Can Alkyl Quaternary Ammonium Cations Substitute H2O2 in Controlling Cyanobacterial Blooms—Laboratory and Mesocosm Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms and Materials
2.2. Laboratory Experiment
2.2.1. Alkyl Chain Length and Inhibition of Photosynthesis in Microcystis Colonies
2.2.2. Exploring the Algaecidal Selectivity of ODTMA-Br (C18)
2.3. Mesocosm Experiment
2.4. Sample Analyses
2.4.1. Measurement of Pigments Contents
2.4.2. Measurement of Chl a Fluorescence Transient
2.4.3. Determination of Phytoplankton Community
2.5. Data Analyses
3. Results and Discussion
3.1. The Effect of the Alkyl Chain Length on Photosynthetic Activity
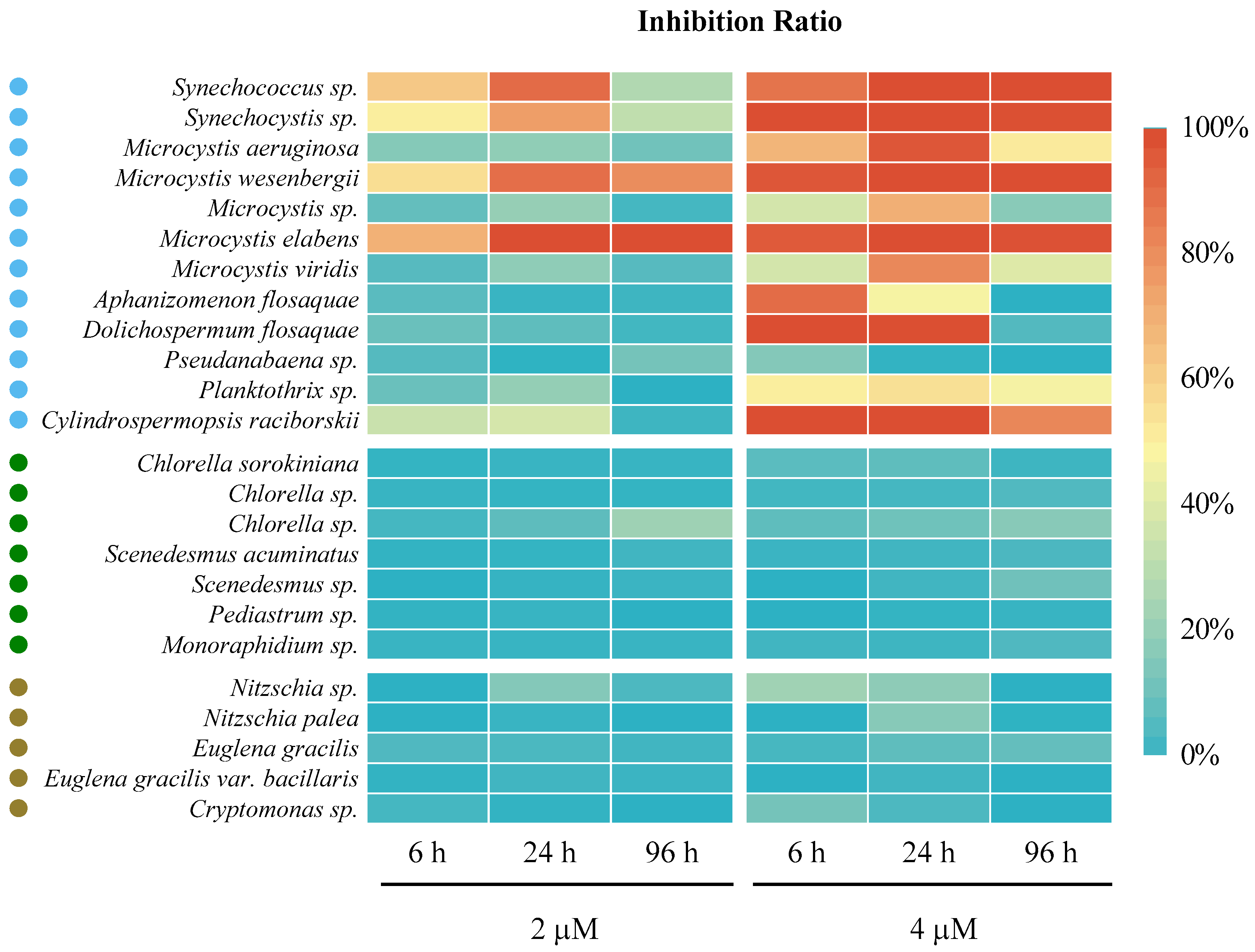
3.1.1. The Algaecidal Effect of ODTMA-Br (C18) on Cyanobacteria and Eukaryotic Algae
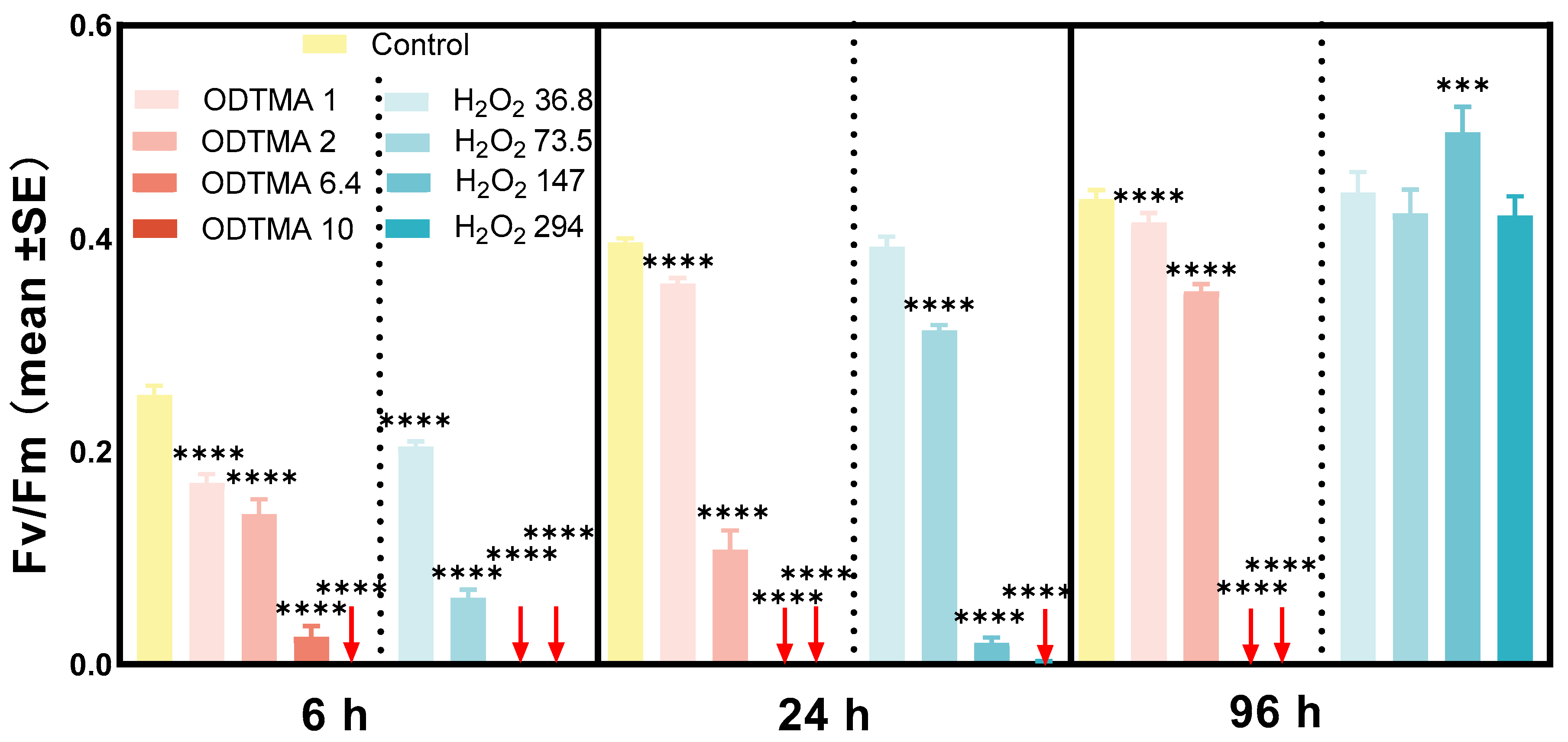
3.1.2. Laboratory-Scale Comparison between ODTMA-Br (C18) and H2O2 Efficacies as Cyanocides
3.2. Mesocosm Experiment
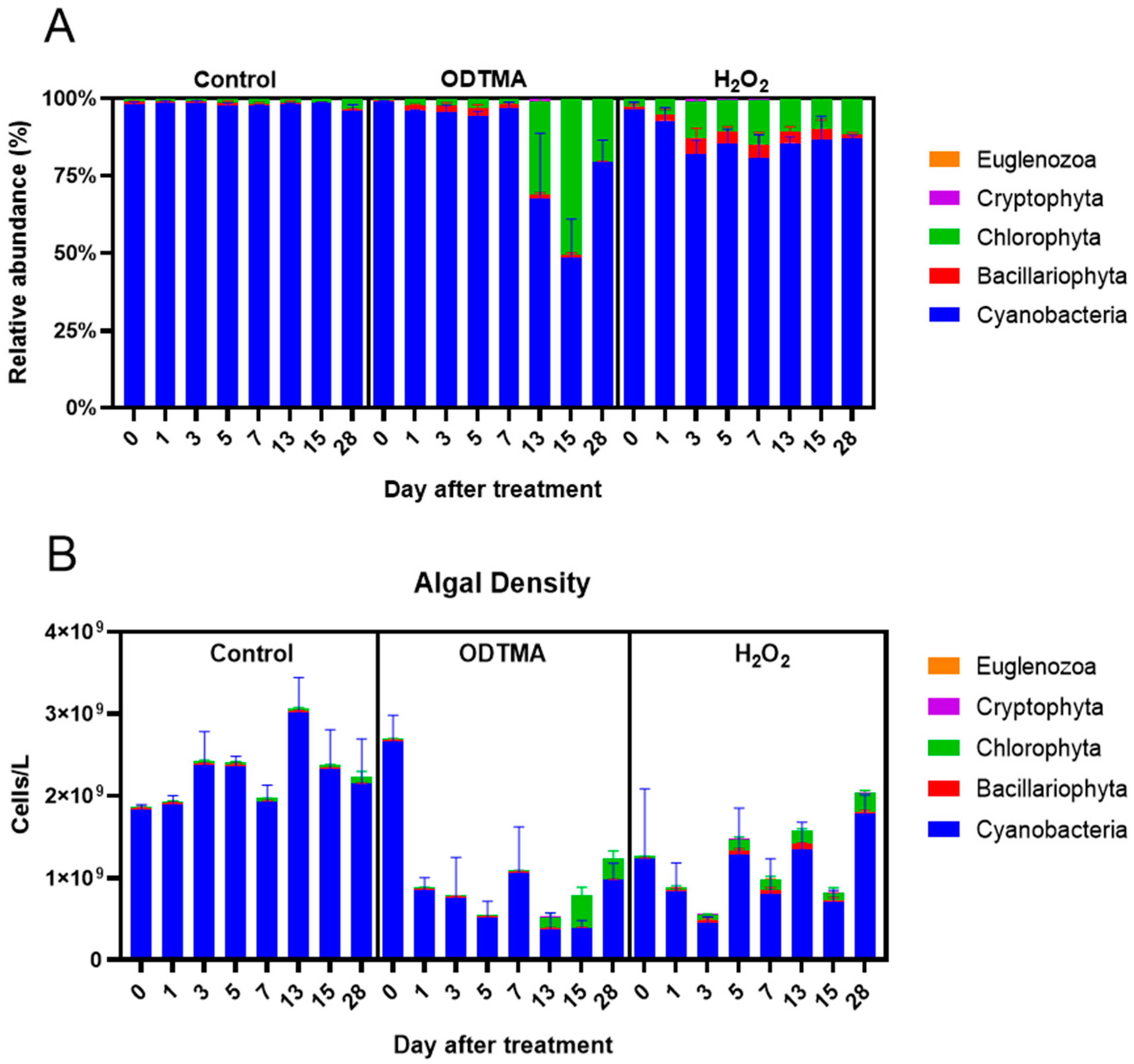
3.2.1. Morphological and Physiological Changes in Microcystis Colonies
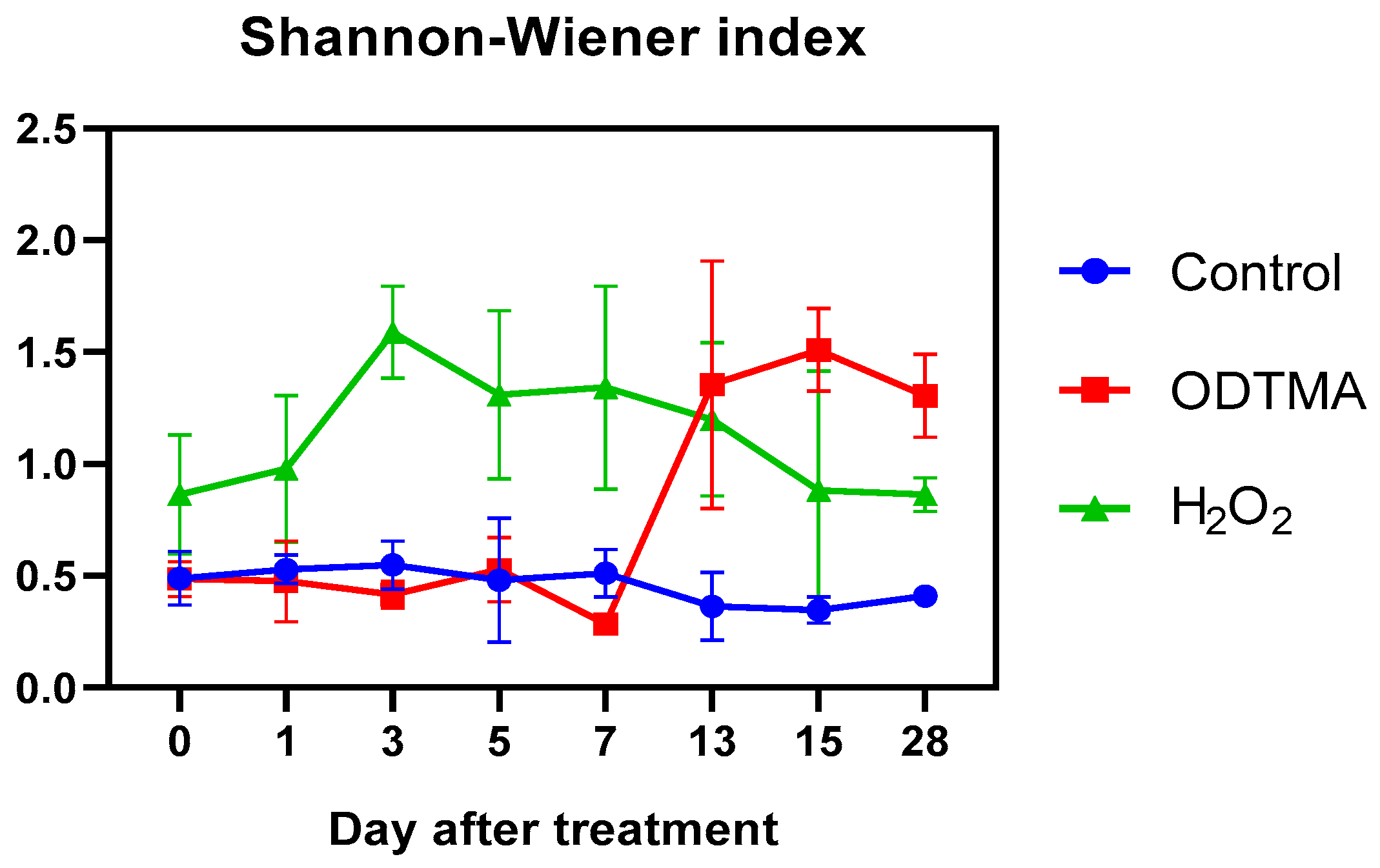
3.2.2. Impacts on the Long-Term Biodiversity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukharevich, V.I.; Polyak, Y.M. Global Occurrence of Cyanobacteria: Causes and Effects (Review). Inland Water Biol. 2020, 13, 566–575. [Google Scholar] [CrossRef]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 86, 139–209. [Google Scholar] [CrossRef]
- Buley, R.P.; Adams, C.; Belfiore, A.P.; Fernandez-Figueroa, E.G.; Gladfelter, M.F.; Garner, B.; Wilson, A.E. Field evaluation of seven products to control cyanobacterial blooms in aquaculture. Environ. Sci. Pollut. Res. 2021, 28, 29971–29983. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Adams, M.S. The macrophyte tissue nutrient pool of a hardwater eutrophic lake: Implications for macrophyte harvesting. Aquat. Bot. 1977, 3, 239–255. [Google Scholar] [CrossRef]
- Shi, W.; Lei, B.; Gan, G.P. Effect of algal flocculation on dissolved organic matters using cationic starch modified soils. J. Environ. Sci. 2016, 45, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Chen, W.; Shan, K.; Zheng, L.; Song, L. Influence of sunlight on the proliferation of cyanobacterial blooms and its potential applications in Lake Taihu, China. J. Environ. Sci. 2014, 26, 626–635. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.M.; Richardson, R.; Willis, B.E. Comparison of Partitioning and Efficacy between Copper Algaecide Formulations: Refining the Critical Burden Concept. Water Air Soil Pollut. 2018, 229, 300. [Google Scholar] [CrossRef]
- Matthijs, H.C.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Ghadouani, A. Application of Hydrogen Peroxide for the Removal of Toxic Cyanobacteria and Other Phytoplankton from Wastewater. Environ. Sci. Technol. 2008, 42, 8916–8921. [Google Scholar] [CrossRef] [PubMed]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; Van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Mucci, M. Mitigating eutrophication nuisance: In-lake measures are becoming inevitable in eutrophic waters in the Netherlands. Hydrobiologia 2020, 847, 4447–4467. [Google Scholar] [CrossRef]
- Triest, L.; Stiers, I.; Van Onsem, S. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquat. Ecol. 2015, 50, 461–483. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Jančula, D.; Visser, P.M.; Maršálek, B. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016, 50, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Ash, M.; Ash, I. Handbook of Preservatives; Synapse Information Resources Inc: Endicott, NY, USA, 2009. [Google Scholar]
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. RTP 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Wu, X.; Viner-Mozzini, Y.; Jia, Y.; Song, L.; Sukenik, A. Alkyltrimethylammonium (ATMA) surfactants as cyanocides-Effects on photosynthesis and growth of cyanobacteria. Chemosphere 2021, 274, 129778. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Tavassi, M.; Nir, S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation octadecyltrimethyl ammonium (ODTMA). Water Res. 2017, 120, 165–173. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Jia, Y.; Yang, C.; Li, T.; Wu, Z.; Liu, J.; Song, L. Multiple physiological response analyses aid the understanding of sensitivity variation between Microcystis aeruginosa and Chlorella sp. under paraquat exposures. Environ. Sci. Eur. 2019, 31, 83. [Google Scholar] [CrossRef]
- Hu, H. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Long, J.; Song, G.; Mi, W.; Bi, Y. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef]
- Barrington, D.; Reichwaldt, E.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The Efficacy of Hydrogen Peroxide in Mitigating Cyanobacterial Blooms and Altering Microbial Communities across Four Lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Daniel, E.; Weiss, G.; Murik, O.; Sukenik, A.; Lieman-Hurwitz, J.; Kaplan, A. The response of Microcystis aeruginosa strain MGK to a single or two consecutive H 2 O 2 applications. Environ. Microbiol. Rep. 2019, 11, 621–629. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Drábková, M.; Admiraal, W.; Mar?Álek, B. Combined exposure to hydrogen peroxide and light-selective effects on cyanobacteria, green algae and diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Piel, T.; Sandrini, G.; Muyzer, G.; Brussaard, C.; Slot, P.; van Herk, M.; Huisman, J.; Visser, P. Resilience of Microbial Communities after Hydrogen Peroxide Treatment of a Eutrophic Lake to Suppress Harmful Cyanobacterial Blooms. Microorganisms 2021, 9, 1495. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Jung, J.-W.; Kim, Y.J.; Park, C.-B.; Shon, J.C.; Kim, J.-H.; Park, J.-W.; Kim, S.G.; Seo, J.-S. Proteomic analysis of whole-body responses in medaka (Oryzias latipes) exposed to benzalkonium chloride. J. Environ. Sci. Health Part A 2020, 55, 1387–1397. [Google Scholar] [CrossRef]
- Pérez, P.; Fernandez, E.; Beiras, R. Toxicity of Benzalkonium Chloride on Monoalgal Cultures and Natural Assemblages of Marine Phytoplankton. Water Air Soil Pollut. 2008, 201, 319–330. [Google Scholar] [CrossRef]
- Kümmerer, K.; Al-Ahmad, A.; Henninger, A. Use of Chemotaxonomy to Study the Influence of Benzalkonium Chloride on Bacterial Populations in Biodegradation Testing. Acta Hydrochim. Hydrobiol. 2002, 30, 171–178. [Google Scholar] [CrossRef]
- Van de Voorde, A.; Lorgeoux, C.; Gromaire, M.-C.; Chebbo, G. Analysis of quaternary ammonium compounds in urban stormwater samples. Environ. Pollut. 2012, 164, 150–157. [Google Scholar] [CrossRef]
- Khan, A.H.; Macfie, S.M.; Ray, M.B. Sorption and leaching of benzalkonium chlorides in agricultural soils. J. Environ. Manag. 2017, 196, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kurt, Z.; Tsementzi, D.; Weigand, M.R.; Kim, M.; Hatt, J.K.; Tandukar, M.; Pavlostathis, S.G.; Spain, J.C.; Konstantinidis, K.T. Microbial Community Degradation of Widely Used Quaternary Ammonium Disinfectants. Appl. Environ. Microbiol. 2014, 80, 5892–5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Acronyms of ATMA Bromides | Chemical Formula | MW (g/mol) |
|---|---|---|
| ODTMA—Br (C18) | CH3(CH2)17N(Br)(CH3) | 392.51 |
| HDTMA—Br (C16) | CH3(CH2)15N(Br)(CH3) | 364.45 |
| TDTMA—Br (C14) | CH3(CH2)13N(Br)(CH3) | 336.40 |
| DDTMA—Br (C12) | CH3(CH2)11N(Br)(CH3) | 280.29 |
| Number | Genus | Species | Culture Medium | |
|---|---|---|---|---|
| 1 | FACHB805 | Synechococcus | Synechococcus sp. | BG11 |
| 2 | PCC6803 | Synechocystis | Synechocystis sp. | BG11 |
| 3 | FACHB905 | Microcystis | Microcystis aeruginosa | BG11 |
| 4 | FACHB908 | Microcystis | Microcystis wesenbergii | BG11 |
| 5 | FACHB915 | Microcystis | Microcystis sp. | BG11 |
| 6 | FACHB917 | Microcystis | Microcystis elabens | BG11 |
| 7 | FACHB979 | Microcystis | Microcystis viridis | BG11 |
| 8 | FACHB1171 | Aphanizomenon | Aphanizomenon flos-aquae | BG11 |
| 9 | FACHB1255 | Dolichospermum | Dolichospermum flos-aquae | BG11 |
| 10 | FACHB1277 | Pseudanabaena | Pseudanabaena sp. | BG11 |
| 11 | FACHB1365 | Planktothrix | Planktothrix sp. | BG11 |
| 12 | FACHB1503 | Cylindrospermopsis | Cylindrospermopsis raciborskii | BG11 |
| 13 | FACHB26 | Chlorella | Chlorella sorokiniana | BG11 |
| 14 | FACHB1552 | Chlorella | Chlorella sp. | BG11 |
| 15 | FACHB1580 | Chlorella | Chlorella sp. | BG11 |
| 16 | FACHB1235 | Scenedesmus | Scenedesmus acuminatus | BG11 |
| 17 | FACHB2944 | Scenedesmus | Scenedesmus sp. | BG11 |
| 18 | FACHB2945 | Pediastrum | Pediastrum sp. | BG11 |
| 19 | FACHB2952 | Monoraphidium | Monoraphidium sp. | BG11 |
| 20 | FACHB512 | Nitzschia | Nitzschia sp. | CSI |
| 21 | FACHB2935 | Nitzschia | Nitzschia palea | CSI |
| 22 | FACHB848 | Euglena | Euglena gracilis | HUT |
| 23 | FACHB850 | Euglena | Euglena gracilis var. bacillaris | HUT |
| 24 | FACHB1943 | Cryptomonas | Cryptomonas sp. | AF-6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xia, Y.; Jia, Y.; Sukenik, A.; Kaplan, A.; Song, C.; Dai, G.; Bai, F.; Li, L.; Song, L. Can Alkyl Quaternary Ammonium Cations Substitute H2O2 in Controlling Cyanobacterial Blooms—Laboratory and Mesocosm Studies. Microorganisms 2021, 9, 2258. https://doi.org/10.3390/microorganisms9112258
Zhang X, Xia Y, Jia Y, Sukenik A, Kaplan A, Song C, Dai G, Bai F, Li L, Song L. Can Alkyl Quaternary Ammonium Cations Substitute H2O2 in Controlling Cyanobacterial Blooms—Laboratory and Mesocosm Studies. Microorganisms. 2021; 9(11):2258. https://doi.org/10.3390/microorganisms9112258
Chicago/Turabian StyleZhang, Xinya, Yiruo Xia, Yunlu Jia, Assaf Sukenik, Aaron Kaplan, Chanyuan Song, Guofei Dai, Fang Bai, Lin Li, and Lirong Song. 2021. "Can Alkyl Quaternary Ammonium Cations Substitute H2O2 in Controlling Cyanobacterial Blooms—Laboratory and Mesocosm Studies" Microorganisms 9, no. 11: 2258. https://doi.org/10.3390/microorganisms9112258
APA StyleZhang, X., Xia, Y., Jia, Y., Sukenik, A., Kaplan, A., Song, C., Dai, G., Bai, F., Li, L., & Song, L. (2021). Can Alkyl Quaternary Ammonium Cations Substitute H2O2 in Controlling Cyanobacterial Blooms—Laboratory and Mesocosm Studies. Microorganisms, 9(11), 2258. https://doi.org/10.3390/microorganisms9112258









