Investigations on the Degradation of the Bile Salt Cholate via the 9,10-Seco-Pathway Reveals the Formation of a Novel Recalcitrant Steroid Compound by a Side Reaction in Sphingobium sp. Strain Chol11
Abstract
1. Introduction
2. Material and Methods
2.1. Cultivation of Bacteria
2.2. Growth Experiments and Co-Cultures
2.3. Cell Suspension Experiments
2.4. Abiotic Transformation of Steroid Compounds
2.5. Enrichment of Bacteria
2.6. Soil Microcosms
2.7. Cloning Techniques and Construction of Unmarked Gene Deletions
2.8. Preparation of Steroid Compounds
2.9. HPLC-MS Analysis
2.10. NMR Analysis of MDTETD
2.11. Modified Zebrafish Embryo Toxicity Test and Transcriptomics
3. Results
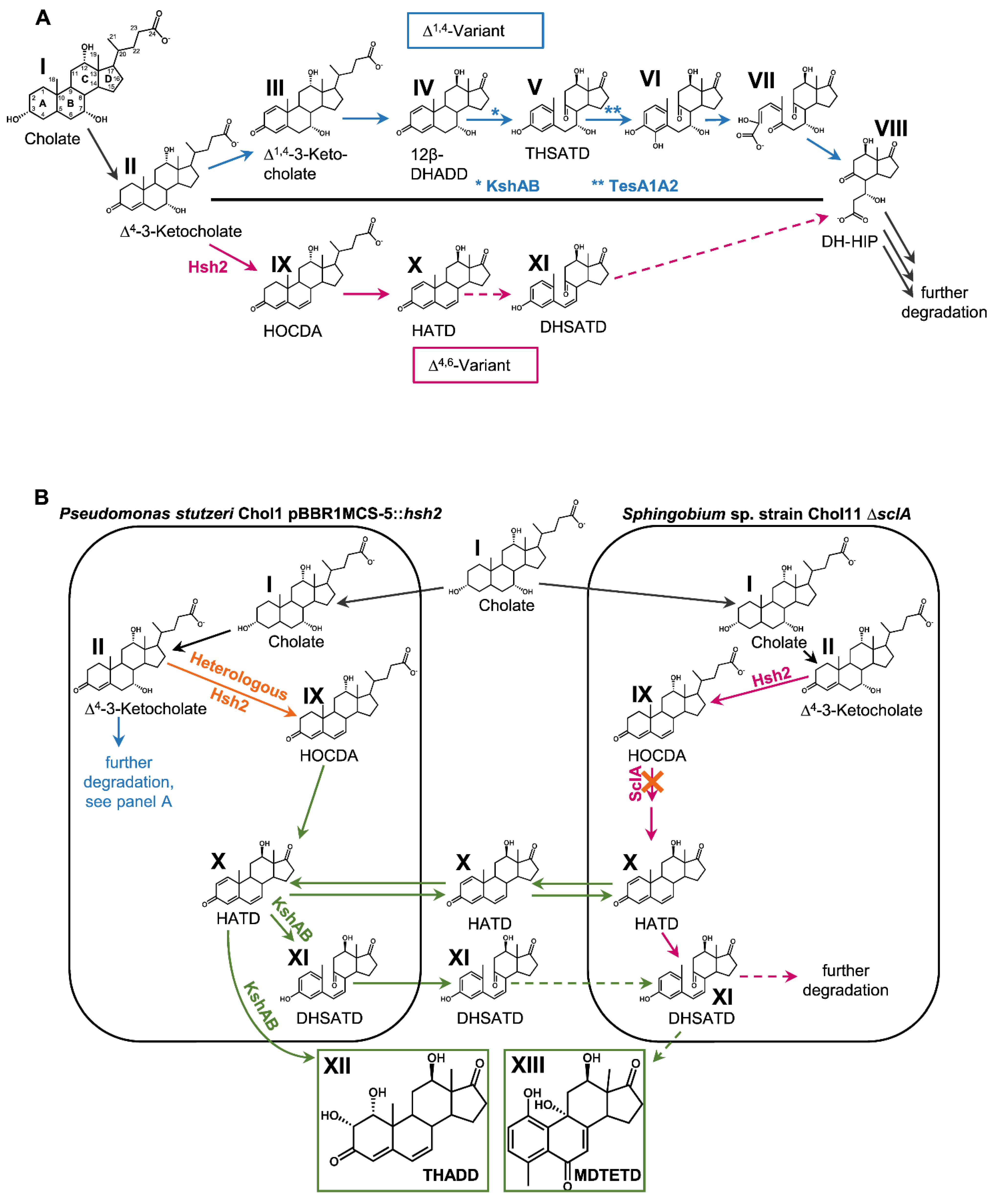
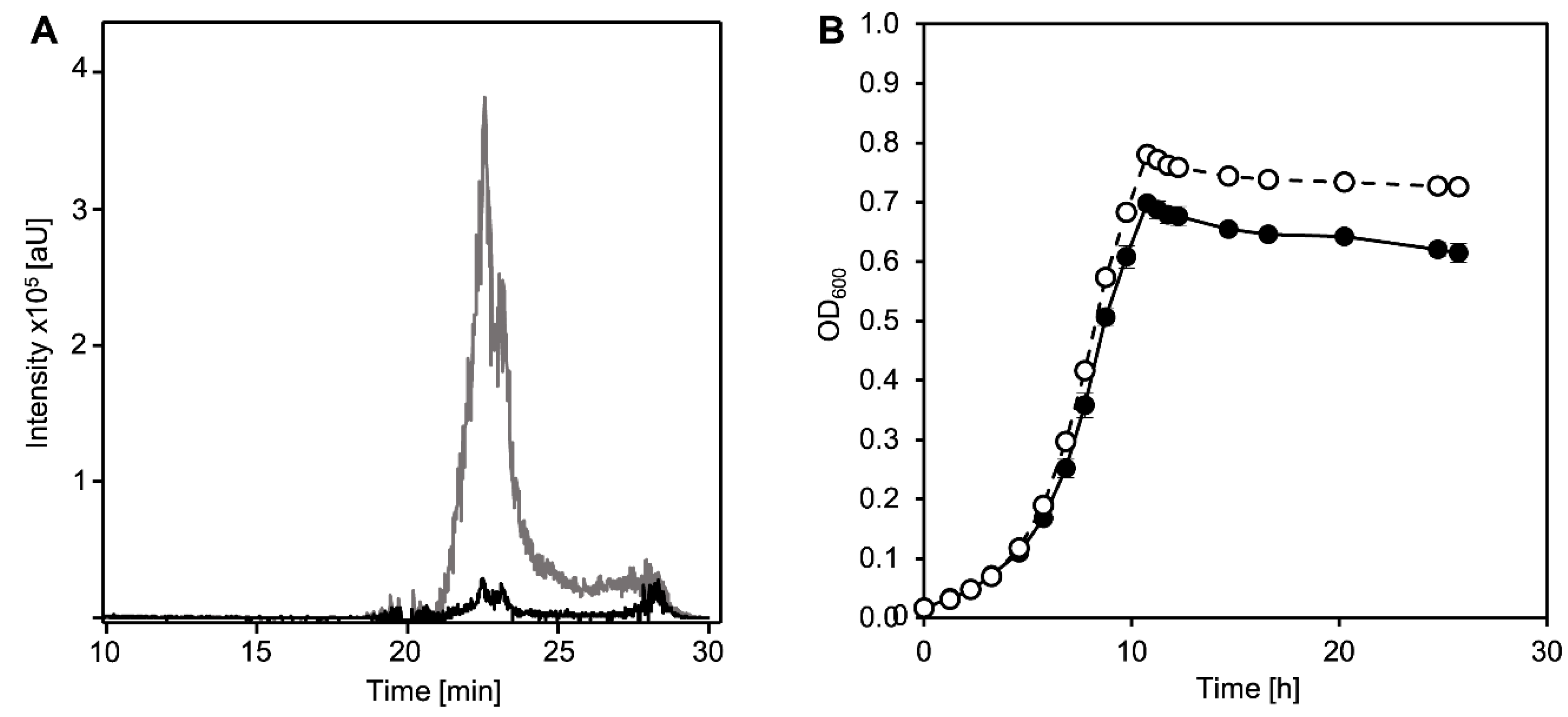
3.1. Ring Cleavage Intermediate DHSATD Transiently Accumulates in Supernatants of Sphingobium sp. Strain Chol11 in Very Low Concentrations
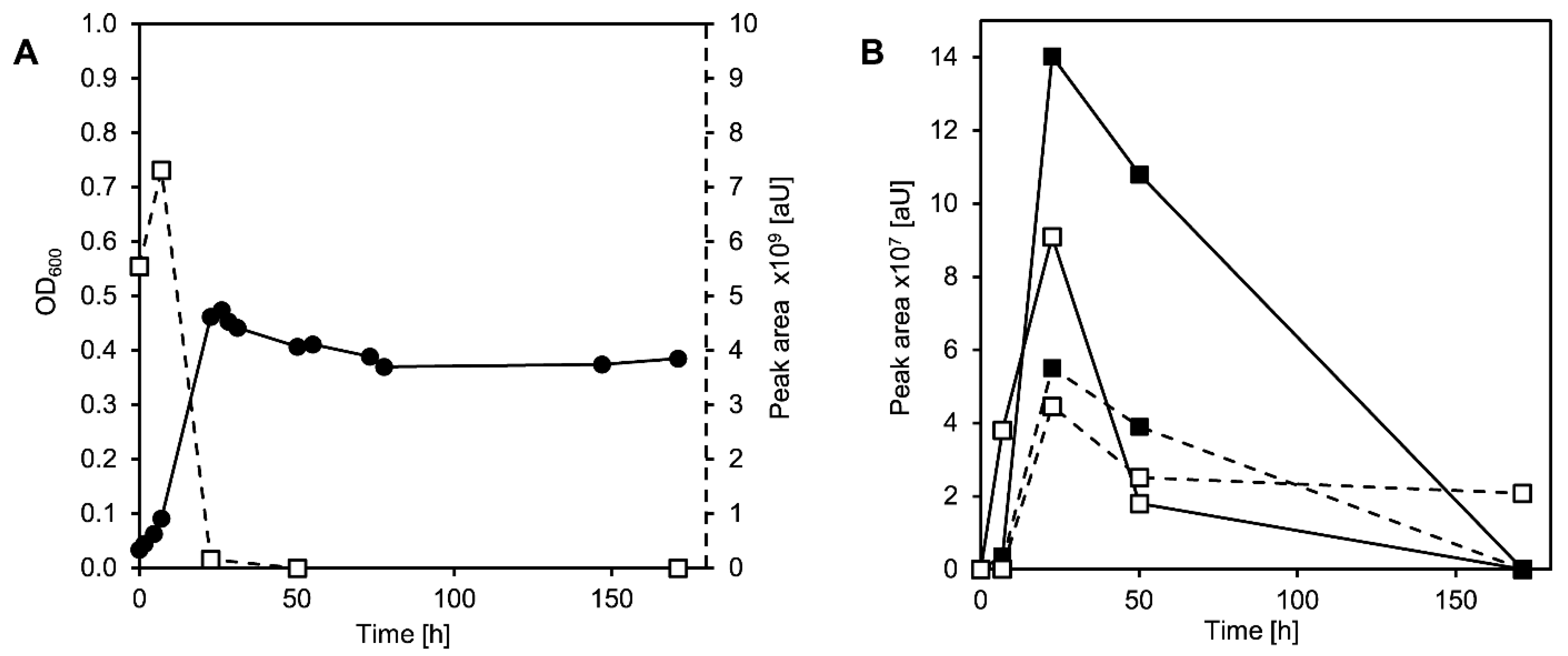
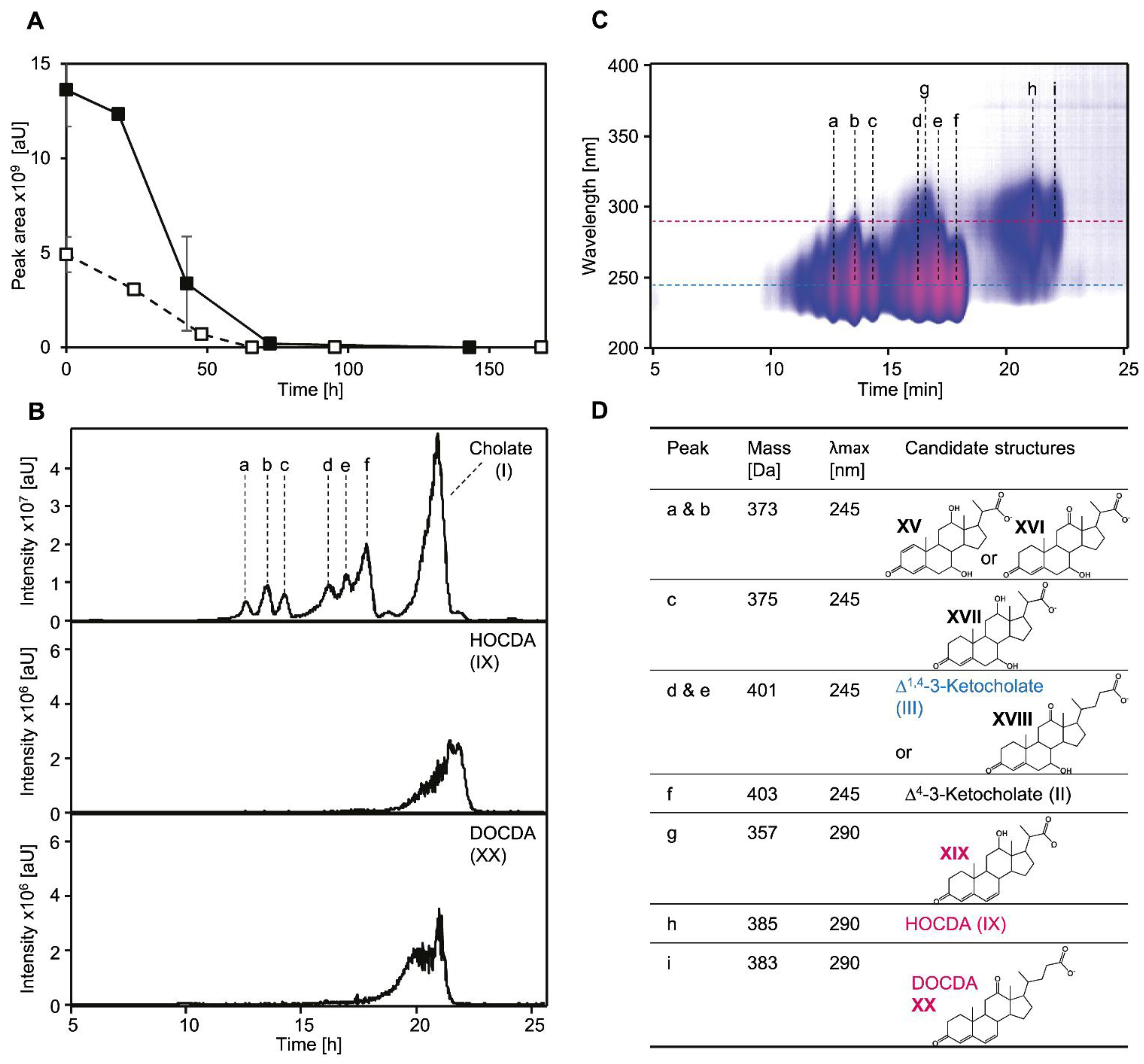
3.2. Cholate Degradation in Co-Cultures of Sphingobium sp. Strain Chol11 and P. stutzeri Chol1 Results in Accumulation of a Novel Steroid Compound
3.3. The Novel Steroid Compound Named MDTETD Has an Unusual Ring Structure
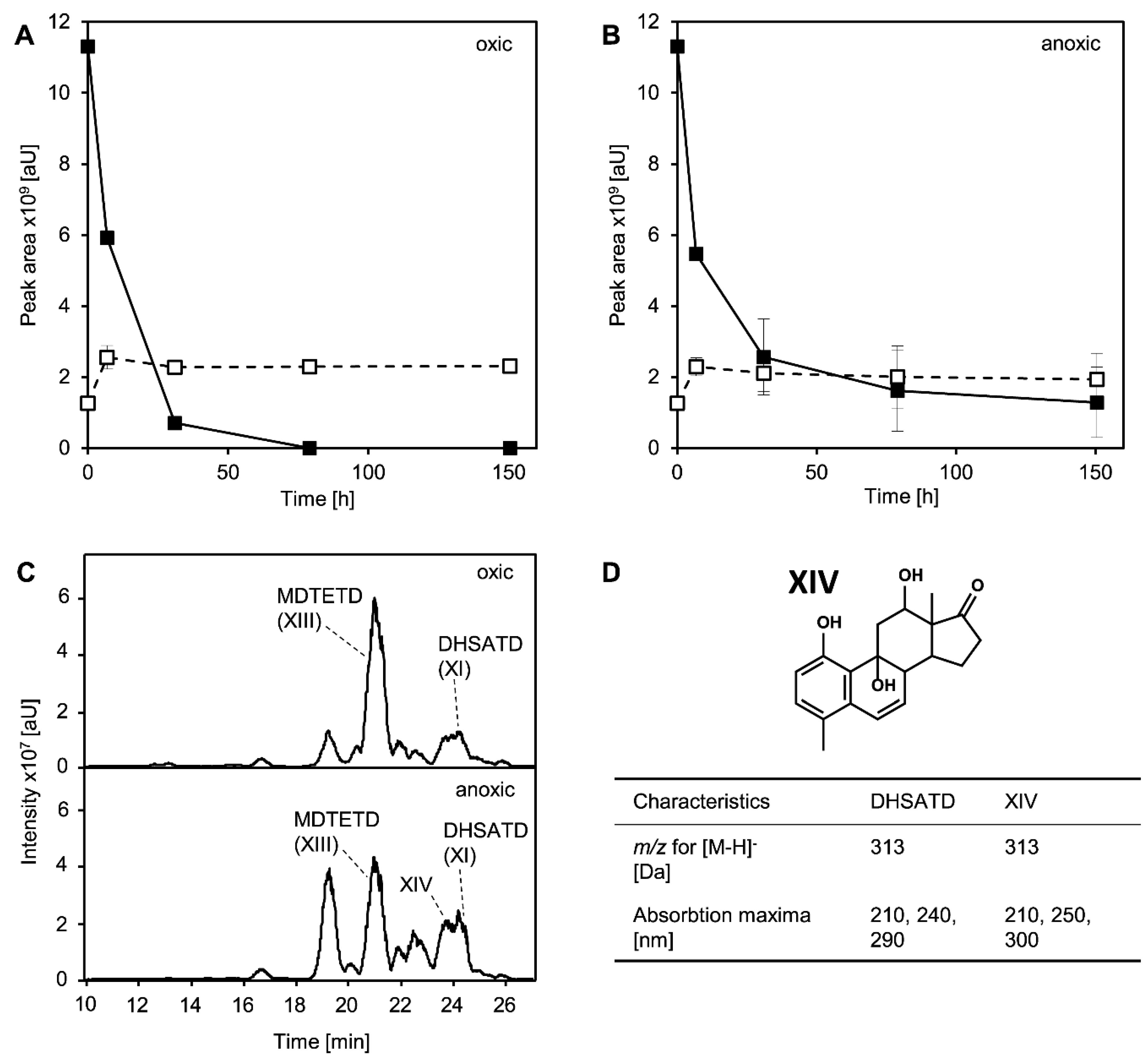
3.4. MDTETD Is Formed by Sphingobium sp. Strain Chol11 When Incubated with DHSATD
3.5. MDTETD Is Produced from DHSATD by Sphingobium sp. Strain Chol11 via so far Unknown Reactions
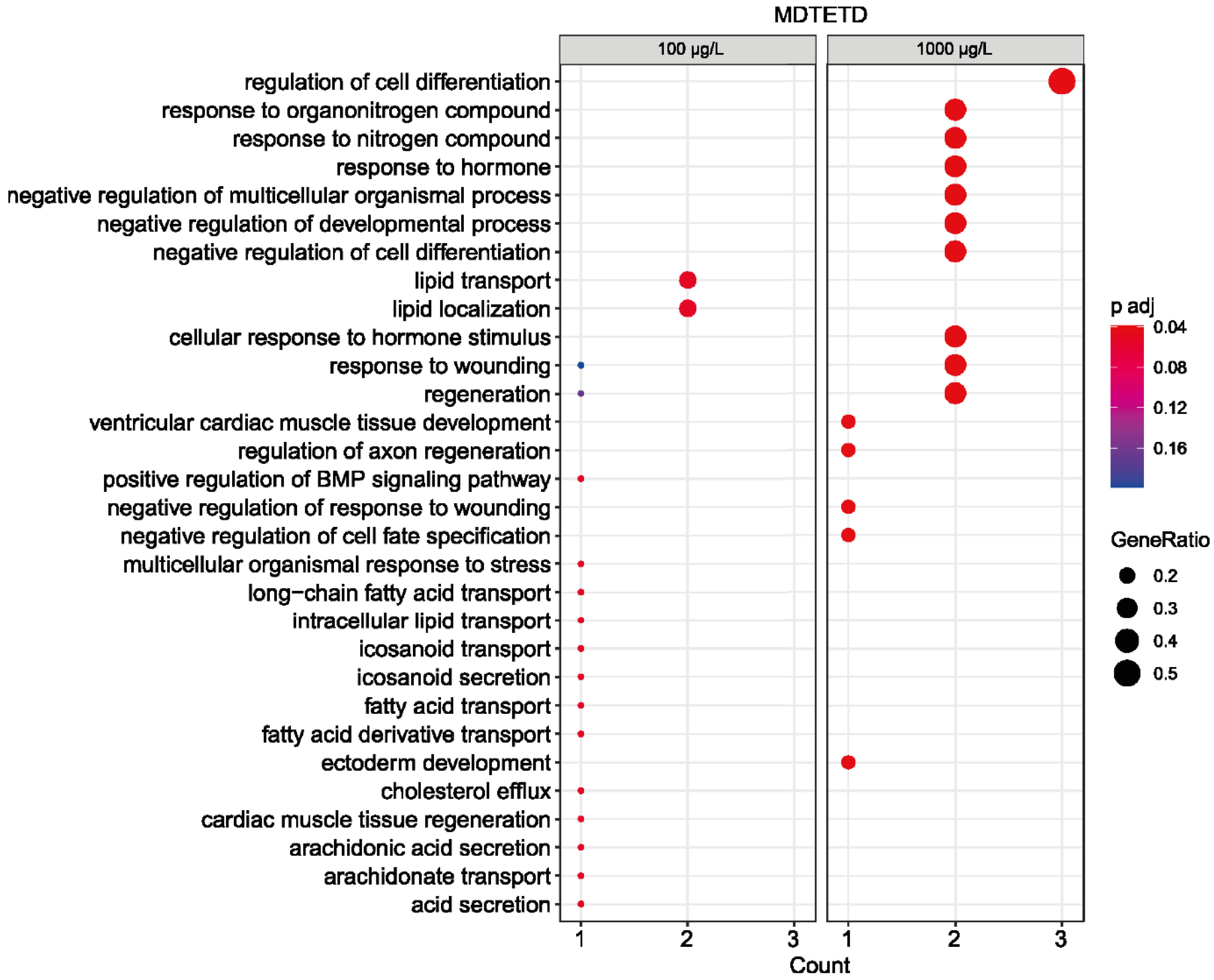
3.6. MDTETD Is Not Degraded in Enrichment Cultures and May Affect Physiological Functions of Fish
3.7. Presence of Both Bile Salt Degradation Variants in Soils Indicates Potential for Cross-Feeding
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Vítek, L.; Haluzík, M. The role of bile acids in metabolic regulation. J. Endocrinol. 2016, 228, R85–R96. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.-J.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Philipp, B. Bacterial degradation of bile salts. Appl. Microbiol. Biotechnol. 2011, 89, 903–915. [Google Scholar] [CrossRef]
- Hayakawa, S. Microbial transformation of bile acids. A unified scheme for bile acid degradation, and hydroxylation of bile acids. Z. Allg. Mikrobiol. 1982, 22, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Feller, F.M.; Holert, J.; Yücel, O.; Philipp, B.; Holert, J.; Feller, F.M.; Philipp, B. Degradation of bile acids by soil and water bacteria. Microorganisms 2021, 9, 1759. [Google Scholar] [CrossRef]
- Philipp, B.; Erdbrink, H.; Suter, M.J.F.; Schink, B. Degradation of and sensitivity to cholate in Pseudomonas sp. strain Chol1. Arch. Microbiol. 2006, 185, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, M.; Hayashi, T.; Kudo, T. Steroid degradation in Comamonas testosteroni. J. Steroid Biochem. Mol. Biol. 2012, 129, 4–14. [Google Scholar] [CrossRef]
- McLeod, M.P.; Warren, R.L.; Hsiao, W.W.L.; Araki, N.; Myhre, M.; Fernandes, C.; Miyazawa, D.; Wong, W.; Lillquist, A.L.; Wang, D.; et al. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. USA 2006, 103, 15582–15587. [Google Scholar] [CrossRef]
- Olivera, E.R.; Luengo, J.M. Steroids as environmental compounds recalcitrant to degradation: Genetic mechanisms of bacterial biodegradation pathways. Genes 2019, 10, 512. [Google Scholar] [CrossRef]
- Feller, F.M.; Richtsmeier, P.; Wege, M.; Philipp, B. Comparative analysis of bile-salt degradation in Sphingobium sp. strain Chol11 and P. stutzeri Chol1 reveals functional diversity of proteobacterial steroid degradation enzymes and suggests a novel pathway for side-chain degradation. Appl. Environ. Microbiol. 2021, AEM0145321. online ahead of print. [Google Scholar] [CrossRef]
- Petrusma, M.; Hessels, G.; Dijkhuizen, L.; van der Geize, R. Multiplicity of 3-ketosteroid-9α-hydroxylase enzymes in Rhodococcus rhodochrous DSM43269 for specific degradation of different classes of steroids. J. Bacteriol. 2011, 193, 3931–3940. [Google Scholar] [CrossRef] [PubMed]
- Capyk, J.K.; Casabon, I.; Gruninger, R.; Strynadka, N.C.; Eltis, L.D. Activity of 3-ketosteroid 9α-hydroxylase (KshAB) indicates cholesterol side chain and ring degradation occur simultaneously in Mycobacterium tuberculosis. J. Biol. Chem. 2011, 286, 40717–40724. [Google Scholar] [CrossRef] [PubMed]
- Penfield, J.S.; Worrall, L.J.; Strynadka, N.C.; Eltis, L.D. Substrate specificities and conformational flexibility of 3-ketosteroid 9α-hydroxylases. J. Biol. Chem. 2014, 289, 25523–25536. [Google Scholar] [CrossRef] [PubMed]
- Dresen, C.; Lin, L.Y.C.; D’Angelo, I.; Tocheva, E.I.; Strynadka, N.; Eltis, L.D. A flavin-dependent monooxygenase from Mycobacterium tuberculosis involved in cholesterol catabolism. J. Biol. Chem. 2010, 285, 22264–22275. [Google Scholar] [CrossRef]
- Horinouchi, M.; Hayashi, T.; Kudo, T. The genes encoding the hydroxylase of 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione in steroid degradation in Comamonas testosteroni TA441. J. Steroid Biochem. Mol. Biol. 2004, 92, 143–154. [Google Scholar] [CrossRef]
- Horinouchi, M.; Yamamoto, T.; Taguchi, K.; Arai, H.; Kudo, T. meta-cleavage enzyme gene tesB is necessary for testosterone degradation in Comamonas testosteroni TA441. Microbiology 2001, 147, 3367–3375. [Google Scholar] [CrossRef][Green Version]
- Van der Geize, R.; Yam, K.; Heuser, T.; Wilbrink, M.H.; Hara, H.; Anderton, M.C.; Sim, E.; Dijkhuizen, L.; Davies, J.E.; Mohn, W.W.; et al. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 1947–1952. [Google Scholar] [CrossRef]
- Bergstrand, L.H.; Cardenas, E.; Holert, J.; van Hamme, J.D.; Mohn, W.W. Delineation of steroid-degrading microorganisms through comparative genomic analysis. MBio 2016, 7. [Google Scholar] [CrossRef]
- Holert, J.; Cardenas, E.; Bergstrand, L.H.; Zaikova, E.; Hahn, A.S.; Hallam, S.J.; Mohn, W.W. Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. MBio 2018, 9, e02345-17. [Google Scholar] [CrossRef]
- Holert, J.; Yücel, O.; Suvekbala, V.; Kulić, Ž.; Möller, H.; Philipp, B. Evidence of distinct pathways for bacterial degradation of the steroid compound cholate suggests the potential for metabolic interactions by interspecies cross-feeding. Environ. Microbiol. 2014, 16, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Yücel, O.; Drees, S.; Jagmann, N.; Patschkowski, T.; Philipp, B. An unexplored pathway for degradation of cholate requires a 7α-hydroxysteroid dehydratase and contributes to a broad metabolic repertoire for the utilization of bile salts in Novosphingobium sp. strain Chol11. Environ. Microbiol. 2016, 18, 5187–5203. [Google Scholar] [CrossRef] [PubMed]
- Feller, F.M.; Wöhlbrand, L.; Holert, J.; Schnaars, V.; Elsner, L.; Mohn, W.W.; Rabus, R.; Philipp, B. Proteome, bioinformatic and functional analyses reveal a distinct and conserved metabolic pathway for bile salt degradation in the Sphingomonadaceae. Appl. Environ. Microbiol. 2021, 87, e00987-21. [Google Scholar] [CrossRef] [PubMed]
- Feller, F.M.; Marke, G.; Drees, S.L.; Wöhlbrand, L.; Rabus, R.; Philipp, B. Substrate inhibition of 5β-Δ4-3-ketosteroid dehydrogenase in Sphingobium sp. strain Chol11 acts as circuit breaker during growth with toxic bile salts. Front. Microbiol. 2021, 12, 655312. [Google Scholar] [CrossRef] [PubMed]
- Yücel, O.; Holert, J.; Ludwig, K.C.; Thierbach, S.; Philipp, B. A novel steroidcoenzyme A ligase from Novosphingobium sp. strain Chol11 is essential for an alternative degradation pathway for bile salts. Appl. Environ. Microbiol. 2018, 84, e01492-17. [Google Scholar] [CrossRef]
- Swain, K.; Casabon, I.; Eltis, L.D.; Mohn, W.W. Two transporters essential for reassimilation of novel cholate metabolites by Rhodococcus jostii RHA1. J. Bacteriol. 2012, 194, 6720–6727. [Google Scholar] [CrossRef]
- Mendelski, M.N.; Dölling, R.; Feller, F.M.; Hoffmann, D.; Ramos Fangmeier, L.; Ludwig, K.C.; Yücel, O.; Mährlein, A.; Paul, R.J.; Philipp, B. Steroids originating from bacterial bile acid degradation affect Caenorhabditis elegans and indicate potential risks for the fauna of manured soils. Sci. Rep. 2019, 9, 11120. [Google Scholar] [CrossRef] [PubMed]
- Gravert, T.K.O.; Fauser, P.; Olsen, P.; Hansen, M. In situ formation of environmental endocrine disruptors from phytosterol degradation: A temporal model for agricultural soils. Environ. Sci. Process. Impacts 2021, 23, 855–866. [Google Scholar] [CrossRef]
- Jagmann, N.; Brachvogel, H.-P.; Philipp, B. Parasitic growth of Pseudomonas aeruginosa in co-culture with the chitinolytic bacterium Aeromonas hydrophila. Environ. Microbiol. 2010, 12, 1787–1802. [Google Scholar] [CrossRef]
- Fritts, R.K.; McCully, A.L.; McKinlay, J.B. Extracellular metabolism sets the table for microbial cross-feeding. Microbiol. Mol. Biol. Rev. 2021, 85, e00135-20. [Google Scholar] [CrossRef]
- Smith, N.W.; Shorten, P.R.; Altermann, E.; Roy, N.C.; McNabb, W.C. The classification and evolution of bacterial cross-feeding. Front. Ecol. Evol. 2019, 7, 153. [Google Scholar] [CrossRef]
- Milton, D.L.; O’Toole, R.; Hörstedt, P.; Wolf-Watz, H. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.E.; Elzer, P.H.; Steven Hill, D.; Robertson, G.T.; Farris, M.A.; Roop, R.M.; Peterson, K.M.; Elzer A’, P.H.; Steven Hill, D.; Robertson, G.T.; et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Thoma, S.; Schobert, M. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol. Lett. 2009, 294, 127–132. [Google Scholar] [CrossRef]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Holert, J.; Kulić, Ž.; Yücel, O.; Suvekbala, V.; Suter, M.J.F.; Möller, H.M.; Philipp, B. Degradation of the acyl side chain of the steroid compound cholate in Pseudomonas sp. strain Chol1 proceeds via an aldehyde intermediate. J. Bacteriol. 2013, 195, 585–595. [Google Scholar] [CrossRef]
- Reinwald, H.; König, A.; Ayobahan, S.U.; Alvincz, J.; Sipos, L.; Göckener, B.; Böhle, G.; Shomroni, O.; Hollert, H.; Salinas, G.; et al. Toxicogenomic fin(ger)prints for thyroid disruption AOP refinement and biomarker identification in zebrafish embryos. Sci. Total Environ. 2021, 760. [Google Scholar] [CrossRef]
- Athar, A.; Füllgrabe, A.; George, N.; Iqbal, H.; Huerta, L.; Ali, A.; Snow, C.; Fonseca, N.A.; Petryszak, R.; Papatheodorou, I.; et al. ArrayExpress update—From bulk to single-cell expression data. Nucleic Acids Res. 2019, 47, D711–D715. [Google Scholar] [CrossRef]
- Carbon, S.; Douglass, E.; Dunn, N.; Good, B.; Harris, N.L.; Lewis, S.E.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; et al. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omi. A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Yu, G.; He, Q.Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef]
- Reif, B.; Köck, M.; Kerssebaum, R.; Kang, H.; Fenical, W.; Griesinger, C. Adequate, a new set of experiments to determine the constitution of small molecules at natural abundance. J. Magn. Reson. Ser. A 1996, 118, 282–285. [Google Scholar] [CrossRef]
- Grieneisen, M.L.; Warren, J.T.; Gilbert, L.I. Early steps in ecdysteroid biosynthesis: Evidence for the involvement of cytochrome P-450 enzymes. Insect Biochem. Mol. Biol. 1993, 23, 13–23. [Google Scholar] [CrossRef]
- Niwa, R.; Niwa, Y.S. The fruit fly Drosophila melanogaster as a model system to study cholesterol metabolism and homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama-Yanagawa, T.; Enya, S.; Shimada-Niwa, Y.; Yaguchi, S.; Haramoto, Y.; Matsuya, T.; Shiomi, K.; Sasakura, Y.; Takahashi, S.; Asashima, M.; et al. The conserved rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 2011, 286, 25756–25762. [Google Scholar] [CrossRef] [PubMed]
- Brueggemeier, R.W. Aromatase inhibitors—Mechanisms of steroidal inhibitors. Breast Cancer Res. Treat. 1994, 30, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Shagufta. Recent developments in steroidal and nonsteroidal aromatase inhibitors for the chemoprevention of estrogen-dependent breast cancer. Eur. J. Med. Chem. 2015, 102, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Palmer, R.B. Anabolic—Androgenic steroids. Disease-a-Month 2013, 59, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Blickenstaff, R.; Ghosh, A.; Wolf, G. Total Synthesis of Steroids: Organic Chemistry: A Series of Monographs, 1st ed.; Blomquist, A., Wassermann, H., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 30. [Google Scholar]
- Chen, Y.L.; Yu, C.P.; Lee, T.H.; Goh, K.S.; Chu, K.H.; Wang, P.H.; Ismail, W.; Shih, C.J.; Chiang, Y.R. Biochemical mechanisms and catabolic enzymes involved in bacterial estrogen degradation pathways. Cell Chem. Biol. 2017, 24, 712–724. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochromes P450. In Metabolism of Drugs and Other Xenobiotics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 27–66. ISBN 9783527630905. [Google Scholar]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Dagley, S. Catabolism of aromatic compounds by micro-organisms. Adv. Microb. Physiol. 1971, 6, 1–46. [Google Scholar] [CrossRef]
- Stolz, A. Molecular characteristics of xenobiotic-degrading sphingomonads. Appl. Microbiol. Biotechnol. 2009, 81, 793–811. [Google Scholar] [CrossRef]
- Pieper, D.H. Aerobic degradation of polychlorinated biphenyls. Appl. Microbiol. Biotechnol. 2005, 67, 170–191. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Haller, M.Y.; Müller, S.R.; McArdell, C.S.; Alder, A.C.; Suter, M.J.-F. Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography–mass spectrometry. J. Chromatogr. A 2002, 952, 111–120. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 2011, 213, 1–26. [Google Scholar] [CrossRef]
- Kungolos, A.; Emmanouil, C.; Tsiridis, V.; Tsiropoulos, N. Evaluation of toxic and interactive toxic effects of three agrochemicals and copper using a battery of microbiotests. Sci. Total Environ. 2009, 407, 4610–4615. [Google Scholar] [CrossRef]
- De Oliveira-Filho, E.C.; Lopes, R.M.; Paumgartten, F.J.R. Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 2004, 56, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, A. Microbial physiology of sidechain degradation of sterols. J. Ind. Microbiol. 1990, 6, 101–115. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Prior, S.D.; Dalton, H. Copper ions as inhibitors of protein C of soluble methane monooxygenase of Methylococcus capsulatus (Bath). Eur. J. Biochem. 1985, 153, 137–144. [Google Scholar] [CrossRef]


| Name | Sequence | Restriction Sites |
|---|---|---|
| upfor_nov2c349 | TTTTTTTTCTAGACGGTCTGCGAGAAGGTGAGG | XbaI |
| uprev_nov2c349 | TCGCGCATATGGCATCTGGCA | |
| dnfor_nov2c349 | TGCCAGATGCCATATGCGCGACATCTTCTCCATTGTAGGCGA | |
| dnrev_nov2c349 | TTTTTTTGTCGACCGTAGAAGAGCTCCATCGGG | SalI |
| pDM4_MCS_for | AAGATGTGGCGTGTTACGGT | |
| pDM4_MCS_rev | AGGCTCTGGGAGGCAGAATA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feller, F.M.; Eilebrecht, S.; Nedielkov, R.; Yücel, O.; Alvincz, J.; Salinas, G.; Ludwig, K.C.; Möller, H.; Philipp, B. Investigations on the Degradation of the Bile Salt Cholate via the 9,10-Seco-Pathway Reveals the Formation of a Novel Recalcitrant Steroid Compound by a Side Reaction in Sphingobium sp. Strain Chol11. Microorganisms 2021, 9, 2146. https://doi.org/10.3390/microorganisms9102146
Feller FM, Eilebrecht S, Nedielkov R, Yücel O, Alvincz J, Salinas G, Ludwig KC, Möller H, Philipp B. Investigations on the Degradation of the Bile Salt Cholate via the 9,10-Seco-Pathway Reveals the Formation of a Novel Recalcitrant Steroid Compound by a Side Reaction in Sphingobium sp. Strain Chol11. Microorganisms. 2021; 9(10):2146. https://doi.org/10.3390/microorganisms9102146
Chicago/Turabian StyleFeller, Franziska Maria, Sebastian Eilebrecht, Ruslan Nedielkov, Onur Yücel, Julia Alvincz, Gabriela Salinas, Kevin Christopher Ludwig, Heiko Möller, and Bodo Philipp. 2021. "Investigations on the Degradation of the Bile Salt Cholate via the 9,10-Seco-Pathway Reveals the Formation of a Novel Recalcitrant Steroid Compound by a Side Reaction in Sphingobium sp. Strain Chol11" Microorganisms 9, no. 10: 2146. https://doi.org/10.3390/microorganisms9102146
APA StyleFeller, F. M., Eilebrecht, S., Nedielkov, R., Yücel, O., Alvincz, J., Salinas, G., Ludwig, K. C., Möller, H., & Philipp, B. (2021). Investigations on the Degradation of the Bile Salt Cholate via the 9,10-Seco-Pathway Reveals the Formation of a Novel Recalcitrant Steroid Compound by a Side Reaction in Sphingobium sp. Strain Chol11. Microorganisms, 9(10), 2146. https://doi.org/10.3390/microorganisms9102146






