Abstract
Accurate, prompt, and reliable tools for the diagnosis of malaria are crucial for tracking the successes or drawbacks of control and elimination efforts, and for future programs aimed at global malaria eradication. Although microscopy remains the gold standard method, the number of imported malaria cases and the risk of reappearance of autochthonous cases stimulated several laboratories located in European countries to evaluate methods and algorithms suited to non-endemic settings, where skilled microscopists are not always available. In this review, an overview of the field evaluation and a comparison of the methods used for the diagnosis of malaria by European laboratories is reported, showing that the development of numerous innovations is continuous. In particular, the combination of rapid diagnostic tests and molecular assays with microscopy represents a reliable system for the early diagnosis of malaria in non-endemic settings.
1. Introduction
The genus Plasmodium consists of over 200 widely distributed species, of which at least six regularly infect humans: Plasmodium falciparum (Pf), P. vivax (Pv), P. malariae (Pm), P. ovale wallikeri (Pow), P. ovale curtisi (Poc), and P. knowlesi (Pk) [1]. However, recently, cases of susceptibility to the non-human primate Plasmodia, such as P. cynomolgi in Southeast Asia and P. brasilianum and P. simium in South America, have been described [2,3,4].
Among species causing malaria in humans, P. falciparum and P. vivax pose the greatest threat: in 2018 P. falciparum accounted for 99.7% of estimated cases in the World Health Organization (WHO) African regions; and P. vivax is the most common species in the WHO regions of Americas, accounting for 75% of infections [5].
Malaria is a febrile illness and clinical symptoms of uncomplicated malaria include fatigue, headaches, muscle aches, malaise, abdominal discomfort, fever, nausea, and vomiting [6]. Specific diagnostic methods are needed to differentiate between malaria and other febrile illnesses. An early diagnosis can prevent further progression and lower the severity of the disease, especially for children under 5 years of age who accounted for about 67% of deaths in 2018 due to severe malaria worldwide [5]. For the most effective treatment of malaria, it is important to know the species of Plasmodium interested and the parasitic burden in the blood. Parasite count is mandatory in cases of infection with P. falciparum, because it is one of the criteria used to define severe malaria (parasitemia >4% in adults and >10% in children). Different patient management modalities are applied if the parasitemia is >2% [7]. The presence of mature asexual forms (>20% of parasites) is another criterion for the definition of severe P. falciparum malaria [6,7].
Accurate, prompt, and affordable diagnostic tools are also pivotal for tracking the successes or drawbacks of control and elimination efforts, and for future programs aimed at global malaria eradication. Active surveillance of the disease in each geographical area is essential for a program to succeed. The WHO Global Technical Strategy for Malaria aims, by 2030, to reduce malaria case incidence and mortality rates by 90%, compared to the 2015 baseline, to interrupt malaria transmission in at least 35 countries and to prevent its re-establishment in all malaria-free countries. The aim of surveillance is to detect all malaria infections and to investigate each individual case of infection, to differentiate imported cases, namely, infections acquired outside the areas in which they are diagnosed, from those acquired locally [8,9].
In fact, with 229 million cases and 409,000 deaths, especially among children (estimated in 2019), malaria is one of the most severe public health problems worldwide [5,10]. Although it occurs mostly in poor tropical and subtropical areas of the world [5,10], a high number of cases are also reported in non-endemic settings, such as Europe, where it is a medical emergency.
Malaria is thought to have arrived in South Europe via the Nile Valley during the Neolithic period, from whence it has been spread to the entire continent, where it remained endemic for more than 2000 years until its elimination by 1978 [11]. During 2011–2012, outbreaks were reported in an agricultural area of South Greece, and sporadic locally acquired cases were recorded throughout the country [12]. During 2019, the European Centre for Disease Prevention and Control (ECDC) reported 8641 malaria cases in the EU/EAA (99% confirmed). Among the episodes with known importation status, 99.8% were travel-related. Nine confirmed cases were reported as acquired in the EU (2 in Germany, 2 in Greece, 2 in Spain, 2 in France, and 1 in the Netherlands) [13].
The consistent number of imported malaria cases and the risk of reappearance of autochthonous cases stimulated several laboratories located in European countries to evaluate methods and algorithms for the diagnosis that are best suited to non-endemic settings, where skilled microscopists are not always available, especially when the diagnosis is required in emergencies outside laboratory opening hours [10,14,15].
In this review, an overview of the studies performed in the period 1999–2021 by European laboratories, concerning the evaluation and the comparison of methods for the diagnosis of malaria, was reported.
2. Gold-Standard Method
Microscopic examination of blood films was the first technique used, and remains the “gold standard” and the most widely used method for the diagnosis of malaria [16,17]. Thick and thin blood smears stained with Giemsa, Wright’s, or Field’s allows to rapidly detect and differentiate, when possible, the various species and the parasite stages, and quantify the parasite density, known as parasitemia (Figure 1) [16,17,18]. Thick blood film is a concentration technique that provides enhanced sensitivity in case of low level parasitemia [18]. Stained thin blood film is less sensitive; however, it is the most used technique for the diagnosis of malaria and for the parasitemia determination because the organisms are easier to see and count [16,18]. The sensitivity and specificity for this method are 95% and 98%, respectively, when the polymerase chain reaction (PCR) is used for comparison; the limit of detection for this method is approximately 50–200 parasites per μL of blood [19]. To enhance the detection of Plasmodia in blood film, alternative methods can be used in areas where training and expensive equipment can be introduced, such as staining with fluorescent dyes having affinity for the nucleic acid (especially acridine orange and benzothiocarboxypurine) (Figure 2) directly on blood smears or using quantitative buffy coat (QBC), a concentration method associated with fluorescent staining [16,18,20].
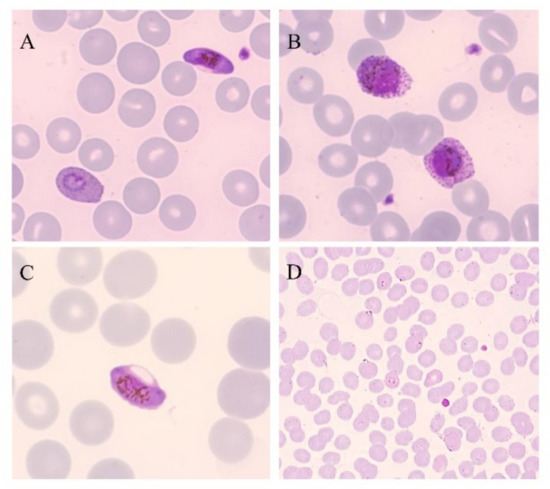
Figure 1.
Thin blood smears of blood samples from malaria cases prepared and stained with Giemsa. (A) P. falciparum gametocyte and P. ovale trophozoite (100×). (B) P. ovale gametocytes (100×). (C) P. falciparum gametocyte (100×). (D) P. falciparum trophozoites (40×). (Picture by A. Calderaro, Department of Medicine and Surgery, University of Parma, Parma, Italy).
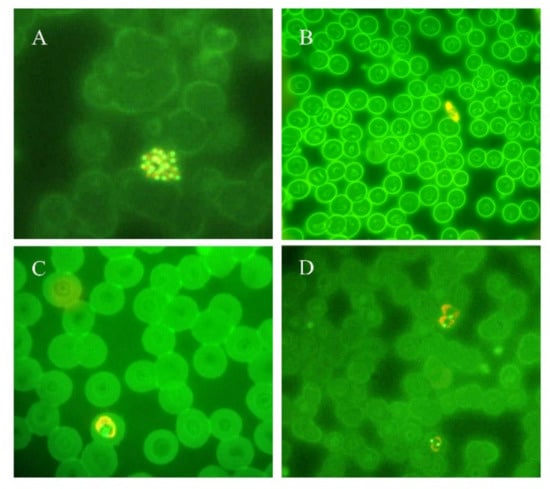
Figure 2.
Thin blood smears of blood samples from malaria cases prepared and stained with acridine orange. (A) P. vivax schizont (100×). (B) P. falciparum gametocyte (40×). (C) P. ovale trophozoite (100×). (D) P. vivax trophozoites (100×). (Picture by A. Calderaro, Department of Medicine and Surgery, University of Parma, Parma, Italy).
Overall, microscopic examination provides rapid and inexpensive detection and identification of Plasmodia at the species and stage levels, and allows their quantification in peripheral blood in order to monitor patients with malaria, including follow-up during specific therapy. It is noteworthy that microscopy requires specific skills rarely available in non-endemic settings, especially when cases of mixed or sub-microscopic infection occur [16]. Although microscopy remains the gold standard method, most of the laboratories located in non-endemic countries evaluated further techniques that can be used for malaria diagnosis.
3. Rapid Diagnostic Tests
Rapid diagnostic tests (RDT) are immunochromatographic assays for quickly (15–20 min) establishing the diagnosis of malaria infection by detecting specific malaria antigens in blood [17,18]. The first commercial RDT was distributed in 1994 to improve the diagnosis of malaria, particularly in endemic remote areas, and since then more than 200 devices have been marketed [21,22].
The availability of commercial kits (Figure 3) providing all the necessary reagents and their ease of performance and interpretation have made them an increasingly common tool to support microscopy in non-endemic areas where the low prevalence of malaria does not give the microscopists the chance to maintain their interpretation skills [16,18].
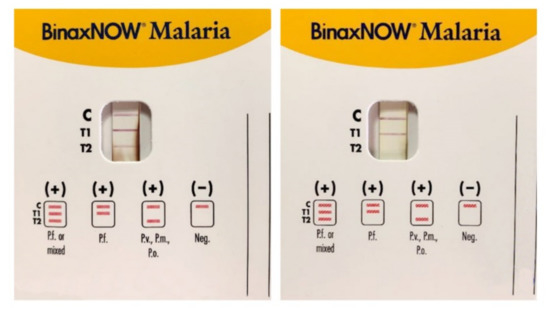
Figure 3.
Immunocromatographic assay for the search of Plasmodia antigens in blood samples: P. falciparum (Pf), P. malariae (Pm), P. vivax (Pv), and P. ovale (Po). C is the control band, T1 band corresponds to P.falciparum histidine-rich protein 2 (HRP2), and T2 band corresponds to parasite lactate aldolase. A Pf or mixed infection on the left and a Pf infection on the right. (Picture by A. Calderaro, Department of Medicine and Surgery, University of Parma, Parma, Italy).
The antigens currently used in RDTs available are Plasmodium falciparum-specific histidine-rich protein 2 (HRP2), Plasmodium pan-specific lactate dehydrogenase (pLDH), and pan-malarial aldolase for Plasmodia infecting humans [23]. HRP2 was the first antigen selected to develop an RDT because of its abundance in P. falciparum: it is produced by asexual stages and gametocytes of such Plasmodium, and it is expressed on red blood cells’ (RBCs) surface. pLDH is expressed at high level in asexual stages of P. falciparum, P. ovale, P. vivax, and P. malariae human malaria parasites. Aldolase is a pan-specific enzyme involved in the glycolytic pathway of the malaria parasites [18]. The limit of detection of RDTs is approximately 200–2000 parasites per μL of blood [24].
Several European laboratories evaluated the performance and/or the usefulness of commercial RDTs for their inclusion in the malaria diagnosis workflow, as reported in Table 1 [15,21,25,26,27,28,29,30,31,32,33].

Table 1.
Rapid diagnostic tests field evaluated by European diagnostic laboratories.
Different performances among the commercial assays were observed; however, overall, the authors concluded that RDTs are useful supporting tools for the diagnosis of malaria in non-endemic settings. Though they cannot be considered as unique diagnostic methods, these tests help the operator to achieve a rapid and easy to perform interpretation, especially if a trained microscopist is not always available; this can avoid delay in the management of life-threatening malaria cases [12,15,25,26,27,28,31,32]. However, if a negative result is obtained, the disease cannot be ruled out [12,25]. As expected, false-positive and false-negative results were observed. Concerning P. falciparum malaria, the false-negative results observed can be attributed to a low level of parasitemia that appears to be critical for this assay [26]. Furthermore, mutations/deletions in HRP2 gene have been reported to affect the results of RDTs based on the detection of this antigen [25,26,31]. In some cases, a prozone effect could be the explanation of false-negative results, although not observed in the studies reported above [15,31]. False-positive results could have various explanations. Although rarely, a cross-reaction with rheumatoid factor can occur. More frequently, in the case of HRP2 based assays, the antigen can persist for weeks following the eradication of the asexual-stage parasitemia [31,32] because of the delayed clearance of circulating antigen and because of the persistence of sexual-stage forms producing antigen [31]. On the contrary, pLDH is produced only by viable parasites; it is detected earlier than HRP2 and it appears to be cleared from the bloodstream within 24 h of a treatment [31,32]. However, it cannot be ignored that microscopy, despite being the reference test, could result negative when asexual-stage parasitemia runs at a level below its detection limit, and a related result by RDT could be misinterpreted as a false positive [31].
Together with the risk of false negative and false positive results, RDTs could miss double infections and are not able to quantify the parasitemia and distinguish among the parasitic stages [31].
However, based on their results and the scientific literature in the topic, Grobush and colleagues [31] conclude that the combination of HRP2 for P. falciparum detection and pLDH antigens for P. vivax detection might be the best way to realize a reliable RDT for malaria diagnosis. In this light, the sensitivity in detecting species other than P. falciparum and P. vivax is very low [25,33].
The observations reported by these authors meet with the Guideline for the laboratory diagnosis of malaria by Bailey et al. in 2013 [34], intended for UK and applicable to other non-endemic areas, suggesting the use of RDTs to confirm the presence or absence of P. falciparum assessed by microscopy, particularly when an inexperienced observer is involved in the diagnosis. However, for the reasons already exposed, they cannot substitute microscopy and their use is not recommended for following the response to antimalarial treatment. Furthermore, the currently available RDTs are not able to detect P. knowlesi [34,35].
RDTs have been proposed to be used as self-diagnosis technique for high-risk groups, such as travellers in endemic areas after appropriate instructions and training to allow prompt treatment and avoid over-diagnosis of malaria on-site; although some recent results are encouraging, this application is still controversial [28,36].
4. Molecular Assays
Although microscopy is still the reference method because of the reasons described above, and RDTs provide valid support for diagnosing malaria, molecular assays have been proposed as a confirmatory method. In particular, they are crucial in cases of sub-microscopic parasitemia and when morphologic characteristics overlap, and/or when parasite morphology has been altered by drug treatment or improper storage of the sample [17]. CDC suggests the use of the real-time PCR assay developed by Rougemont et al., 2004, and when a mixed infection is suspected, a nested-PCR assay by Snounou et al., 1993, which could improve the resolution [17].
In Europe, molecular methods have been largely evaluated [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
Overall, nucleic acid amplification tests (NAATs) are at least 10-fold more sensitive than microscopy [34]. The limit of detection for NAATs is approximately 0.2–6 parasites per μL of blood, depending on the assay and the species of Plasmodia involved [57].
The first target considered—and it is still used as a reference target—is the 18S-rRNA gene, present in 5–8 copies per Plasmodium genome. In particular, this reference target includes a genus-specific sequence of approximately 1.2 kb containing all the Plasmodium human-infecting species-specific sequences, which have been characterized and sequenced [42,53,58,59].
Newly developed NAATs include additional target genes, such as mitochondrial DNA (mtDNA), which allows the detection of all human malaria species together with 18S-rRNA, and other targets focusing on single species detection, such as P. falciparum stevor multigene family, telomere-associated repetitive element, and P. vivax Pvr64 sequence [60]. The 18S-rRNA gene exists in the chromosomal genome 5–8 copies depending on the strain; mitochondrial DNA exists in about 20 copies in the mitochondrial organelle. In the early ring stage, P. falciparum parasite has one mitochondrion, whereas mature gametocytes have 4–8 mitochondrial organelles [42]. In a study performed in 2013, mitochondrial PCR demonstrated to have sensitivity non-inferior to that of 18S-PCR, and interestingly, the short product size allows easy full-length sequencing [42]. The different features of these different targets were used to observe the presence of plasmodial DNA in follow-up samples post-treatment, and to determine the proportion of positive PCRs due to gametocytes in an observational study of the same research group using PCR assays targeting the var acidic terminal sequence (varATS) gene, located on the chromosomal genome, and cytochrome b (cytb) on the mitochondrial genome. The authors assumed that, as previously demonstrated, most individuals with asexual parasites also have sub-microscopic gametocyte carriage. Interestingly, cytb PCR detection in follow-up samples later than varATS PCR may be due to the detection of gametocytes, as hypothesized by the authors. However, based on their observations, the authors concluded that it is unclear whether the DNA detected after treatment originated from residuals of destroyed parasites or live gametocytes [61].
In all the studies cited in Table 2, the evaluated molecular assays, as expected, demonstrated better performance than conventional methods. Besides higher sensitivity, specificity, and accuracy, they allow to detect Plasmodia not only at the genus level, but at the species level too [37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,53,54,55,56] and they allow species identification in cases of mixed infections [37,56]. This is particularly evident in non-P. falciparum infections with low parasite density and it is important to P. malariae and P. ovale malaria because the sensitivity of RDTs can be very low [44].
Microscopy is the gold-standard method, and that cannot be avoided; however, different laboratories include in their workflows the molecular assays in ways that best suit their needs. In some laboratories, for example, the molecular assay is performed when species identification is problematic or in cases of strong suspicion of malaria with negative results by conventional methods [52,55]. Rougemont and colleagues [52] affirm that the development of automated PCR platforms and the unavailability of skilled microscopists will make molecular diagnosis more appealing at a reasonable cost, even or especially during nights and weekends. Conventional PCR has been the starting point for more sensitive, specific, and complex assays, such as nested-PCR and the application of Southern blot for the identification of Plasmodia species [37,40,49,50]. New PCR protocols evolving from conventional PCR are always in development to simplify the analysis and to reduce the possibility of contamination. As a matter of fact, conventional PCR (including the nested-PCR) is labor-intensive, time consuming, susceptible to cross-contamination by PCR products, and vulnerable to false-positive results [40,48]. This problem could be tackled by adopting several precautions [40] or developing more “safe” techniques, such as real-time PCR (Figure 4).
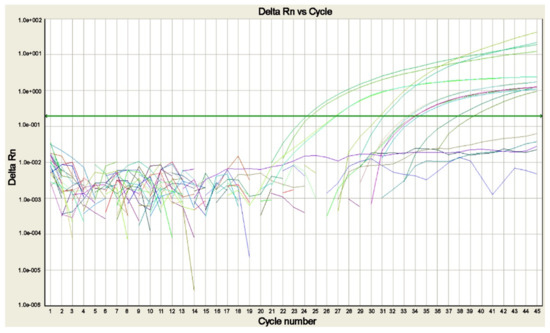
Figure 4.
Real-time PCR amplification plot for the search of Plasmodia DNA in blood samples of cases of suspected malaria. The plot shows the amplification of P. falciparum, P. malariae, P. ovale curtisi, P. ovale wallikeri, and P. vivax positive controls and of the sample positive for P. falciparum, each tested in duplicate. The green line corresponds to the threshold (picture by A. Calderaro, Department of Medicine and Surgery, University of Parma, Parma, Italy).
Real-time PCR assays are highly sensitive and specific, and far less labor-intensive. They are performed in a closed system where post-PCR handling is not required and limit the possibility of contamination together with a good rapidity, although they cannot be strictly considered a rapid technique for the initial diagnosis of malaria requiring more than 1 h [52,53]. Furthermore, as they allow DNA quantification too, their use was proposed to potentially determine the reduction of the parasite load to monitor the therapeutic efficacy [52]. In a recent study, besides the successful evaluation of two commercial kits for Plasmodia detection, the correlation between real-time PCR’s cycle threshold and parasitemia was also assessed, as previously performed [62]. Unsatisfactory and weaker results were obtained, maybe because of different storage and carriage conditions [63].
Among the different available molecular techniques, a faster and simpler method than real-time PCR for the diagnosis of malaria is a real-time quantitative nucleic acid sequence-based amplification (QT-NASBA) assay evaluated in Amsterdam [51,54] that proved to be a sensitive and specific technique useful for both the detection and the quantification of Plasmodia 18S-rRNA for diagnostic purposes and epidemiological and drug studies [51,54].
One of the most recent evolutions of DNA amplification for malaria diagnosis is the development of commercial assays based on the DNA loop-mediated isothermal amplification (LAMP) that reduce the analysis time within the 2-h delay recommended for the diagnosis and ensure a simple technical process and a high sensitivity [43,44,47]. An interesting result was obtained in a 2017 study evaluating a commercial LAMP assay (Pan and Pf LoopAMP®-Eiken Chemical Co., Tokyo, Japan) for the detection of P. ovale malaria. The LAMP results were discordant in 2.6% of samples as compared to the nested-PCR used as a reference method: it remains to be determined whether there were false positives by LAMP, or false negatives with very low parasitemia by nested-PCR, as already reported [64]. The authors were satisfied by the assay’s performance. They judged it as a useful tool for malaria control and elimination programs and in targeting returning travellers from P. ovale endemic areas [44]. In the same study, an evaluation of the LAMP results by the naked eye in comparison with the use of turbidimeter was performed; there was good correspondence, as deemed by the authors [44]. However, for such an assay [47], the target sequence is not declared, and this remains a bias for its use in the practice and its comparison with other assays.
Dakić and colleagues [38] encourage the use of molecular assays, especially in non-endemic settings, as a complementary method to microscopy, particularly in cases of low parasitemia and for species determination, taking into account that most instances of misdiagnosis occur in cases of malaria by Plasmodia other than P. falciparum. Although the improved sensitivity is evident, their adoption and inclusion in the workflow should be deeply evaluated.
It cannot be ignored that they detected the parasitic DNA while not distinguishing among DNA belonging to live parasites, residual DNA of destroyed asexual blood stage parasites, and circulating gametocytes which can remain in sub-microscopic quantities after successful therapy, thereby risking false positives due to the persistence of DNA after a malaria episode’s resolution, and as a consequence, unnecessary malaria treatment interventions [38,45,46]. However, a control experiment performed in an animal model [65] demonstrated the clearance of parasite DNA from blood within 48 h after malaricides treatment; thus, it can be inferred that Plasmodium DNA detected in blood is probably a sign of active infection, even if no parasites are detected by microscopy [50]. Further disadvantages are the requirements for a sophisticated laboratory setting and trained operators, and the higher costs [45]. The wide spread of different molecular assays for the diagnosis of malaria, often developed in-house, laid the foundations in 2008 for the establishment by WHO of an International Standard for Plasmodium falciparum DNA for (NAT)-based assays that can be used for quality control and in the determination of the analytical sensitivity of different assays [66].
These considerations strengthen the need to carefully apply molecular techniques to the diagnosis of malaria.
One of the main current challenges is the detection of P. knowlesi in travellers with suspected malaria returning from Southeast Asia. The detection of P. knowlesi is mandatory, since the infection can be fatal if not treated promptly; however, its identification by microscopy is particularly difficult because of the morphological resemblance of early trophozoites to P. falciparum and later erythrocytic stages to P. malariae [41]. In this light, the inclusion of molecular assays in the malaria diagnostic workflow in Europe became essential, and as reported in Table 2, it was applied successfully by different authors [41,63].


Table 2.
Molecular assays field evaluated by European diagnostic laboratories.
Table 2.
Molecular assays field evaluated by European diagnostic laboratories.
| Evaluated Assay | Type of Amplification | Target | Country | Samples/ Patients Tested | Period | Reference Test | Performance/ Agreement with Reference Test | Reference |
|---|---|---|---|---|---|---|---|---|
| In-house species-specific PCR | Nested PCR | 18S rRNA | Spain | 192 samples/patients with suspected malaria | 1997–1998 | Microscopy | 12.4% more malaria cases detected by PCR | [37] |
| In-house genus-specific PCR | Conventional genus-specific PCR | 18S rRNA | Italy | 101 samples/ patients with suspected malaria | 1994–1999 | Microscopy | Sensitivity 100% Specificity 100% | [49] |
| species-specific PCR by [67] and by [68] | species-specific Southern blot | Agreement 94% | ||||||
| In-house species-specific PCR by [58] | Nested PCR | 18S rRNA | Poland | 216 patients with suspected malaria | / | Microscopy | Agreement 83.8% | [50] |
| In-house genus-specific PCR | QT-NASBA | 18S rRNA | The Netherlands | 113 patients with suspected malaria | 4 months | Microscopy | Sensitivity 100% Specificity 94% Agreement 94.7% | [51] |
| In-house genus-specific qPCR | TaqMan | 18S rRNA | Switzerland | 97 samples/ 66 from patients with suspected malaria + 31 from patients with known Pf malaria | 2002–2003 | Microscopy | 86% agreement | [52] |
| In-house species-specific qPCR | 71% agreement | |||||||
| In-house genus-specific PCR+ species-specific PCR (Pf, Pv, Po) | TaqMan | 18S rRNA | Italy | 122 samples/patients with suspected malaria | / | 18S rRNA nested PCR | Sensitivity 100% Specificity 100% | [53] |
| In-house species-specific PCR | QT-NASBA | 18S rRNA | The Netherlands | 79 samples of patients with malaria | / | Microscopy | Perfect agreement (evaluated by Cohen’s Kappa coefficient) | [54] |
| Pf qReal Time-PCR by [69] | Sybr Green | Pf CoxI gene | France | 192 patients with suspected malaria | 2005–2007 | Microscopy +RDT | 93.3% agreement | [55] |
| In-house genus-specific qPCR | Plasmodium mitochondrial sequence | 99% agreement | ||||||
| In-house species-specific qPCR | 18S rRNA | 98% agreement | ||||||
| Genus-specific qPCR by [52] with modifications | TaqMan | 18S rRNA Plasmodium gene | Belgium | 351 samples | 1995–2009 | Microscopy | 8.3% cases detected only by PCR | [56] |
| In-house species-specific PCR | 18S rRNA specific genes | 1.3% species identification only by PCR | ||||||
| In-house species-specific PCR by [70] | Seminested PCR | 18S rRNA | Italy | 1226 patients with suspected malaria | 1998–2003 | Microscopy | Sensitivity 100% Specificity 100% PPV 100% NPV 100% | [40] |
| In-house species-specific PCR for Poc and Pow | TaqMan | 18S rRNA | Italy | 31 samples from patients with P.ovale malaria | / | 18S rRNA nested PCR | 100% agreement | [39] |
| Genus-specific PCR by [58] | TaqMan | 18S rRNA | Norway | 135 samples/patients with suspected malaria | 2006–2011 | Nested SSU rRNA PCR | 93% agreement | [42] |
| Genus-specific PCR by [71] | Mitochondrial DNA sequence | 97% agreement | ||||||
| Species-specific PCR [72] | 18S rRNA | 87% agreement | ||||||
| In-house genus-specific PCR + species -specific PCR | TaqMan | 18S rRNA (including Pk, Poc and Pow) | Italy | 398 samples/ patients with suspected malaria | 2000–2012 | Microscopy | 6.3% species identification only by PCR:1.5% samples disagreement with microscopy | [41] |
| Genus-specific qPCR [52] | TaqMan | 18S rRNA Plasmodium gene | Serbia | 109 samples/patients with suspected malaria | 2010–2013 | Microscopy | 95.5% agreement | [38] |
| Species-specific qPCR by [53] (for Pf, Pv, Po) and by [52] (for Pm) | 18S rRNA specific genes | 73.3% agreement | ||||||
| Pan and Pf LoopAMP® (Eiken Chemical Co.) | LAMP | Mitochondrial DNA sequence | Switzerland | 210 samples/patients with suspected malaria | March–October 2012 | Microscopy | Sensitivity 100% Specificity 97.5% PPV 91.5% NPV 100% | [43] |
| 18S rRNA qPCR | Sensitivity 100% Specificity 100% PPV 100% NPV 100% | |||||||
| Pan and Pf LoopAMP® (Eiken Chemical Co.) | LAMP | Mitochondrial DNA sequence | Spain | 427 samples: 29 Po positive samples+ 398 negative samples stored | 2014–2016 | Nested SSU rRNA PCR | Sensitivity 100% Specificity 97.2% PPV 72.5% NPV 100% | [44] |
| Genus-specific PCR and Species-specific PCR (FTD Malaria, Fast-Track Diagnostics®) | TaqMan | / | Spain | 250 patients: 86 with suspected malaria+ 164 asymptomatic immigrants from endemic areas | 2015–2017 | In-house genus-and species-specific PCR | Sensitivity 96% Specificity 97.4% PPV 93.6% NPV 98% | [45] |
| Species-specific PCR by RealStar Malaria S&T PCR Kit 1.0 | TaqMan | Plasmodium spp. DNA (including Pk) | Germany | 179 samples positive by microscopy and genus-specific PCR | April–December 2017 | Microscopy+ genus-specific PCR | Sensitivity 95.1% | [46] |
| Genus-specific PCR and Species-specific PCR (FTD Malaria, Fast-Track Diagnostics®) | Sensitivity 96.8% | |||||||
| In-house species-specific duplex PCR for Poc and Pow by [73] | TaqMan | 18S rRNA | Germany | 77 samples/patients with P.ovale malaria | 2010–2019 | / | 100% agreement among the 2 evaluated assays | [63] |
| In-house species-specific singleplex PCR for Poc and Pow described by [74] and [39] | ||||||||
| Alethia assay (Meridian Bioscience) | LAMP | Undeclared target: segments of the Plasmodium genome | France | 331 samples/patients with suspected malaria | 2017–2018 | Real-time PCR | Sensitivity 97.3% Specificity 99.6% PPV 94.8% NPV 99.8% | [47] |
| In-house Pfhrp2 PCR | TaqMan | Pfhrp2 gene | United Kingdom/ Switzerland/ Portugal | 50 DNA samples from suspected Pf patients from Eritrea | / | Conventional qPCR | Sensitivity 100% | [48] |
| In-house Pfhrp3 PCR | Pfhrp3 gene | Specificity 100% |
Performance: calculation of diagnostic sensitivity, specificity, positive predictive value, and/or negative predictive value. CoxI: mitochondrial cytochrome C oxidase. Pf: Plasmodium falciparum, Po: P. ovale, Pv: P. vivax, Pm: P.malariae, Poc: P. ovale curtisi, Pow: P. ovale wallikeri, Pk: P. knowlesi. PCR: Polymerase Chain Reaction. PPV: Positive Predictive Value. NPV: Negative Predictive Value. LAMP: loop-mediated isothermal amplification. SSU: small subunit. QT-NASBA: Quantitative Nucleic Acid Sequence-Based Amplification. /: Not reported.
A summary of the key features of, and the desired improvements for, microscopic examination, RDTs, and NAATs for the diagnosis of malaria, are reported in Table 3. Moreover, the milestones in the introduction of the methods currently used, since the discovery of malaria parasites in 1880 by microscopy [75], and herein described, are shown in Figure 5, highlighting that the novelties proposed in the last 22 years are improvements and evolutions of previously developed assays.

Table 3.
Key features and future desired improvements of the methods for the diagnosis of malaria.

Figure 5.
Milestones of the introduction of diagnostic assays for malaria (the red rectangle shows the milestones included in this review) [21,39,41,43,49,51,52,53,55,58,67].
5. Other Diagnostic Methods
Together with molecular assays, other novel techniques have been developed for the diagnosis of malaria, particularly those detecting hemozoin [76,77,78,79,80,81] in both endemic and non-endemic areas, which can be mutually exported. The starting point is the assumption that the detection in a patient’s leukocytes of hemozoin, generated through the digestion of the globin part of hemoglobin by Plasmodia, is indicative of malaria infection [76]. Hänscheid and colleagues [77,82] have developed a flow-cytometry assay by using an automated full blood counts (FBC) instrument that, taking advantages from the anisotropic properties of hemozoin, allows to detect the Plasmodium sp. pigment in those laboratories where FBC is routinely performed. Although promising, if applied in addition to conventional methods, this approach still requires extensive field evaluation [82].
In 2010, Mens et al. [76] evaluated the magneto-optical technology (MOT) exploiting the paramagnetic features of hemozoin. When the samples are submitted to a magnetic field, the hemozoin crystals, if present, align with the magnetic field. A laser-based instrument able to quantify this phenomenon allows to understand whether hemozoin is present or not in a sample. The results obtained demonstrated a performance not yet at a competitive level compared to other diagnostic tests [76]. A technical improvement in the magneto-optical detection of hemozoin crystals has been recently proposed by Arndt et al. [78] in Papua New Guinea. The authors hope it will be used in other settings too. The novel diagnostic technique named rotating-crystal magneto-optical detection (RMOD) maximizes the MO signal, rapidly providing a measurement of the magnetically induced linear dichroism of hemozoin. Furthermore, RMOD demonstrated to be able to quantify the amount of the pigment in a sample. However, RMOD, by revealing the presence of residual hemozoin, is not able to discriminate between current and previous infections. The authors affirmed that this limitation is expected to be reduced in low-transmission settings. Moreover, in the current state of development, RMOD cannot distinguish between parasite species in P. falciparum and P.vivax co-endemic settings. Thus, according to the authors’ conclusions, this technique requires further evaluation and potential further improvements for both endemic and non-endemic settings [78].
The magnetic susceptibility of hemozoin has led to the development of innovative detection methods based on nuclear magnetic resonance (NMR) and on magnetic resonance relaxometry (MRR) ([79,80], respectively). Gupta et al., in 2020 [80], proposed a portable banchtop assay based on NMR that turned out to be sensitive, easy to handle, cost-effective, and able to work with only a small sample volume. In the same year, Di Gregorio et al. [79] developed an MRR assay that appears to be an efficient tool for the detection of P. falciparum-parasitized RBC and that could be useful to assess the effects of dihydroartemisinin and chloroquine.
The detection of hemozoin in RBC parasitized by P. falciparum has been investigated also by using a novel photoacustics (PA) excited surface acoustic wave (SAW) sensor [81]. The authors demonstrated the good potential of a PA-SAW sensor in the diagnosis of malaria at early stages and at a concentration of 1%. They aimed to improve the performance of the developed technique and to extend its use to other parasite species.
In conclusion, therefore, the described novel techniques that search for hemozoin are not yet tools applicable to the diagnosis of malaria, but they could be promising solutions, after improvements, for future diagnostic systems.
In Figure 6, an algorithm for the laboratory diagnosis of malaria is proposed for both endemic and non-endemic areas, on the basis of that reported by WHO, based on microscopic examination and rapid diagnostic tests [83].
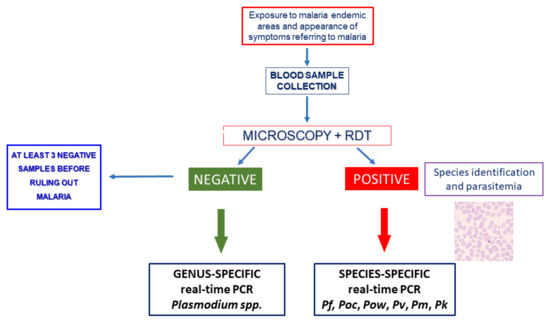
Figure 6.
A diagnostic algorithm for malaria for non-endemic areas. Pf: Plasmodium falciparum, Pv: P. vivax, Pm: P. malariae, Poc: P. ovale curtisi, Pow: P. ovale wallikeri, Pk: P. knowlesi. PCR: polymerase chain reaction. RDT: rapid diagnostic tests.
6. Conclusions
Malaria is a rare diagnosis in Europe, but it is a medical emergency. A travel history is the key when malaria is suspected, and it is mandatory in patients with fever. There are no specific clinical signs or symptoms of malaria, although fever is seen in almost all non-immune patients. Migrants from malaria-endemic areas may have few symptoms.
Malaria diagnostics should be performed immediately on suspicion of malaria, and the gold-standard is microscopy of Giemsa-stained thick and thin blood films. The quantification of malaria parasites can be used to make clinical management decisions and to monitor responses to treatment. Microscopy diagnosis is prone to human error, owing to its subjective nature. An inherent weakness of microscopy is the dependence on morphological features when Plasmodium species are being distinguished. Even under ideal conditions, reliable distinction of the infecting Plasmodium species can be very difficult, if not impossible. Particularly, P. vivax and P. ovale cannot always be easily differentiated based on morphology; distinguishing P. knowlesi from P. malariae can be very challenging; P. ovale wallikeri and P. ovale curtisi are morphologically identical; P. cynomolgi is morphologically indistinguishable from P. vivax; and P. simium and P. brasilianum cannot be distinguished by microscopy from P. vivax and P. malariae, respectively. The limit of detection is also not ideal, because sub-microscopic asymptomatic individuals with low parasitemia remain undiagnosed and untreated, and also enable the transmission cycle to continue in the community.
A RDT may be used in parallel, but should not replace microscopy [20]. It is a fast and affordable method for malaria diagnosis; the personnel training required is much less intensive as compared to microscopy and PCR. However, it does not allow for the quantification of parasitemia, and consequently, monitoring therapeutic effectiveness is difficult [20,84]. Microscopy remains the gold-standard technique for diagnosis but RDTs, originally limited to endemic areas and returning travellers from endemic areas, are now more widely used as a complement to microscopy [85].
Molecular methods have demonstrated to be more sensitive and specific than microscopy, allowing the detection of missed cases and correctly identifying the species of Plasmodia of medical interest, with the final result of improving the early diagnosis of all cases of imported malaria [14,20]. However, their application should be deeply evaluated because of the risk of false positives due to the persistence of DNA after malaria episodes resolve [38,45,63].
The proposed algorithm takes into account these observations and the essential contribution of the genus- and species-specific DNA amplification assays for accurate diagnosis of malaria.
According to WHO Global Technical Strategy for Malaria 2016–2030 [8], the future direction for the diagnosis of infectious diseases, including malaria, in both endemic and non-endemic settings, is the development of point-of-care testing (POCT) in response to the request for rapid diagnosis, together with “on-site” results, which would be helpful for prompt and accurate treatment and for preventing the transmission of infectious diseases [86]. Several research groups developed new generation assays, or adapted pre-existing assays to smart devices. Furthermore, at present, efforts are being made to support POCT by using devices derived from innovations in the field of Internet of Medical Things (IoMT), offering wireless-based operations and connectivity of such devices with medical centers [86].
This review showed that diagnostic laboratories in malaria non-endemic settings provide excellent diagnosis of malaria, especially regarding the detection of P. falciparum.
Despite the limitations of current diagnostic methods, they continue to play important roles in dealing with the current global malaria situation, including decreasing its incidence.
Diagnostic tools are critical for ensuring the appropriate care for each patient, and in this light, the development of numerous innovations continues.
Author Contributions
Conceptualization, A.C.; methodology, A.C.; data curation, writing—original draft preparation, S.M., M.B., G.P. and S.R. writing—review and editing, A.C., S.M., M.B., G.P., M.C.A., B.F., F.D.C. and C.C.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of University and Scientific Research grant FIL, University of Parma, Parma, Italy and the grant “Fondo di finanziamento per le attività base di ricerca (FFABR)" from the Italian Ministry for the University and Research (Ministero dell’Università e della Ricerca, MUR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Anstey, N.M.; Grigg, M.J. Zoonotic Malaria: The Better You Look, the More You Find. J. Infect. Dis. 2019, 219, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Madmanee, W.; Suwannasin, K.; Kunasol, C.; Peto, T.J.; Tripura, R.; von Seidlein, L.; Nguon, C.; Davoeung, C.; Day, N.P.J.; et al. Asymptomatic Natural Human Infections with the Simian Malaria Parasites Plasmodium cynomolgi and Plasmodium knowlesi. J. Infect. Dis. 2019, 219, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.J.; Snounou, G. Plasmodium simium: A Brazilian focus of anthropozoonotic vivax malaria? Lancet Glob. Health 2017, 5, e961–e962. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 28 September 2021).
- WHO. Severe malaria. Trop. Med. Int. Health 2014, 19 (Suppl. 1), 7–131. [Google Scholar] [CrossRef] [PubMed]
- Kamaliddin, C.; Le Bouar, M.; Berry, A.; Fenneteau, O.; Gillet, P.; Godineau, N.; Candolfi, E.; Houzé, S. Assessment of diagnostic methods for imported malaria in mainland France. Med. Mal. Infect. 2020, 50, 141–160. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Technical Strategy for Malaria 2016–2030. 2021. Available online: https://www.who.int/publications/i/item/9789240031357 (accessed on 28 September 2021).
- WHO. WHO Malaria Terminology. 2019. Available online: https://www.who.int/publications/i/item/WHO-HTM-GMP-2016.6 (accessed on 29 August 2021).
- Centers for Disease Control and Prevention. Malaria’s Impact Worldwide. 2021. Available online: https://www.cdc.gov/malaria/malaria_worldwide/impact.html (accessed on 28 September 2021).
- Piperaki, E.T.; Daikos, G.L. Malaria in Europe: Emerging threat or minor nuisance? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Tseroni, M.; Pervanidou, D.; Tserkezou, P.; Rachiotis, G.; Pinaka, O.; Baka, A.; Georgakopoulou, T.; Vakali, A.; Dionysopoulou, M.; Terzaki, I.; et al. Field application of SD bioline malaria Ag Pf/Pan rapid diagnostic test for malaria in Greece. PLoS ONE 2015, 10, e0120367. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. 2021 Malaria-Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2019#no-link (accessed on 28 September 2021).
- Calderaro, A.; Gorrini, C.; Peruzzi, S.; Piccolo, G.; Dettori, G.; Chezzi, C. An 8-year survey on the occurrence of imported malaria in a nonendemic area by microscopy and molecular assays. Diagn. Microbiol. Infect. Dis. 2008, 61, 434–439. [Google Scholar] [CrossRef]
- Rossi, I.A.; D’Acremont, V.; Prod’Hom, G.; Genton, B. Safety of falciparum malaria diagnostic strategy based on rapid diagnostic tests in returning travelers and migrants: A retrospective study. Malar. J. 2012, 11, 377. [Google Scholar] [CrossRef]
- Kamaliddin, C.; Salnot, V.; Leduc, M.; Ezinmegnon, S.; Broussard, C.; Fievet, N.; Deloron, P.; Guillonneau, F.; Bertin, G.I. PFI1785w: A highly conserved protein associated with pregnancy associated malaria. PLoS ONE 2017, 12, e0187817. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2020 Malaria Diagnostic Tests. Available online: https://www.cdc.gov/malaria/diagnosis_treatment/diagnostic_tools.html (accessed on 28 September 2021).
- Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Dhorda, M.; Ba, E.H.; Kevin Baird, J.; Barnwell, J.; Bell, D.; Carter, J.Y.; Dondorp, A.; Ekawati, L.; Gatton, M.; González, I.; et al. Towards harmonization of microscopy methods for malaria clinical research studies. Malar. J. 2020, 19, 324. [Google Scholar] [CrossRef]
- Calderaro, A.; Piccolo, G.; Montecchini, S.; Buttrini, M.; Rossi, S.; Dell’Anna, M.L.; De Remigis, V.; Arcangeletti, M.C.; Chezzi, C.; De Conto, F. High prevalence of malaria in a non-endemic setting: Comparison of diagnostic tools and patient outcome during a four-year survey (2013–2017). Malar. J. 2018, 17, 63. [Google Scholar] [CrossRef]
- Eibach, D.; Traore, B.; Bouchrik, M.; Coulibaly, B.; Coulibaly, N.; Siby, F.; Bonnot, G.; Bienvenu, A.-L.; Picot, S. Evaluation of the malaria rapid diagnostic test VIKIA malaria Ag Pf/PanTM in endemic and non-endemic settings. Malar. J. 2013, 12, 188. [Google Scholar] [CrossRef]
- Garcia, L.S. Diagnostic Medical Parasitology; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Gimenez, A.M.; Marques, R.F.; Regiart, M.; Bargieri, D.Y. Diagnostic Methods for Non-Falciparum Malaria. Front. Cell. Infect. Microbiol. 2021, 11, 681063. [Google Scholar] [CrossRef]
- WHO. Malaria Rapid Diagnostic Test Performance. Results of WHO Product Testing of Malaria RDTs: Round 8 (2016–2018). 2018. Available online: https://www.who.int/publications/i/item/9789241514965 (accessed on 25 October 2021).
- Houzé, S.; Boutron, I.; Marmorat, A.; Dalichampt, M.; Choquet, C.; Poilane, I.; Godineau, N.; Le Guern, A.-S.; Thellier, M.; Broutier, H.; et al. Performance of rapid diagnostic tests for imported malaria in clinical practice: Results of a national multicenter study. PLoS ONE 2013, 8, e75486. [Google Scholar] [CrossRef]
- Pasquier, G.; Azoury, V.; Sasso, M.; Laroche, L.; Varlet-Marie, E.; Houzé, S.; Lachaud, L.; Bastien, P.; Sterkers, Y.; Leveque, M.F. Rapid diagnostic tests failing to detect infections by Plasmodium falciparum encoding pfhrp2 and pfhrp3 genes in a non-endemic setting. Malar. J. 2020, 19, 179. [Google Scholar] [CrossRef]
- Cropley, I.M.; Lockwood, D.N.; Mack, D.; Pasvol, G.; Davidson, R.N. Rapid diagnosis of Falciparum malaria by using the ParaSight F test in travellers returning to the United Kingdom: Prospective study. BMJ 2000, 321, 484–485. [Google Scholar] [CrossRef][Green Version]
- Jelinek, T.; Grobusch, M.P.; Nothdurft, H.D. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. J. Travel Med. 2000, 7, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Viani, I.; Piccolo, G.; Fabio, A.; Calderaro, A.; Galati, L.; Perandin, F.; Vecchia, L.; Manca, N.; Dettori, G.; et al. Evaluation of OptiMAL Assay test to detect imported malaria in Italy. New Microbiol. 2000, 23, 391–398. [Google Scholar]
- Rubio, J.M.; Buhigas, I.; Subirats, M.; Baquero, M.; Puente, S.; Benito, A. Limited level of accuracy provided by available rapid diagnosis tests for malaria enhances the need for PCR-based reference laboratories. J. Clin. Microbiol. 2001, 39, 2736–2737. [Google Scholar] [CrossRef] [PubMed]
- Grobusch, M.P.; Hänscheid, T.; Göbels, K.; Slevogt, H.; Zoller, T.; Rögler, G.; Teichmann, D. Comparison of three antigen detection tests for diagnosis and follow-up of falciparum malaria in travellers returning to Berlin, Germany. Parasitol. Res. 2003, 89, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Crassous, B.; Fricker-Hidalgo, H.; Carpentier, F.; Brion, J.-P.; Grillot, R.; Pelloux, H. Performance of the Now Malaria rapid diagnostic test with returned travellers: A 2-year retrospective study in a French teaching hospital. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2005, 11, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, D.P.J.; Gillet, P.; Vlieghe, E.; Cnops, L.; van Esbroeck, M.; Jacobs, J. Evaluation of the Palutop+4 malaria rapid diagnostic test in a non-endemic setting. Malar. J. 2009, 8, 293. [Google Scholar] [CrossRef]
- Bailey, J.W.; Williams, J.; Bain, B.J.; Parker-Williams, J.; Chiodini, P.L. Guideline: The laboratory diagnosis of malaria. General Haematology Task Force of the British Committee for Standards in Haematology. Br. J. Haematol. 2013, 163, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Chilton, D.; Malik, A.N.J.; Armstrong, M.; Kettelhut, M.; Parker-Williams, J.; Chiodini, P.L. Use of rapid diagnostic tests for diagnosis of malaria in the UK. J. Clin. Pathol. 2006, 59, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Berthod, D.; Rochat, J.; Voumard, R.; Rochat, L.; Genton, B.; D’Acremont, V. Self-diagnosis of malaria by travellers: A cohort study on the use of malaria rapid diagnostic tests provided by a Swiss travel clinic. Malar. J. 2017, 16, 436. [Google Scholar] [CrossRef]
- Rubio, J.M.; Benito, A.; Berzosa, P.J.; Roche, J.; Puente, S.; Subirats, M.; López-Vélez, R.; García, L.; Alvar, J. Usefulness of seminested multiplex PCR in surveillance of imported malaria in Spain. J. Clin. Microbiol. 1999, 37, 3260–3264. [Google Scholar] [CrossRef]
- Dakić, Z.; Ivović, V.; Pavlović, M.; Lavadinović, L.; Marković, M.; Djurković-Djaković, O. Clinical significance of molecular methods in the diagnosis of imported malaria in returning travelers in Serbia. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 29, 24–30. [Google Scholar] [CrossRef]
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Montecchini, S.; Rossi, S.; Medici, M.C.; Chezzi, C.; Snounou, G. A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS ONE 2012, 7, e48033. [Google Scholar] [CrossRef]
- Paglia, M.G.; Vairo, F.; Bevilacqua, N.; Ghirga, P.; Narciso, P.; Severini, C.; Nicastri, E. Molecular diagnosis and species identification of imported malaria in returning travellers in Italy. Diagn. Microbiol. Infect. Dis. 2012, 72, 175–180. [Google Scholar] [CrossRef]
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Rossi, S.; Montecchini, S.; Dell’Anna, M.L.; De Conto, F.; Medici, M.C.; Chezzi, C.; Arcangeletti, M.C. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar. J. 2013, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Haanshuus, C.G.; Mohn, S.C.; Mørch, K.; Langeland, N.; Blomberg, B.; Hanevik, K. A novel, single-amplification PCR targeting mitochondrial genome highly sensitive and specific in diagnosing malaria among returned travellers in Bergen, Norway. Malar. J. 2013, 12, 26. [Google Scholar] [CrossRef]
- Marti, H.; Stalder, C.; González, I.J. Diagnostic accuracy of a LAMP kit for diagnosis of imported malaria in Switzerland. Travel Med. Infect. Dis. 2015, 13, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, J.; Martin Ramírez, A.; González, I.J.; Ding, X.C.; Perez Tanoira, R.; Rojo-Marcos, G.; Gómez-Herruz, P.; Rubio, J.M. LAMP kit for diagnosis of non-falciparum malaria in Plasmodium ovale infected patients. Malar. J. 2017, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Martín-Díaz, A.; Rubio, J.M.; Herrero-Martínez, J.M.; Lizasoain, M.; Ruiz-Giardin, J.M.; Jaqueti, J.; Cuadros, J.; Rojo-Marcos, G.; Martín-Rabadán, P.; Calderón, M.; et al. Study of the diagnostic accuracy of microbiological techniques in the diagnosis of malaria in the immigrant population in Madrid. Malar. J. 2018, 17, 314. [Google Scholar] [CrossRef]
- Frickmann, H.; Wegner, C.; Ruben, S.; Behrens, C.; Kollenda, H.; Hinz, R.; Rojak, S.; Schwarz, N.G.; Hagen, R.M.; Tannich, E. Evaluation of the multiplex real-time PCR assays RealStar malaria S&T PCR kit 1.0 and FTD malaria differentiation for the differentiation of Plasmodium species in clinical samples. Travel Med. Infect. Dis. 2019, 31, 101442. [Google Scholar] [CrossRef]
- Charpentier, E.; Benichou, E.; Pagès, A.; Chauvin, P.; Fillaux, J.; Valentin, A.; Guegan, H.; Guemas, E.; Salabert, A.-S.; Armengol, C.; et al. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Grignard, L.; Nolder, D.; Sepúlveda, N.; Berhane, A.; Mihreteab, S.; Kaaya, R.; Phelan, J.; Moser, K.; van Schalkwyk, D.A.; Campino, S.; et al. A novel multiplex qPCR assay for detection of Plasmodium falciparum with histidine-rich protein 2 and 3 (pfhrp2 and pfhrp3) deletions in polyclonal infections. EBioMedicine 2020, 55, 102757. [Google Scholar] [CrossRef]
- Perandin, F.; Manca, N.; Galati, L.; Piccolo, G.; Calderaro, A.; Viani, I.; Ricci, L.; Dettori, G.; Chezzi, C.; Turano, A. Usefulness of genus-specific PCR and Southern blot species-specific hybridization for the detection of imported malaria cases in Italy. New Microbiol. 2001, 24, 69–76. [Google Scholar]
- Myjak, P.; Nahorski, W.; Pieniazek, N.J.; Pietkiewicz, H. Usefulness of PCR for diagnosis of imported malaria in Poland. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2002, 21, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.F.H.; Schoone, G.J.; Lommerse, E.J.M.; Kroon, C.C.M.; de Vries, P.J.; van Gool, T. Usefulness of quantitative nucleic Acid sequence-based amplification for diagnosis of malaria in an academic hospital setting. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2003, 22, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Rougemont, M.; Van Saanen, M.; Sahli, R.; Hinrikson, H.P.; Bille, J.; Jaton, K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 2004, 42, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Perandin, F.; Manca, N.; Calderaro, A.; Piccolo, G.; Galati, L.; Ricci, L.; Medici, M.C.; Arcangeletti, M.C.; Snounou, G.; Dettori, G.; et al. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J. Clin. Microbiol. 2004, 42, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Mens, P.F.; Schoone, G.J.; Kager, P.A.; Schallig, H.D.F.H. Detection and identification of human Plasmodium species with real-time quantitative nucleic acid sequence-based amplification. Malar. J. 2006, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, N.; Boutet, A.; Bousquet, P.-J.; Basset, D.; Douard-Enault, C.; Charachon, S.; Lachaud, L. Comparison of three real-time PCR methods with blood smears and rapid diagnostic test in Plasmodium sp. infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010, 16, 1305–1311. [Google Scholar] [CrossRef]
- Cnops, L.; Jacobs, J.; Van Esbroeck, M. Validation of a four-primer real-time PCR as a diagnostic tool for single and mixed Plasmodium infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2011, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Evidence Review Group on Malaria Diagnosis in Low Transmission Settings. 2014. Available online: https://www.who.int/malaria/mpac/mpac_mar2014_diagnosis_low_transmission_settings_report.pdf?ua=1 (accessed on 25 October 2021).
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Singh, B.; Sung, L.K.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.G.; Cox-singh, J.; Thomas, A. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2003, 362, 1504. [Google Scholar] [CrossRef]
- Zimmerman, P.A.; Howes, R.E. Malaria diagnosis for malaria elimination. Curr. Opin. Infect. Dis. 2015, 28, 446–454. [Google Scholar] [CrossRef]
- Haanshuus, C.G.; Mørch, K. Detection of remaining Plasmodium DNA and gametocytes during follow up after curative malaria treatment among returned travellers in Norway. Malar. J. 2020, 19, 296. [Google Scholar] [CrossRef]
- Mischlinger, J.; Pitzinger, P.; Veletzky, L.; Groger, M.; Zoleko-Manego, R.; Adegnika, A.A.; Agnandji, S.T.; Lell, B.; Kremsner, P.G.; Mombo-Ngoma, G.; et al. Validity and reliability of methods to microscopically detect and quantify malaria parasitaemia. Trop. Med. Int. Health 2018, 23, 980–991. [Google Scholar] [CrossRef]
- Frickmann, H.; Wegner, C.; Ruben, S.; Loderstädt, U.; Tannich, E. A comparison of two PCR protocols for the differentiation of Plasmodium ovale species and implications for clinical management in travellers returning to Germany: A 10-year cross-sectional study. Malar. J. 2019, 18, 272. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-L.; Lai, M.-Y.; Fong, M.-Y.; Jelip, J.; Mahmud, R. Loop-Mediated Isothermal Amplification Assay for Identification of Five Human Plasmodium Species in Malaysia. Am. J. Trop. Med. Hyg. 2016, 94, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Jarra, W.; Snounou, G. Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect. Immun. 1998, 66, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Padley, D.J.; Heath, A.B.; Sutherland, C.; Chiodini, P.L.; Baylis, S.A. Establishment of the 1st World Health Organization International Standard for Plasmodium falciparum DNA for nucleic acid amplification technique (NAT)-based assays. Malar. J. 2008, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kunisada, K.; Kawai, S.; Kimura, M.; Wataya, Y. DNA Diagnosis of Ovale Malaria and Malariae Malaria Using Microtiter Plate-Hybridization. Nucleosides Nucleotides 1994, 13, 1363–1374. [Google Scholar] [CrossRef]
- Kawai, S.; Maekawajiri, S.; Yamane, A. A simple method of detecting amplified DNA with immobilized probes on microtiter wells. Anal. Biochem. 1993, 209, 63–69. [Google Scholar] [CrossRef]
- Elsayed, S.; Plewes, K.; Church, D.; Chow, B.; Zhang, K. Use of molecular beacon probes for real-time PCR detection of Plasmodium falciparum and other plasmodium species in peripheral blood specimens. J. Clin. Microbiol. 2006, 44, 622–624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Wirtz, R.A.; McConkey, G.A.; Sattabongkot, J.; Waters, A.P.; Rogers, M.J.; McCutchan, T.F. Plasmodium: Genus-conserved primers for species identification and quantitation. Exp. Parasitol. 1995, 81, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.D.; Mori, Y.; Watson, J.; Perkins, M.D.; González, I.J.; Notomi, T.; Chiodini, P.L.; Sutherland, C.J. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J. Clin. Microbiol. 2010, 48, 2866–2871. [Google Scholar] [CrossRef]
- Padley, D.; Moody, A.H.; Chiodini, P.L.; Saldanha, J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 2003, 97, 131–137. [Google Scholar] [CrossRef]
- Bauffe, F.; Desplans, J.; Fraisier, C.; Parzy, D. Real-time PCR assay for discrimination of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in the Ivory Coast and in the Comoros Islands. Malar. J. 2012, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Piccolo, G.; Perandin, F.; Gorrini, C.; Peruzzi, S.; Zuelli, C.; Ricci, L.; Manca, N.; Dettori, G.; Chezzi, C.; et al. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J. Clin. Microbiol. 2007, 45, 1624–1627. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Laveran and the Discovery of the Malaria Parasite. 2015. Available online: https://www.cdc.gov/malaria/about/history/laveran.html (accessed on 25 October 2021).
- Mens, P.F.; Matelon, R.J.; Nour, B.Y.M.; Newman, D.M.; Schallig, H.D.F.H. Laboratory evaluation on the sensitivity and specificity of a novel and rapid detection method for malaria diagnosis based on magneto-optical technology (MOT). Malar. J. 2010, 9, 207. [Google Scholar] [CrossRef]
- Hänscheid, T.; Valadas, E.; Grobusch, M.P. Automated malaria diagnosis using pigment detection. Parasitol. Today 2000, 16, 549–551. [Google Scholar] [CrossRef]
- Arndt, L.; Koleala, T.; Orbán, Á.; Ibam, C.; Lufele, E.; Timinao, L.; Lorry, L.; Butykai, Á.; Kaman, P.; Molnár, A.P.; et al. Magneto-optical diagnosis of symptomatic malaria in Papua New Guinea. Nat. Commun. 2021, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, E.; Ferrauto, G.; Schwarzer, E.; Gianolio, E.; Valente, E.; Ulliers, D.; Aime, S.; Skorokhod, O. Relaxometric studies of erythrocyte suspensions infected by Plasmodium falciparum: A tool for staging infection and testing anti-malarial drugs. Magn. Reson. Med. 2020, 84, 3366–3378. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, K.; Lobiyal, D.K.; Safvan, C.P.; Sahu, B.K.; Yadav, P.; Singh, S. A sensitive on-chip probe–based portable nuclear magnetic resonance for detecting low parasitaemia plasmodium falciparum in human blood. Med. Devices Sens. 2020, 3, e10098. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Preiser, P.; Zheng, Y. A Photoacoustic-Surface-Acoustic-Wave Sensor for Ring-Stage Malaria Parasite Detection. IEEE Trans. Circuits Syst. II Express Briefs 2020, 67, 881–885. [Google Scholar] [CrossRef]
- Hänscheid, T.; Melo-Cristino, J.; Pinto, B.G. Automated detection of malaria pigment in white blood cells for the diagnosis of malaria in Portugal. Am. J. Trop. Med. Hyg. 2001, 64, 290–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHO. Universal Access to Malaria Diagnostic Testing: An Operational Manual. 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/44657/9789241502092_eng.pdf?sequence=1&isAllowed=y (accessed on 25 October 2021).
- Mbanefo, A.; Kumar, N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Trop. Med. Infect. Dis. 2020, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- WHO. New Perspectives Malaria Diagnosis. 2000. Available online: https://www.who.int/tdr/publications/documents/malaria-diagnosis.pdf (accessed on 25 October 2021).
- Jain, S.; Nehra, M.; Kumar, R.; Dilbaghi, N.; Hu, T.; Kumar, S.; Kaushik, A.; Li, C.-Z. Internet of medical things (IoMT)-integrated biosensors for point-of-care testing of infectious diseases. Biosens. Bioelectron. 2021, 179, 113074. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).