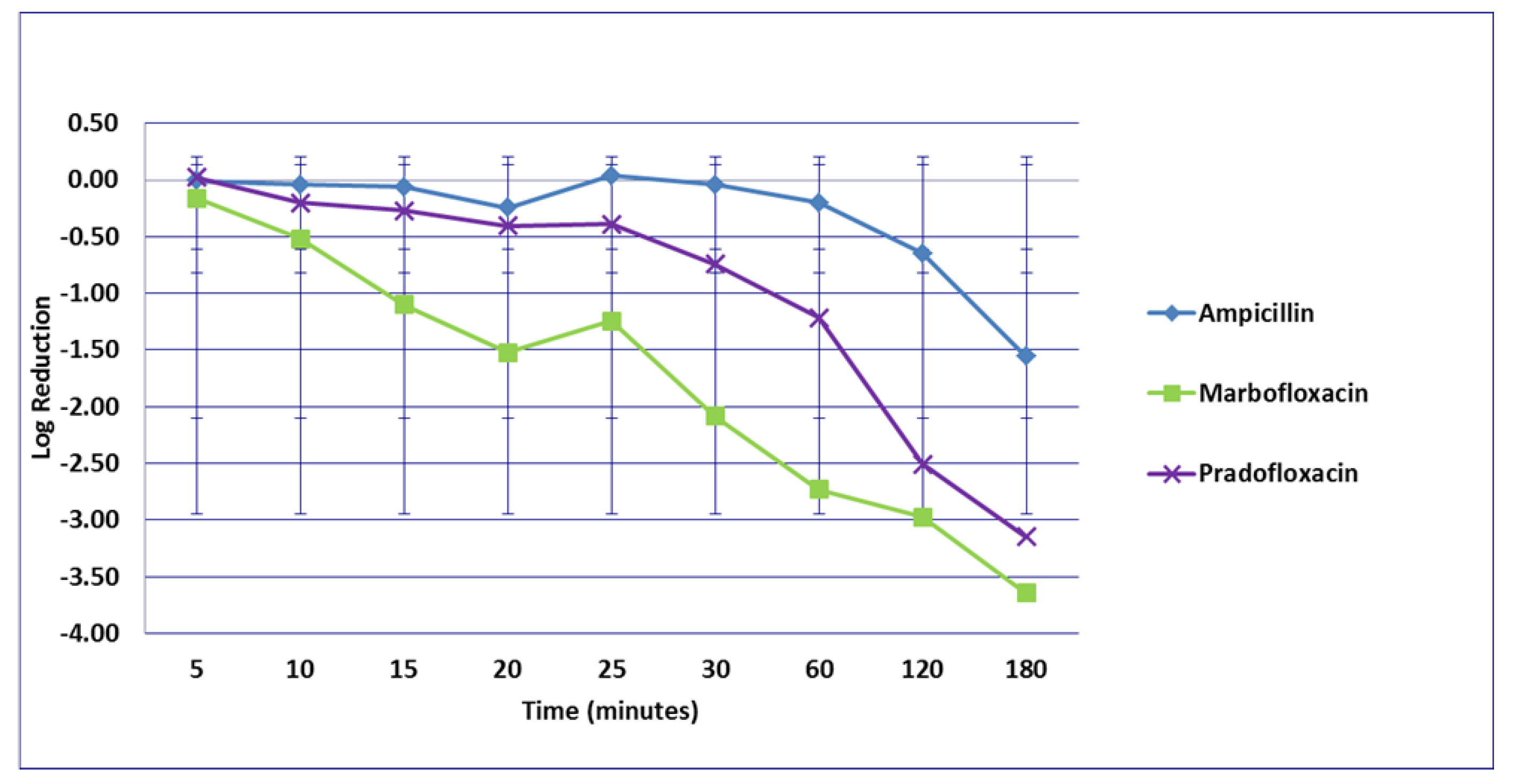
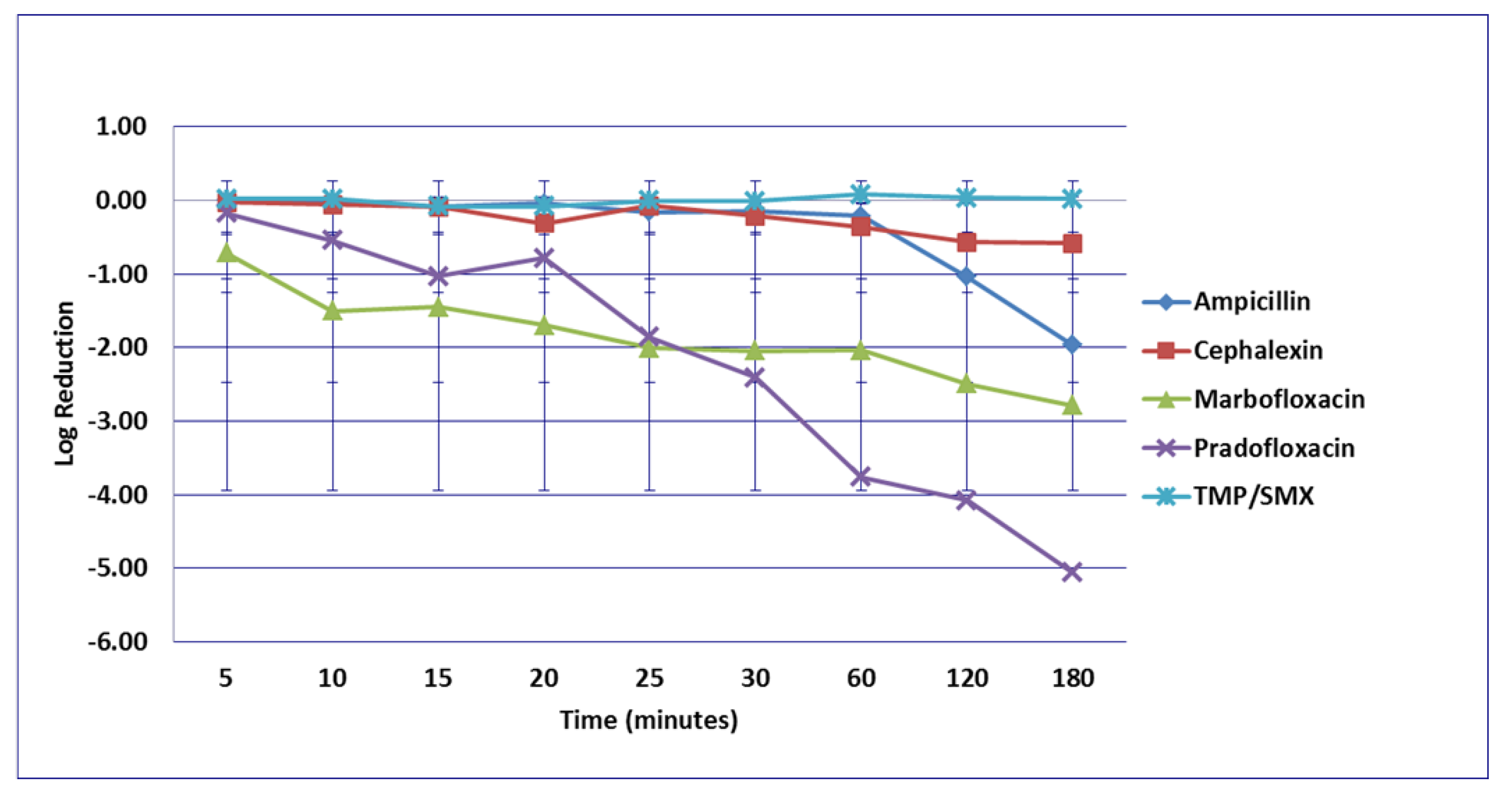
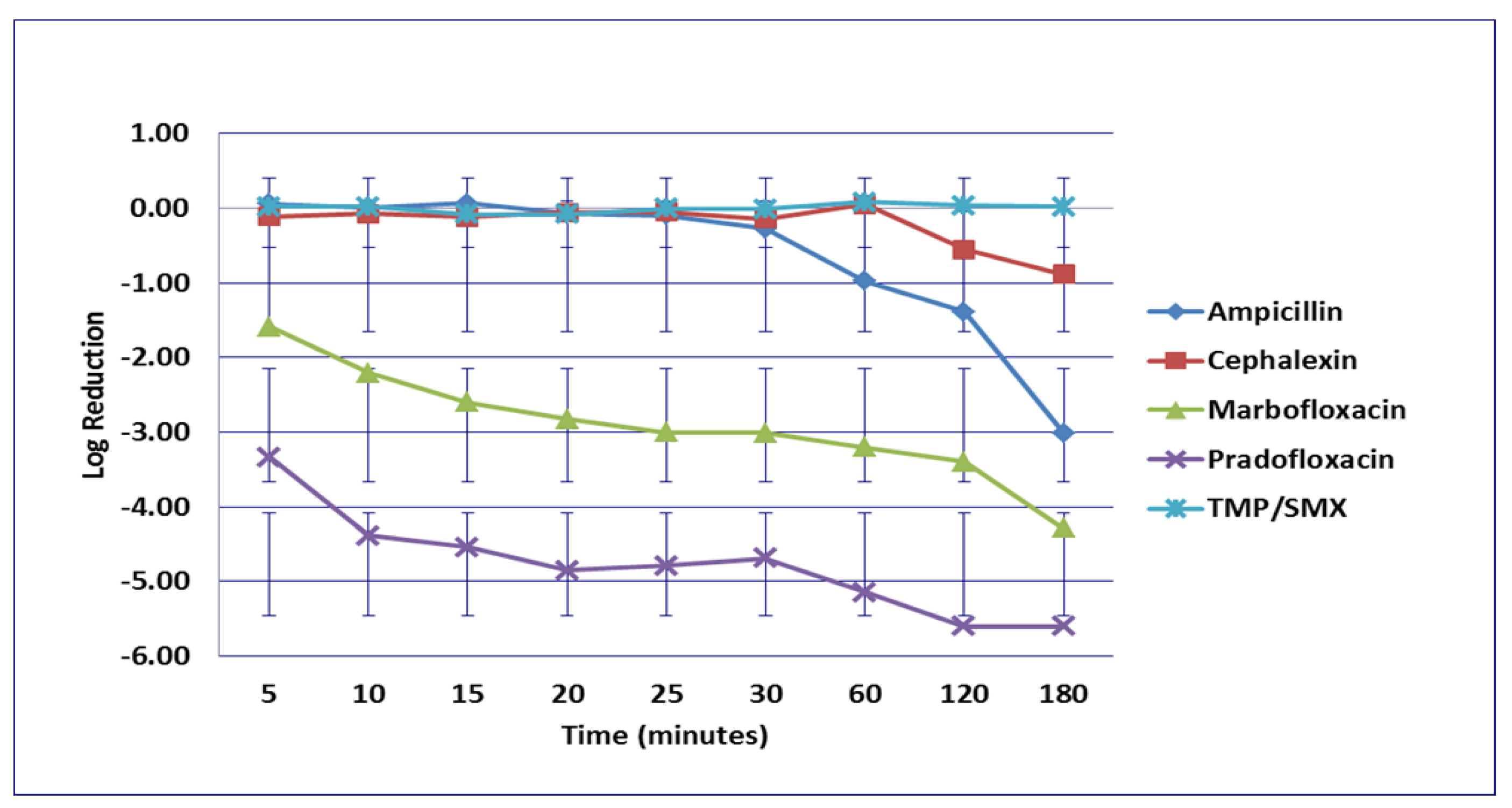
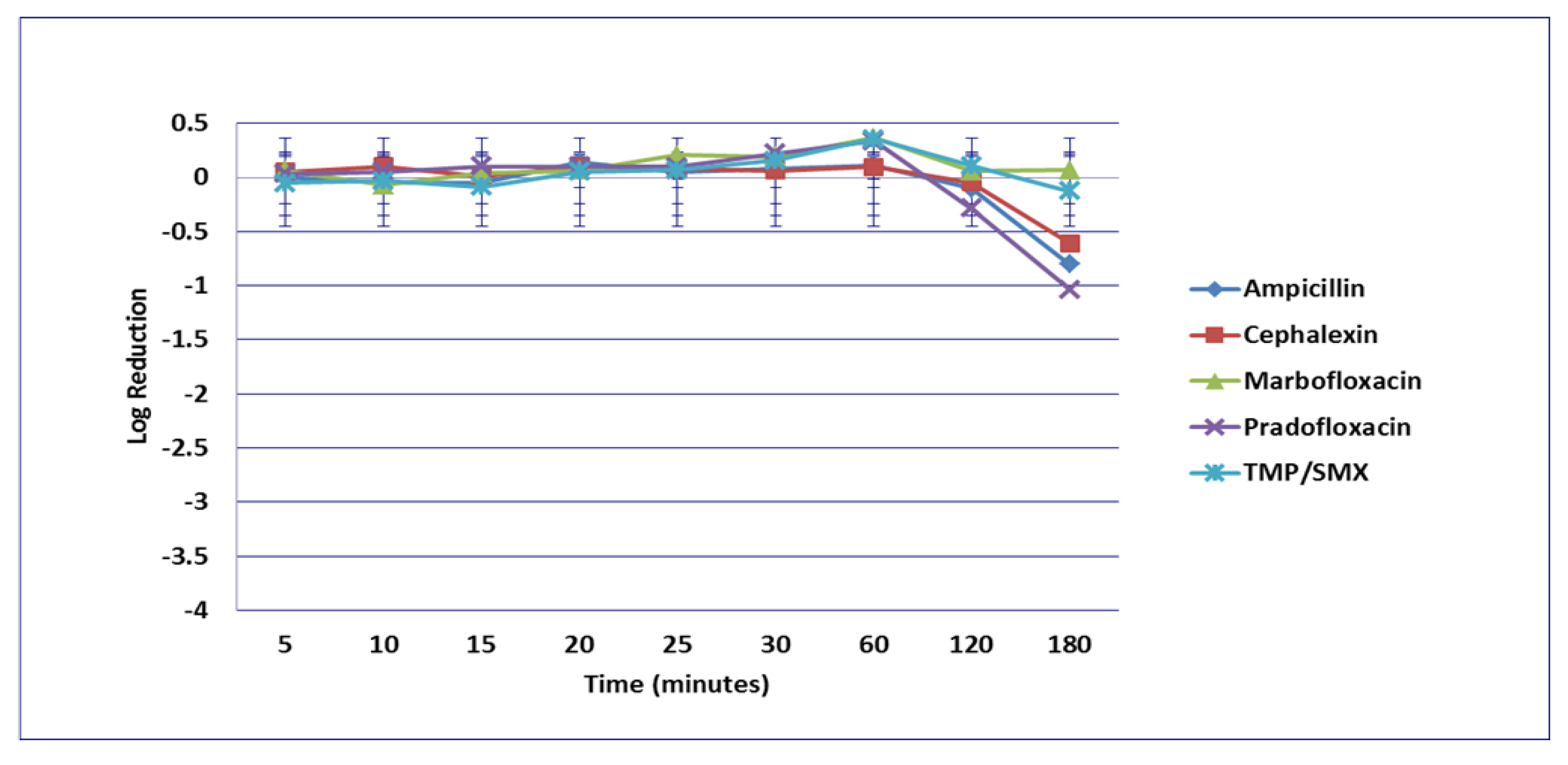
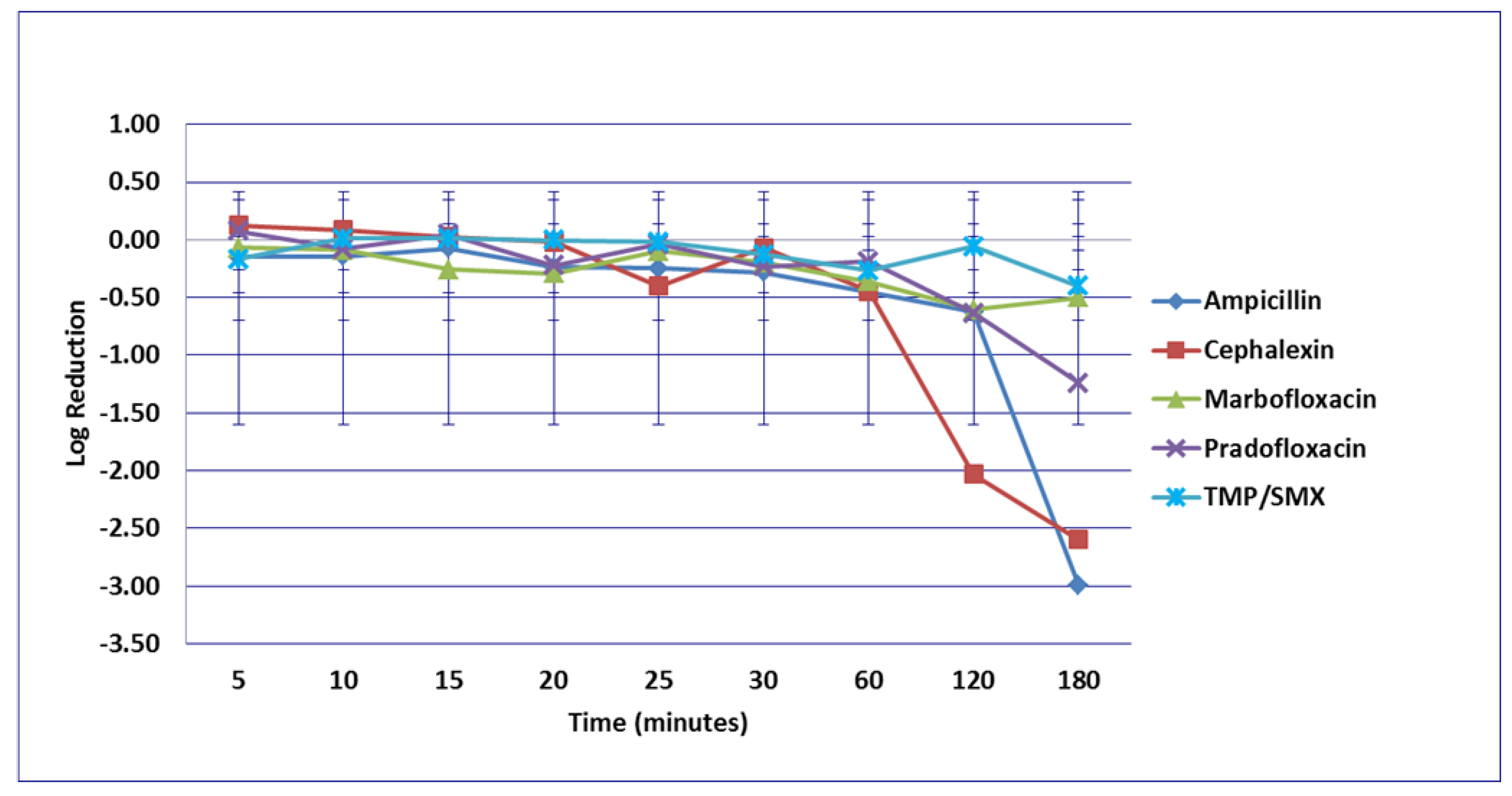
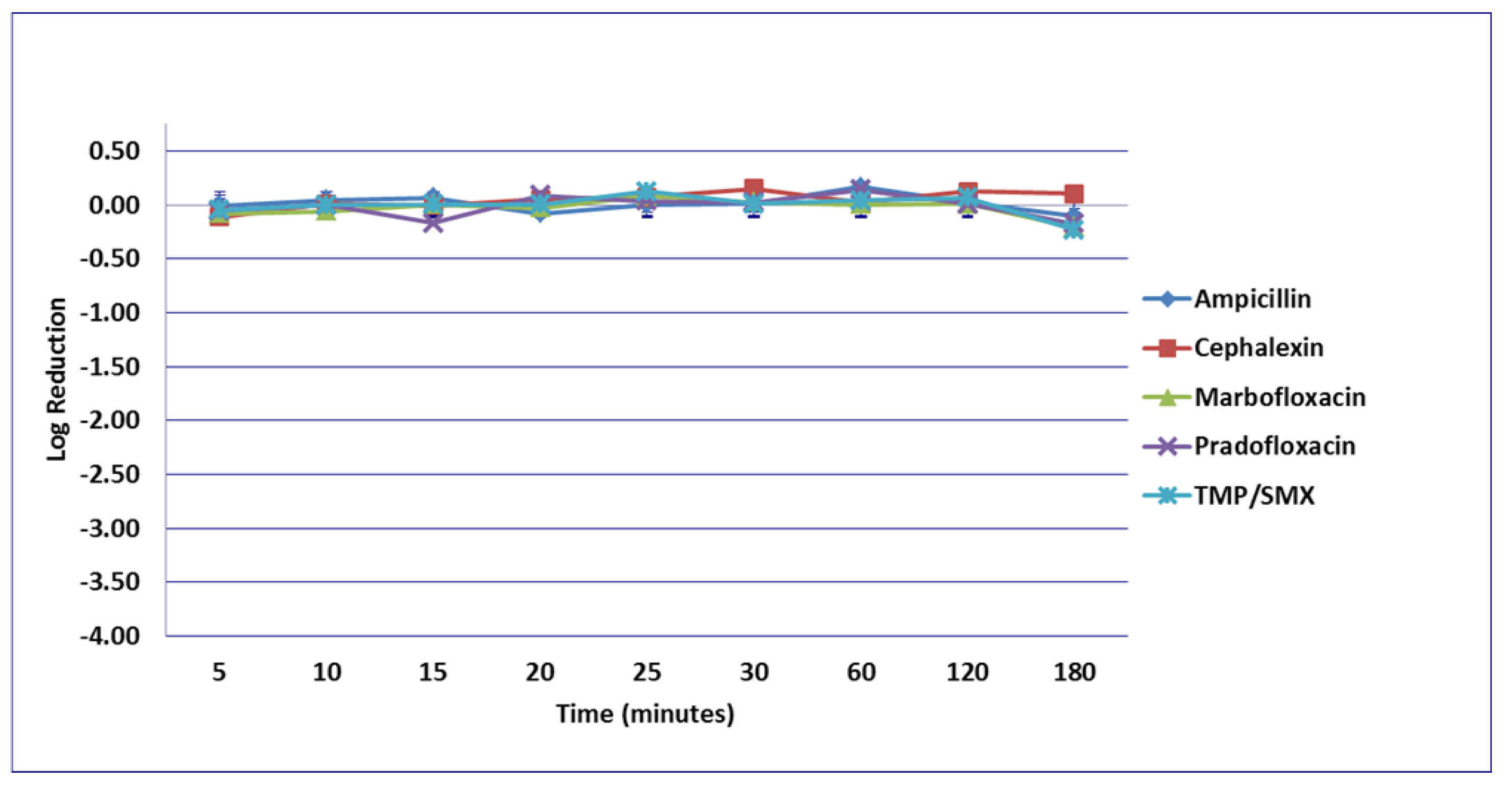
3.1. Escherichia coli
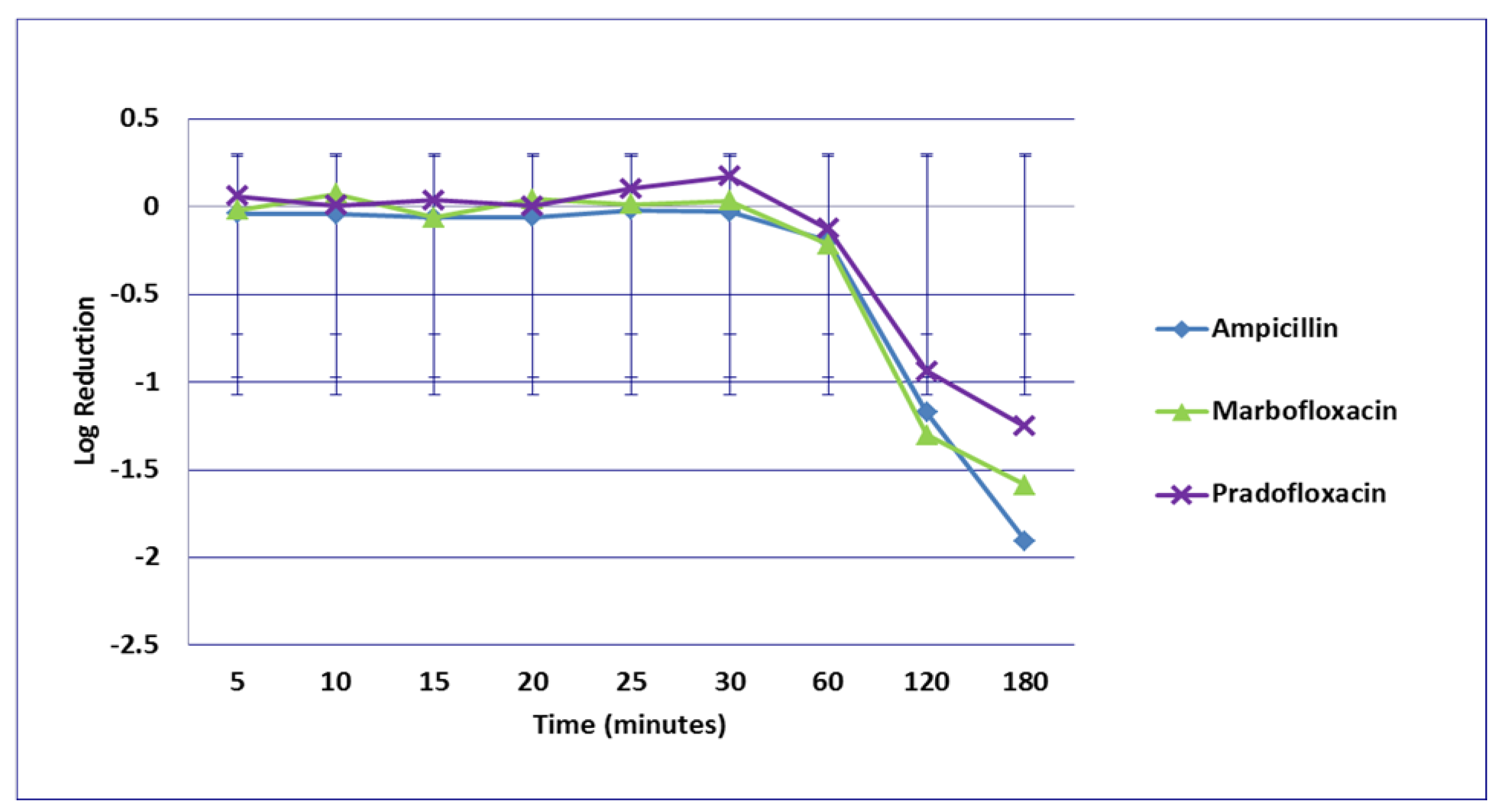
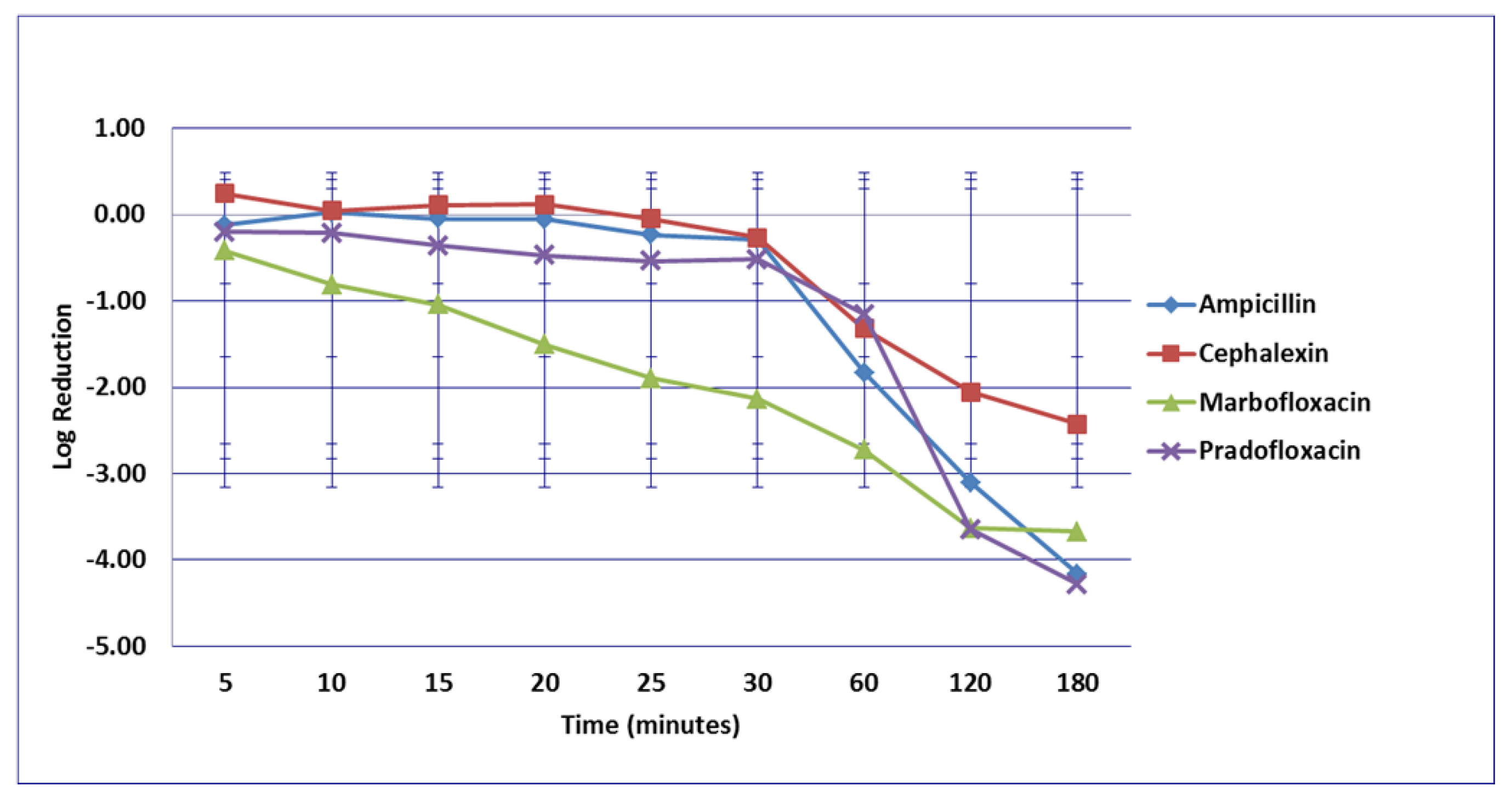
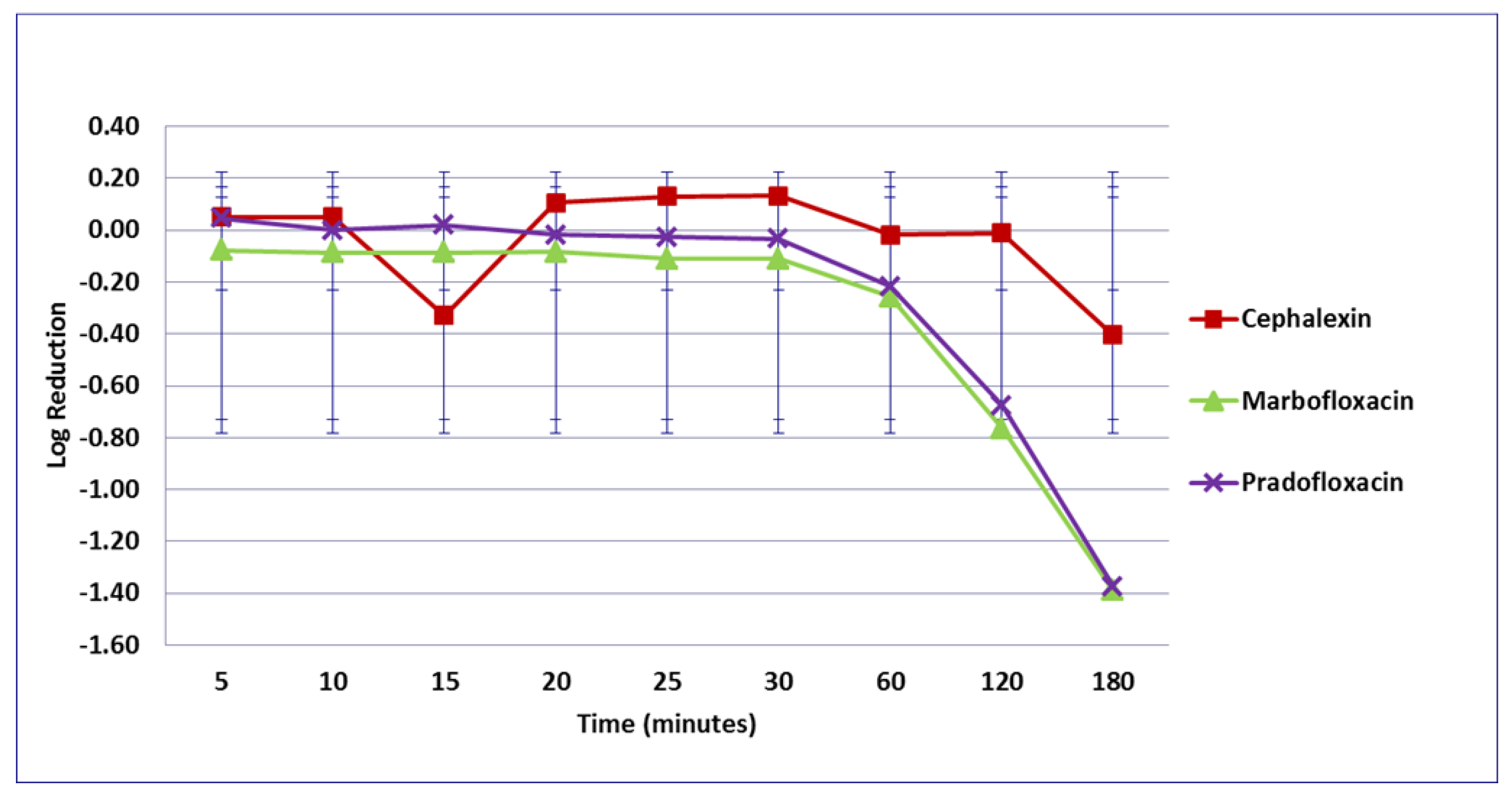
3.2. Enterococcus faecalis
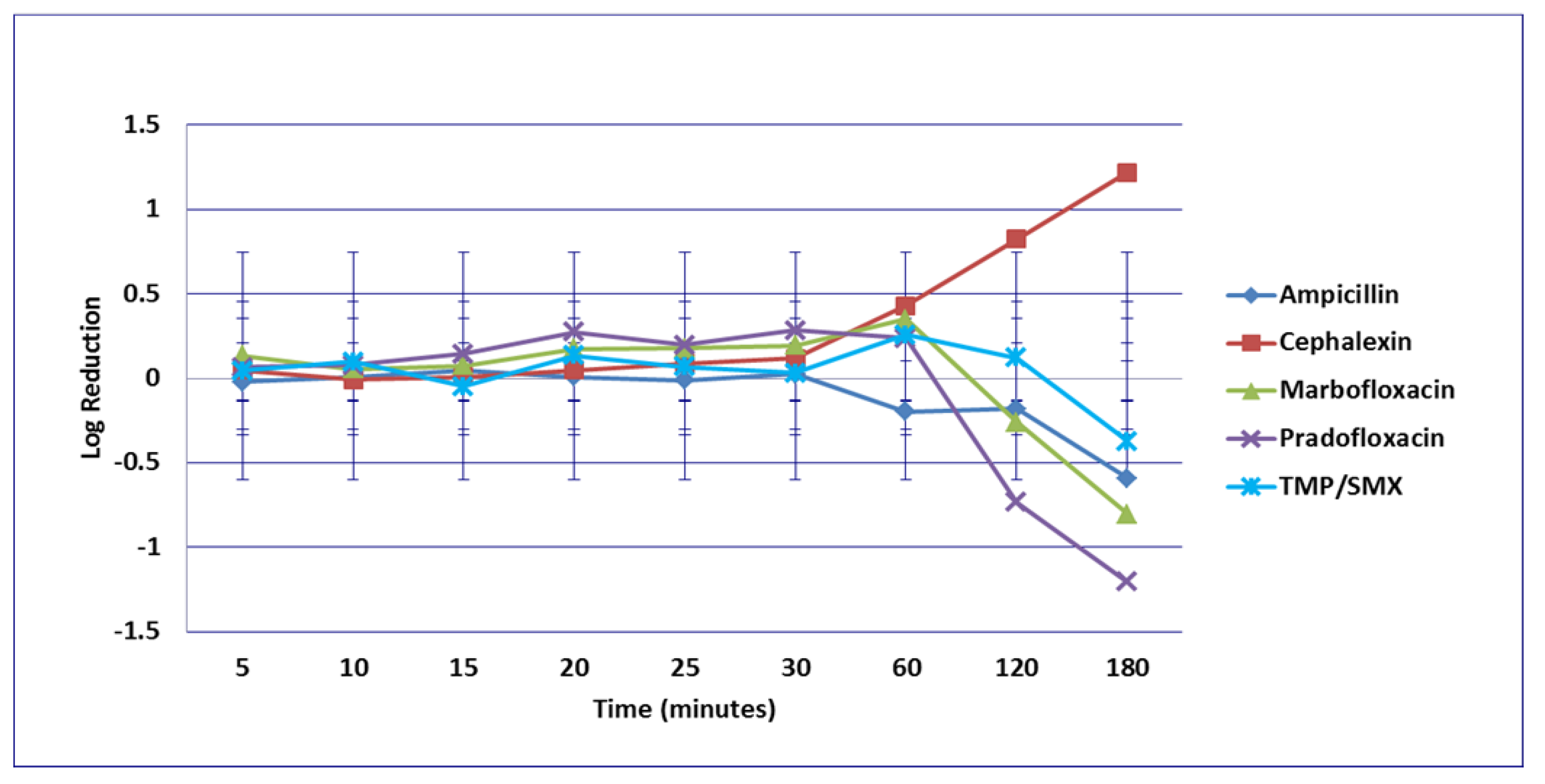
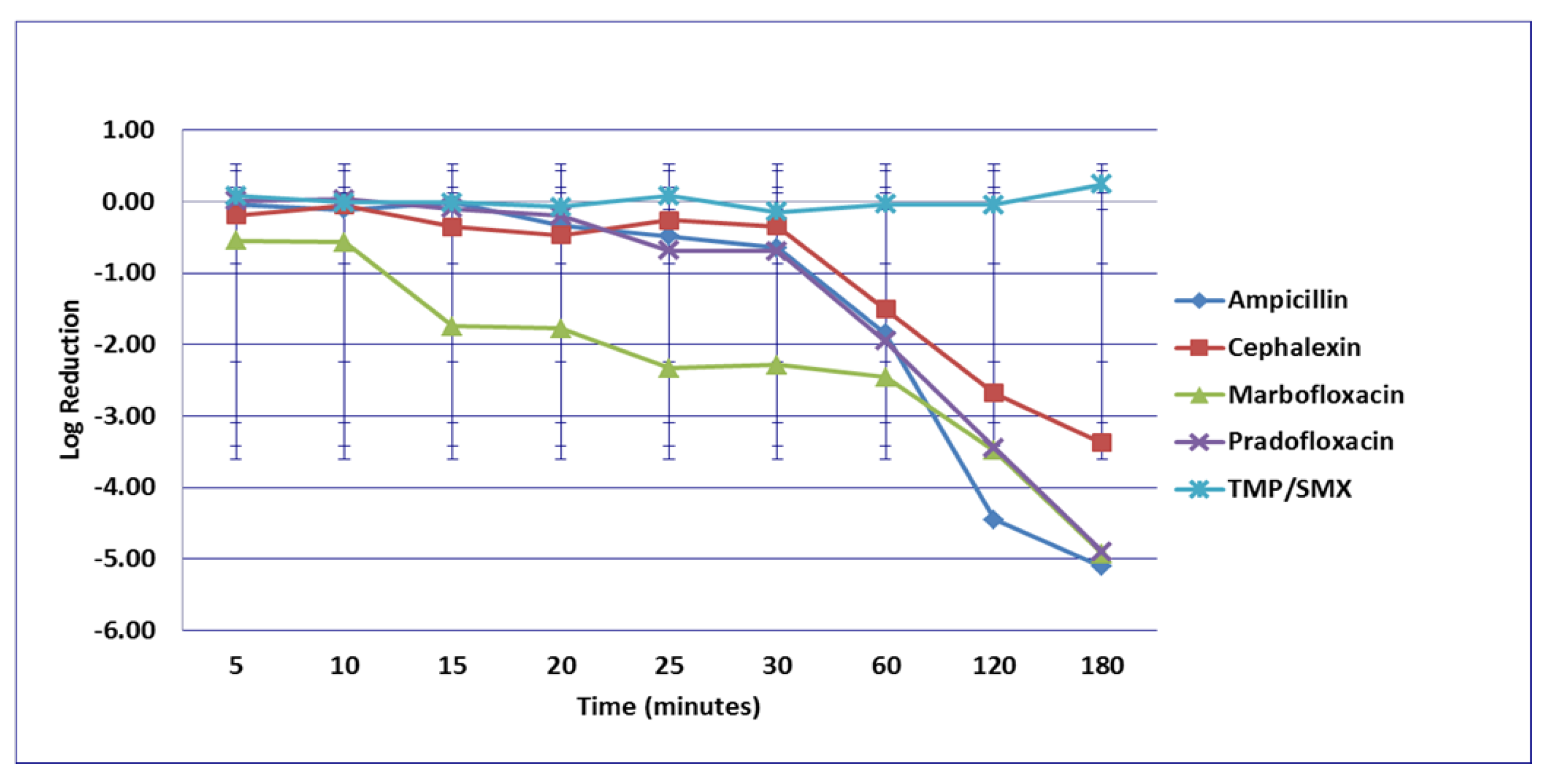
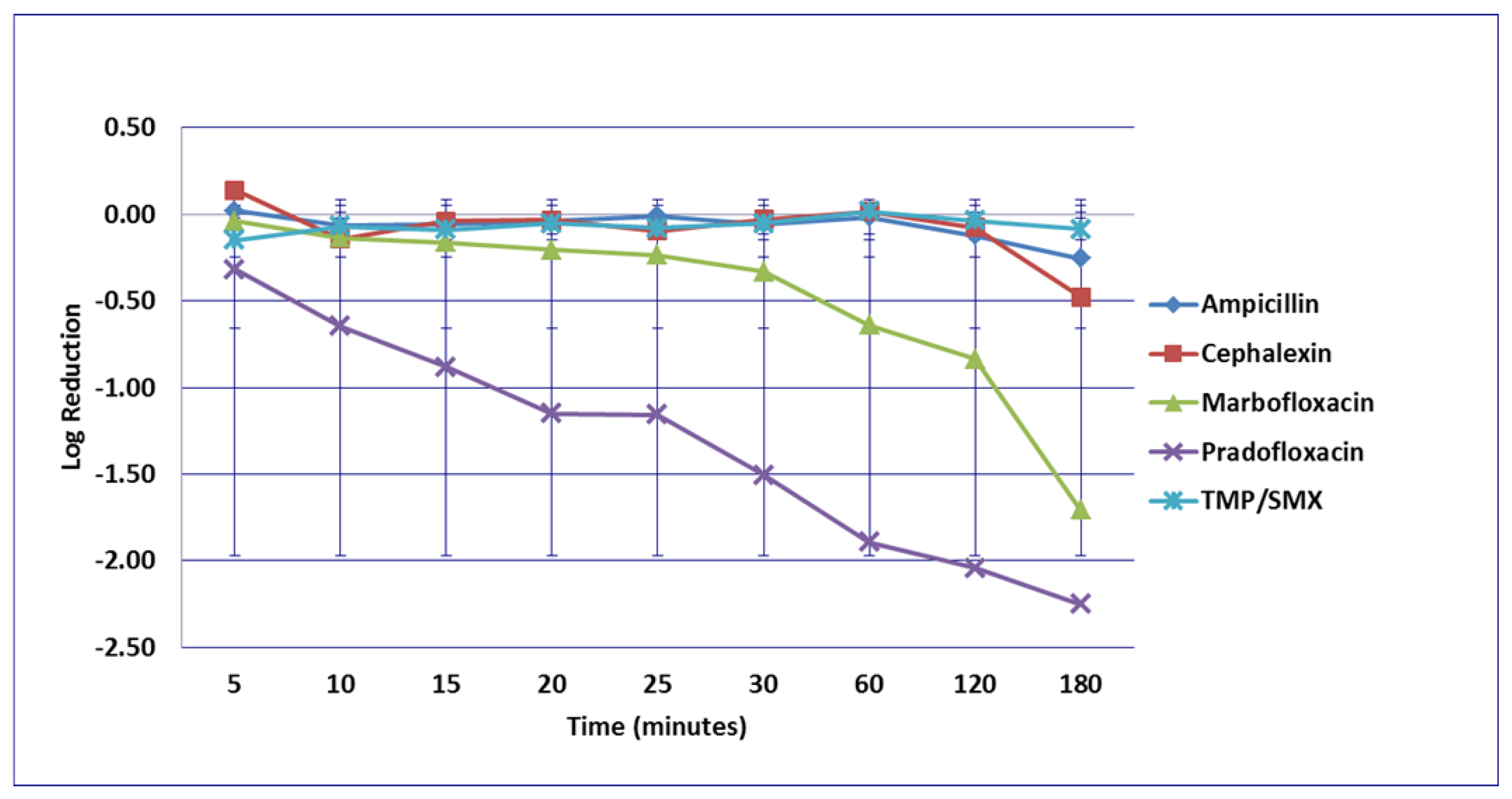
3.3. Proteus mirabilis
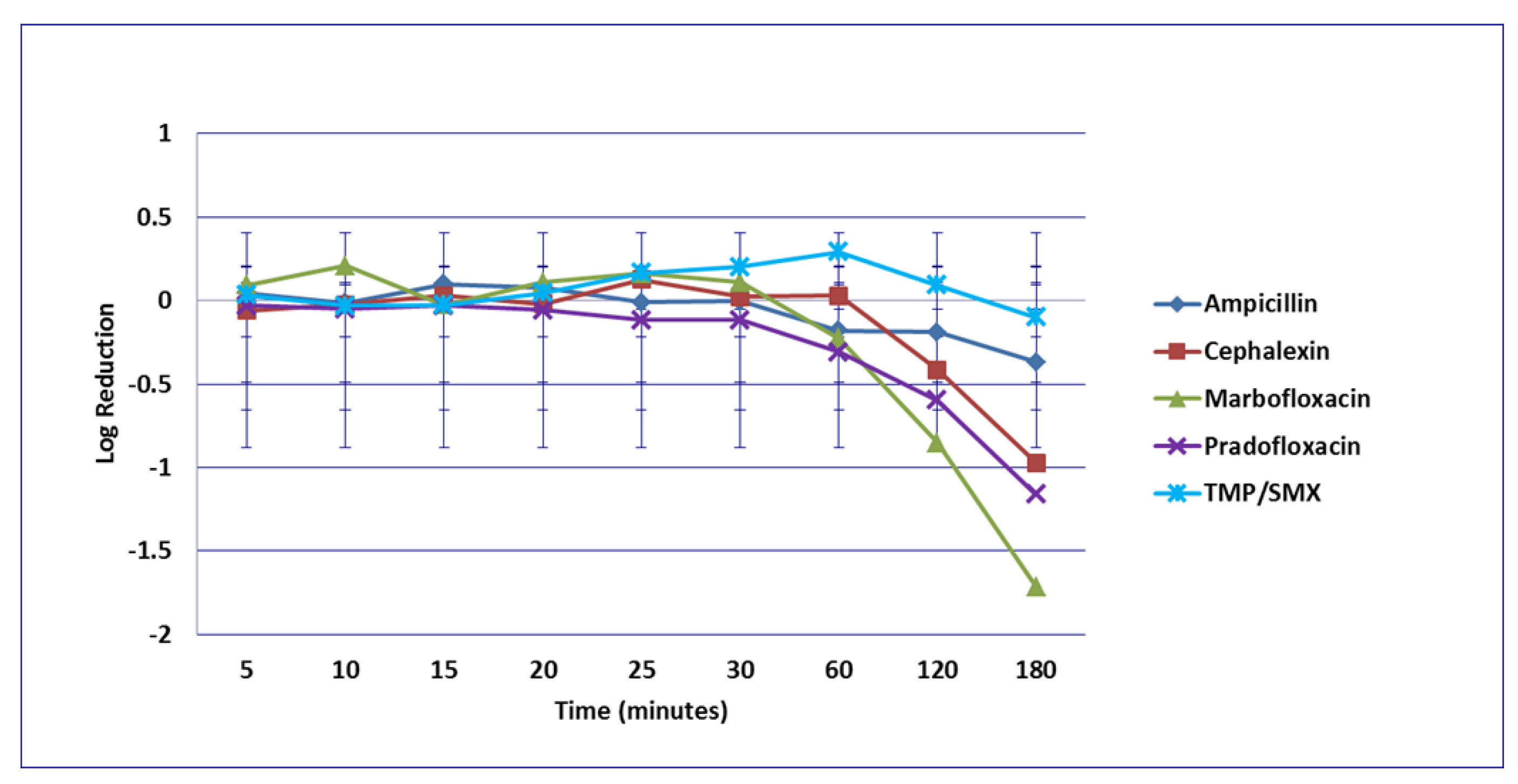
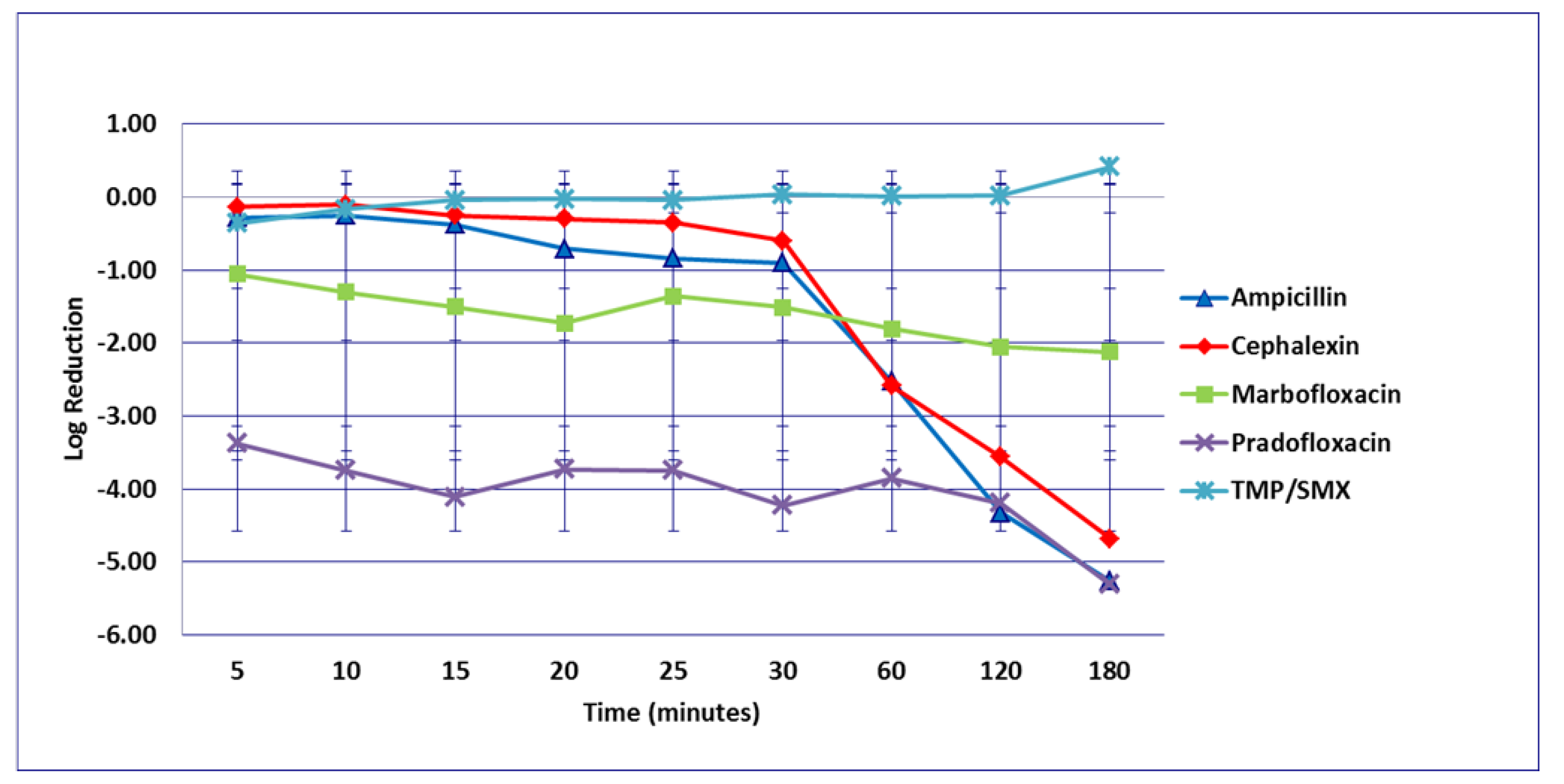
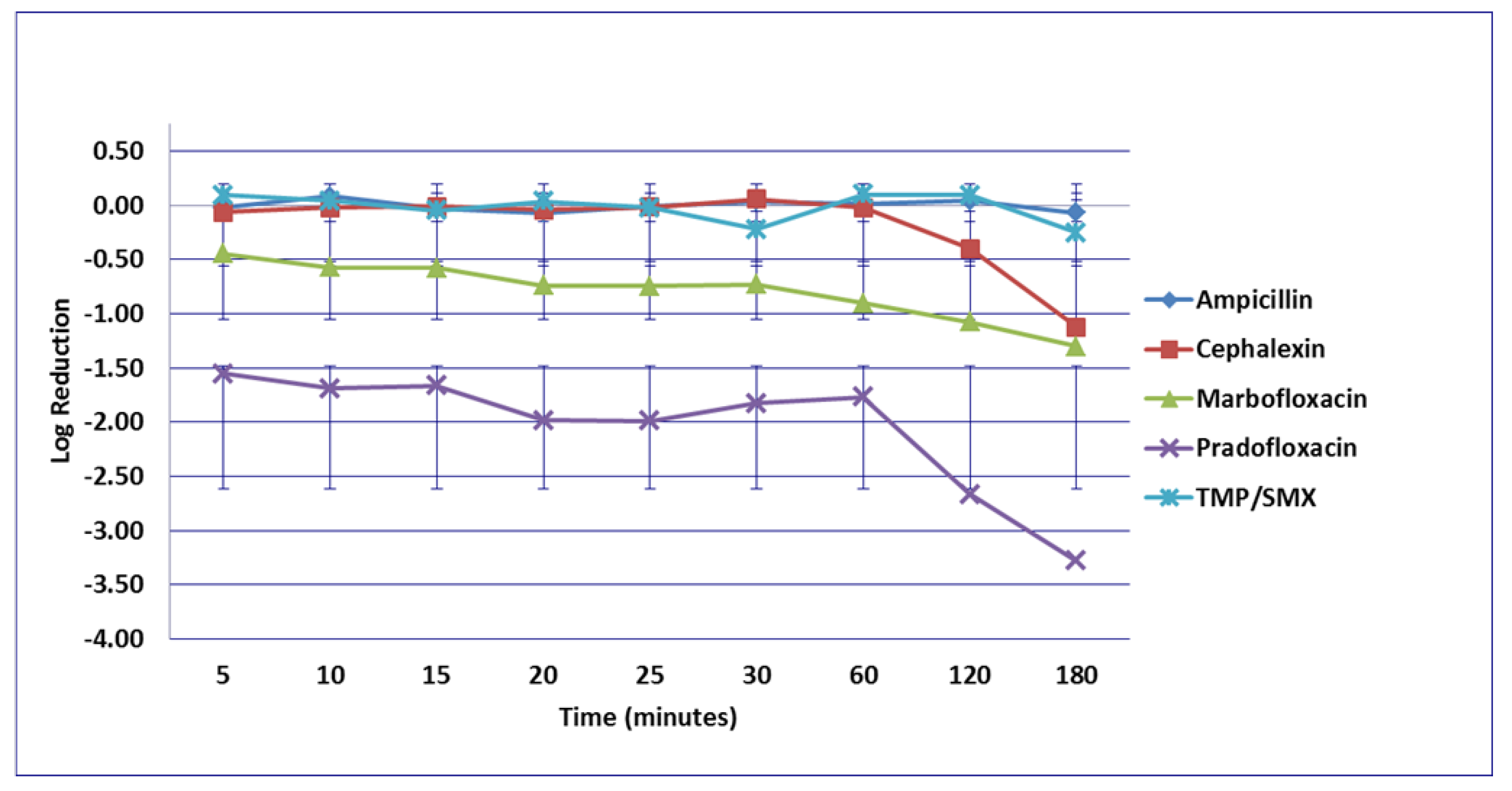
3.4. Staphylococcus pseudintermedius
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef]
- Ling, G.V. Therapeutic strategies involving antimicrobial treatment of the canine urinary tract. J. Am. Vet. Med. Assoc. 1984, 185, 1162–1164. [Google Scholar]
- Olby, N.; MacKillop, E.; Cerda-Gonzalez, S.; Moore, S.; Munana, K.; Grafinger, M.; Osborne, J.; Vaden, S. Prevalence of Urinary Tract Infection in Dogs after Surgery for Thoracolumbar Intervertebral Disc Extrusion. J. Vet. Intern. Med. 2010, 24, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, J.A. Genitorurinary infections. In Infectious Diseases of the Dog and Cat, 3rd ed.; Greene, C.E., Ed.; Saunders: St. Louis, MO, USA; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 935–961. [Google Scholar]
- Baigi, S.R.; Vaden, S.; Olby, N. The Frequency and Clinical Implications of Bacteriuria in Chronically Paralyzed Dogs. J. Vet. Intern. Med. 2017, 31, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- McLellan, L.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Ling, G.V.; Norris, C.R.; Franti, C.E.; Eisele, P.H.; Johnson, D.L.; Ruby, A.L.; Jang, S.S. Interrelations of Organism Prevalence, Specimen Collection Method, and Host Age, Sex, and Breed among 8354 Canine Urinary Tract Infections (1969–1995). J. Vet. Intern. Med. 2001, 15, 341–347. [Google Scholar] [CrossRef]
- Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Hillier, A.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Turnidge, J.D.; et al. Antimicrobial Use Guidelines for Treatment of Urinary Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. Vet. Med. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clare, S.; Hartmann, F.; Jooss, M.; Bachar, E.; Wong, Y.Y.; Trepanier, L.; Viviano, K. Short- and Long-Term Cure Rates of Short-Duration Trimethoprim-Sulfamethoxazole Treatment in Female Dogs with Uncomplicated Bacterial Cystitis. J. Vet. Intern. Med. 2014, 28, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Westropp, J.; Sykes, J.; Irom, S.; Daniels, J.; Smith, A.; Keil, D.; Settje, T.; Wang, Y.; Chew, D. Evaluation of the Efficacy and Safety of High Dose Short Duration Enrofloxacin Treatment Regimen for Uncomplicated Urinary Tract Infections in Dogs. J. Vet. Intern. Med. 2012, 26, 506–512. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.; et al. International Clinical Practice Guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [Green Version]
- Nicolle, L.E. Urinary tract infection: Traditional pharmacologic therapies. Dis. Mon. 2003, 49, 111–128. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Reilly, P.M.; Luppen, K.L.; Weinandt, W.J.; Ellington, L.L.; Bollinger, M.R. Randomized Study of Single-Dose, Three-Day, and Seven-Day Treatment of Cystitis in Women. J. Infect. Dis. 1986, 153, 277–282. [Google Scholar] [CrossRef]
- Warren, J.W.; Abrutyn, E.; Hebel, J.R.; Johnson, J.R.; Schaeffer, A.J.; Stamm, W.E. Guidelines for Antimicrobial Treatment of Uncomplicated Acute Bacterial Cystitis and Acute Pyelonephritis in Women. Clin. Infect. Dis. 1999, 29, 745–758. [Google Scholar] [CrossRef]
- Czaja, C.A.; Hooton, T.M. Update on acute uncomplicated urinary tract infection in women. Postgrad. Med. 2006, 119, 39–45. [Google Scholar] [CrossRef]
- Vogel, T.; Verreault, R.; Gourdeau, M.; Morin, M.; Grenier-Gosselin, L.; Rochette, L. Optimal duration of antibiotic therapy for uncomplicated urinary tract infection in older women: A double-blind randomized controlled trial. Can. Med. Assoc. J. 2004, 170, 469–473. [Google Scholar]
- Blondeau, J.M. New concepts in antimicrobial susceptibility testing: The mutant prevention concentration and mutant selection window approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Hesje, C.K.; Tillotson, G.S.; Blondeau, J.M. MICs, MPCs and PK/PDs: A match (sometimes) made in hosts. Expert Rev. Respir. Med. 2007, 1, 7–16. [Google Scholar] [CrossRef]
- Blondeau, J.; Hansen, G.; Metzler, K.; Hedlin, P. The Role of PK/PD Parameters to Avoid Selection and Increase of Resistance: Mutant Prevention Concentration. J. Chemother. 2004, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.; Shebelski, S.; Hesje, C. Killing of Streptococcus pneumoniae by azithromycin, clarithromycin, erythromycin, telithromycin and gemifloxacin using drug minimum inhibitory concentrations and mutant prevention concentrations. Int. J. Antimicrob. Agents 2015, 45, 594–599. [Google Scholar] [CrossRef]
- Blondeau, J.M.; Shebelski, S.D.; Hesje, C.K. Bactericidal effects of various concentrations of enrofloxacin, florfenicol, tilmicosin phosphate, and tulathromycin on clinical isolates of Mannheimia haemolytica. Am. J. Vet. Res. 2015, 76, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Shebelski, S.D. Comparative in vitro killing of canine strains of Staphylococcus pseudintermedius and Escherichia coli by cefovecin, cefazolin, doxycycline and pradofloxacin. Vet. Dermatol. 2016, 27, 267-e63. [Google Scholar] [CrossRef] [Green Version]
- Boswell, F.J.; Sunderland, J.; Andrews, J.M.; Wise, R. Time-kill kinetics of quinupristin/dalfopristin on Staphylcoccus aureus with and without a raised MBC evaluated by two methods. J. Antimicrob. Chemother. 1997, 39, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald-Dickler, N.; Holtom, P.; Spellberg, B. Busting the Myth of “Static vs Cidal”: A Systemic Literature Review. Clin. Infect. Dis. 2017, 66, 1470–1474. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibililty Tests for Bacteria Isolated from Animals; VET01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Blondeau, J.M.; Zhao, X.; Hansen, G.T.; Drlica, K. Mutant prevention concentrations (MPC) of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 433–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, J.; Borsos, S.; Blondeau, L.; Blondeau, B.; Hesje, C. Comparative minimum inhibitory and mutant prevention drug concentrations of enrofloxacin, ceftiofur, florfenicol, tilmicosin and tulathromycin against bovine clinical isolates of Mannheimia haemolytica. Vet. Microbiol. 2012, 160, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Carli, S.; Anfossi, P.; Villa, R.; Castellani, G.; Mengozzi, G.; Montesissa, C. Absorption kinetics and bioavailability of cephalexin in the dog after oral and intramuscular administration. J. Vet. Pharmacol. Ther. 1999, 22, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Spreng, M.; Deleforge, J.; Thomas, V.; Boisramé, B.; Drugeon, H. Antibacterial activity of marbofloxacin. A new fluoroquinolone for veterinary use against canine and feline isolates. J. Vet. Pharmacol. Ther. 1995, 18, 284–289. [Google Scholar] [CrossRef]
- Williams, A.; Williams, B.; Hansen, C.R.; Coble, K.H. The Impact of Pet Health Insurance on Dog Owners’ Spending for Veterinary Services. Animals 2020, 10, 1162. [Google Scholar] [CrossRef]
- Mettler, J.; Simcock, M.; Sendi, P.; Widmer, A.F.; Bingisser, R.; Battegay, M.; Fluckiger, U.; Bassetti, S. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: A prospective observational study. BMC Infect. Dis. 2007, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Wayne, A.; McCarthy, R.; Lindenmayer, J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. J. Small Anim. Pract. 2011, 52, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.J.; Nichol, K.A.; Hoban, D.J.; Zhanel, G.G. Stretching the mutant prevention concentration (MPC) beyond its limits. J. Antimicrob. Chemother. 2003, 51, 1323–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toutain, P.; Pelligand, L.; Lees, P.; Bousquet-Mélou, A.; Ferran, A.A.; Turnidge, J.D. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 2020, 44, 172–200. [Google Scholar] [CrossRef] [PubMed]
- Frisch, A.W.; Tripp, J.T.; Barrett, C.D., Jr.; Pidgeon, B.E. The specific polysaccharide content of pneumonic lungs. J. Exp. Med. 1942, 76, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Fagon, J.-Y.; Chastre, J.; Trouillet, J.-L.; Domart, Y.; Dombret, M.-C.; Bornet, M.; Gibert, C. Characterization of Distal Bronchial Microflora during Acute Exacerbation of Chronic Bronchitis: Use of the Protected Specimen Brush Technique in 54 Mechanically Ventilated Patients. Am. Rev. Respir. Dis. 1990, 142, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Feldman, W.E. Concentrations of bacteria in cerebrospinal fluid of patients with bacterial meningitis. J. Pediatr. 1976, 88, 549–552. [Google Scholar] [CrossRef]
- Matsukawa, M.; Kunishima, Y.; Takahashi, S.; Takeyama, K.; Tsukamoto, T. Time courses of bacterial density in urine during antibacterial chemotherapy and influential factors in patients having positive bacteriuria with a complicated urinary tract. J. Infect. Chemother. 2007, 13, 99–104. [Google Scholar] [CrossRef]
- Scott, V.C.; Haake, D.A.; Churchill, B.M.; Justice, S.S.; Kim, J.-H. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology 2015, 86, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Jancel, T. Management of uncomplicated urinary tract infections. West. J. Med. 2002, 176, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, B. Diagnosis and treatment of urinary tract infections in children. Am. Fam. Physician 2011, 83, 409–415. [Google Scholar]
- Desforges, J.F.; Stamm, W.E.; Hooton, T.M. Management of Urinary Tract Infections in Adults. N. Engl. J. Med. 1993, 329, 1328–1334. [Google Scholar] [CrossRef]
- Hooton, T.M.; Running, K.; E Stamm, W. Single-dose therapy for cystitis in women. A comparison of trimethoprim-sulfamethoxazole, amoxicillin, and cyclacillin. JAMA 1985, 253, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, A.A.; Notermans, D.W.; Meima, A.; Mulder, M.; Donker, G.A.; Stobberingh, E.E.; Verbon, A. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2151–2158. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, V.; Malini, A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J. Med. Res. 2014, 139, 945–948. [Google Scholar]
- Punia, M.; Kumar, A.; Charaya, G.; Kumar, T. Pathogens isolated from clinical cases of urinary tract infection in dogs and their antibiogram. Vet. World 2018, 11, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.; Epstein, S.; Westropp, J. Antimicrobial Susceptibility Patterns in Urinary Tract Infections in Dogs (2010–2013). J. Vet. Intern. Med. 2015, 29, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, T.; Bjornvad, C.; Cordoba, G.; Damborg, P.P.; Guardabassi, L.; Siersma, V.; Bjerrum, L.; Jessen, L.R. Effects of Diagnostic Work-Up on Medical Decision-Making for Canine Urinary Tract Infection: An Observational Study in Danish Small Animal Practices. J. Vet. Intern. Med. 2018, 32, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.; Torkan, S. Uropathogenic Escherichia coli in the Urine Samples of Iranian Dogs: Antimicrobial Resistance Pattern and Distribution of Antibiotic Resistance Genes. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KuKanich, K.; Lubbers, B.; Salgado, B. Amoxicillin and amoxicillin-clavulanate resistance in urinary Escherichia coli antibiograms of cats and dogs from the Midwestern United States. J. Vet. Intern. Med. 2019, 34, 227–231. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Borriello, S.P.; Pokludová, L. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef] [Green Version]
- Bryskier, A. Penicillins. In Antimicrobial Agents: Antibacterials and Antifungals; Bryskier, A., Ed.; ASM Press: Washington, DC, USA, 2005; pp. 113–162. [Google Scholar]
- Leitner, F.; Goodhines, R.A.; Buck, R.E.; Price, K.E. Bactericidal activity of cefadroxil, cephalexin and cephradine in an in vitro pharmacokinetic model. Infection 1980, 8, S542–S548. [Google Scholar] [CrossRef]
- Baxter, R.; Chapman, J.; Drew, W.L. Comparison of Bactericidal Activity of Five Antibiotics against Staphylococcus aureus. J. Infect. Dis. 1990, 161, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Silley, P.; Stephan, B.; Greife, H.A.; Pridmore, A. Bactericidal properties of pradofloxacin against veterinary pathogens. Vet. Microbiol. 2012, 157, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Silley, P.; Stephan, B.; Greife, H.A.; Pridmore, A. Comparative activity of pradofloxacin against anaerobic bacteria isolated from dogs and cats. J. Antimicrob. Chemother. 2007, 60, 999–1003. [Google Scholar] [CrossRef]
- Stratton, C.W. Dead Bugs Don’t Mutate: Susceptibility Issues in the Emergence of Bacterial Resistance. Emerg. Infect. Dis. 2003, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- I Backhouse, C.; A Matthews, J. Single-dose enoxacin compared with 3-day treatment for urinary tract infection. Antimicrob. Agents Chemother. 1989, 33, 877–880. [Google Scholar] [CrossRef] [Green Version]
- Vercelli, A.; Mottet, J.M. Valutazione della velocita di guarigione clinica e batteriologica della pradofloxacina nei cani affetti da infezioni delle vie urinarie non complicate. SUMMA—Anim. Cia. 2017, 34, 55–62. [Google Scholar]
- Llewelyn, M.J.; Fitzpatrick, J.M.; Darwin, E.; Crine, S.T.; Gorton, C.; Paul, J.; Peto, T.E.A.; Yardley, L.; Hopkins, S.; Walker, A.S. The antibiotic course has had its day. BMJ 2017, 358, j3418. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.; Kamranpour, P.; Gibani, M.; Mughal, N.; Moore, L. Short-course Antibiotic Therapy: A Bespoke Approach Is Required. Clin. Infect. Dis. 2019, 70, 1793–1794. [Google Scholar] [CrossRef]
- Wald-Dickler, N.; Spellberg, B. Short-course Antibiotic Therapy—Replacing Constantine Units With “Shorter Is Better”. Clin. Infect. Dis. 2019, 69, 1476–1479. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, C.I.; Brown, K.A.; Langford, B.J.; Daneman, N.; Garber, G.; Schwartz, K.L. Late-career Physicians Prescribe Longer Courses of Antibiotics. Clin. Infect. Dis. 2019, 69, 1467–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, T.; Skoog, G.; Hermansson, A.B.; Kahlmeter, G.; Kuylenstierna, N.; Lannergård, A.; Otto, G.; Settergren, B.; Ekman, G.S. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: A randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet 2012, 380, 484–490. [Google Scholar] [CrossRef]
- Peterson, J.; Kaul, S.; Khashab, M.; Fisher, A.C.; Kahn, J.B. A Double-Blind, Randomized Comparison of Levofloxacin 750 mg Once-Daily for Five Days with Ciprofloxacin 400/500 mg Twice-Daily for 10 Days for the Treatment of Complicated Urinary Tract Infections and Acute Pyelonephritis. Urology 2008, 71, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.W.; Abraham, S.N. Innate Immune Responses to Bladder Infection. Microbiol. Spectr. 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mariano, L.L.; Ingersoll, M.A. The immune response to infection in the bladder. Nat. Rev. Urol. 2020, 17, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Davidson, L.E. Antimicrobial Stewardship. Mayo Clin. Proc. 2011, 86, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumder, A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Hilaire, M.G.-S. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef] [PubMed]
| Drug | Isolates | Cmax | Urinemax | |||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | ||||||
| MIC | MPC | MIC | MPC | MIC | MPC | |||
| E. coli | ||||||||
| Ampicillin | 8 | 32 | 8 | 32 | 4 | 32 | 87 | 309 |
| Cephalexin | 8 | ≥128 | 8 | ≥128 | 8 | ≥128 | 20.3 | 225 |
| Marbofloxacin | 0.031 | 0.5 | 0.016 | 0.5 | 0.031 | 0.5 | 2.1 | 49.73 |
| Pradofloxacin | 0.031 | 0.125 | 0.25 | 0.5 | 0.016 | 0.125 | 1.4 | 237.9 |
| TMP/SMX | 0.25/4.75 | ≥8/152 | 0.125/2.38 | ≥8/152 | 0.031/0.595 | ≥8/152 | 1.55/29.45 | 26/79 |
| E. faecalis | ||||||||
| Ampicillin | 1 | 4 | NT | NT | 1 | 4 | 87 | 309 |
| Cephalexin | 128 | ≥256 | 128 | ≥256 | 128 | ≥256 | 20.3 | 225 |
| Marbofloxacin | 2 | 8 | 2 | 8 | 2 | 8 | 2.1 | 49.73 |
| Pradofloxacin | 0.25 | 2 | 0.25 | 1 | 0.5 | 2 | 1.4 | 237.9 |
| TMP/SMX | 0.25/4.75 | ≥8/152 | 0.125/2.38 | ≥4/76 | 0.125/2.38 | ≥4/76 | 1.55/29.45 | 26/79 |
| P. mirabilis | ||||||||
| Ampicillin | 1 | 64 | 1 | 64 | 2 | 64 | 87 | 309 |
| Cephalexin | 16 | 64 | 16 | 128 | 16 | 64 | 20.3 | 225 |
| Marbofloxacin | 0.031 | 1 | 0.031 | 1 | 0.063 | 1 | 2.1 | 49.73 |
| Pradofloxacin | 0.063 | 1 | 0.25 | 1 | 0.125 | 1 | 1.4 | 237.9 |
| TMP/SMX | 0.125/2.38 | ≥8/152 | 32/608 | ≥8/152 | 128/2432 | ≥8/152 | 1.55/29.45 | 26/79 |
| S. pseudintermedius | ||||||||
| Ampicillin | 0.125 | NT | 1 | NT | 2 | NT | 87 | 309 |
| Cephalexin | 2 | 64 | 0.5 | 16 | 0.5 | 16 | 20.3 | 225 |
| Marbofloxacin | 0.25 | 0.5 | 0.063 | 0.5 | 0.25 | 0.5 | 2.1 | 49.73 |
| Pradofloxacin | 0.031 | 0.125 | 0.016 | 0.063 | 0.031 | 0.125 | 1.4 | 237.9 |
| TMP/SMX | 0.5/9.5 | ≥8/152 | 0.5/9.5 | ≥8/152 | 1/19 | ≥8/152 | 1.55/29.45 | 26/79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blondeau, J.M.; Fitch, S.D. In Vitro Killing of Canine Urinary Tract Infection Pathogens by Ampicillin, Cephalexin, Marbofloxacin, Pradofloxacin, and Trimethoprim/Sulfamethoxazole. Microorganisms 2021, 9, 2279. https://doi.org/10.3390/microorganisms9112279
Blondeau JM, Fitch SD. In Vitro Killing of Canine Urinary Tract Infection Pathogens by Ampicillin, Cephalexin, Marbofloxacin, Pradofloxacin, and Trimethoprim/Sulfamethoxazole. Microorganisms. 2021; 9(11):2279. https://doi.org/10.3390/microorganisms9112279
Chicago/Turabian StyleBlondeau, Joseph M., and Shantelle D. Fitch. 2021. "In Vitro Killing of Canine Urinary Tract Infection Pathogens by Ampicillin, Cephalexin, Marbofloxacin, Pradofloxacin, and Trimethoprim/Sulfamethoxazole" Microorganisms 9, no. 11: 2279. https://doi.org/10.3390/microorganisms9112279
APA StyleBlondeau, J. M., & Fitch, S. D. (2021). In Vitro Killing of Canine Urinary Tract Infection Pathogens by Ampicillin, Cephalexin, Marbofloxacin, Pradofloxacin, and Trimethoprim/Sulfamethoxazole. Microorganisms, 9(11), 2279. https://doi.org/10.3390/microorganisms9112279






