Aspergillus Section Fumigati in Firefighter Headquarters
Abstract
1. Introduction
2. Material and Methods
2.1. Firefighter Headquarters Characterization
2.2. Sampling Approaches
2.3. Aspergillus Section Fumigati Prevalence
2.4. Molecular Detection of Aspergillus Section Fumigati
2.5. Statistical Analysis
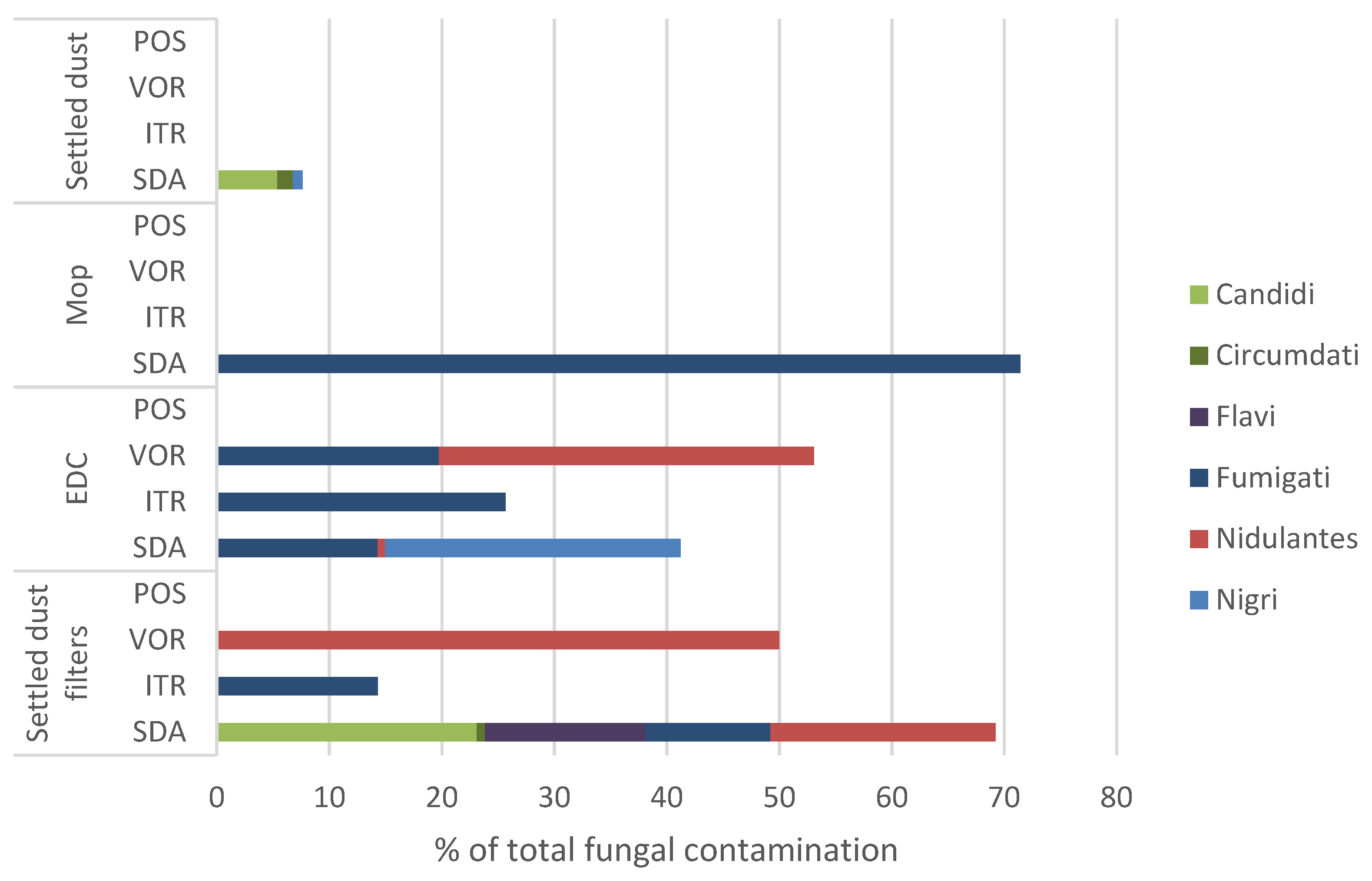
3. Results
3.1. Aspergillus Section Fumigati Distribution
3.2. Screening of Azole Resistance
3.3. Molecular Detection
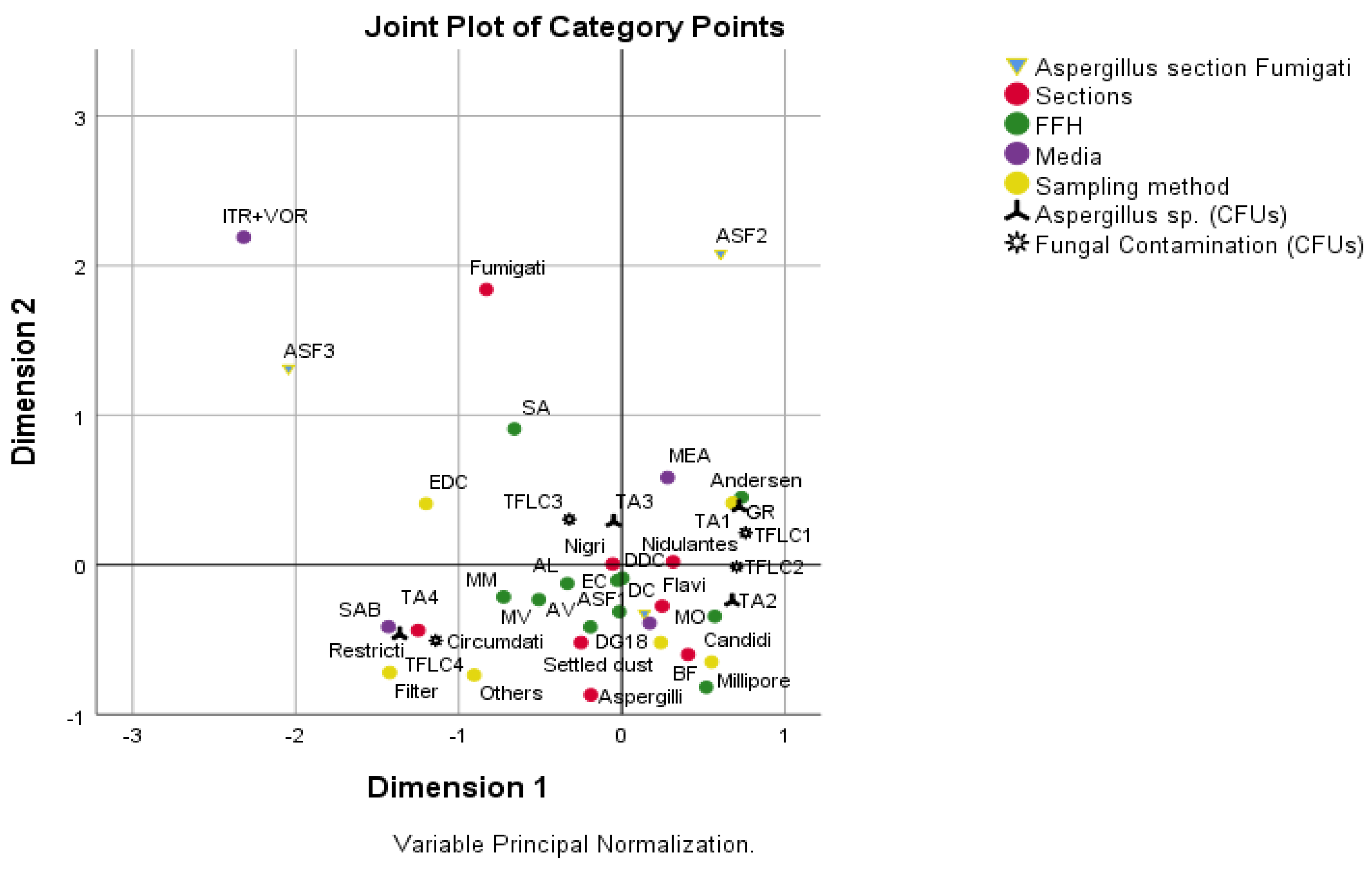
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyedmousavi, S.; Guillot, J.; Arné, P.; de Hoog, G.S.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: A global health concern with parallels to human disease. Med Mycol. 2015, 53, 765–797. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R. Exposure to Fungi in Health Care Facilities. Ref. Modul. Life Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of Aspergillosis: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Aranha Caetano, L.; Viegas, S. Occupational exposure to Aspergillus section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.C. Aspergillus fumigatus: Growth and virulence. Med Mycol. 2006, 44, S77–S81. [Google Scholar] [CrossRef]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. prevalence in different occupational settings. J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef]
- Stop neglecting fungi. Nat. Microbiol. 2017, 2. [CrossRef]
- Viegas, C.; Almeida, B.; Gomes, A.Q.; Carolino, E.; Caetano, L.A. Aspergillus spp. prevalence in Primary Health Care Centres: Assessment by a novel multi-approach sampling protocol. Environ Res. 2019, 175, 133–141. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in healthcare centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Cavaleiro, J.R.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 26, 1–19. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.; Aguiar, L.; Lage, B.; Gonçalves, L.M.D.; Aranha Caetano, L.; Carolino, E.; et al. Exposure assessment in one central Hospital: A multi-approach protocol to achieve an accurate risk characterization. Environ. Res. 2019, 181, 108947. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Viegas, S. Characterization of Occupational Exposure to Fungal Burden in Portuguese Bakeries. Microorganisms 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Caetano, L.A.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Viegas, C.; Caetano, L.A.; Cox, J.; Korkalainen, M.; Haines, S.R.; Dannemiller, K.C.; Viegas, S.; Reponen, T. The effects of waste sorting in environmental microbiome, THP-1 cell viability and inflammatory responses. Environ. Res. 2020, 185. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Almeida, B.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. burden on filtering respiratory protective devices. Is there an occupational health concern? Air Qual. Atmos. Health 2020, 13. [Google Scholar] [CrossRef]
- Andersen, A. New sampler for the collection, sizing and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Sousa, P.; Dias, M.; Caetano, L.A.; Ribeiro, E.; Carolino, E.; Twarużek, M.; Kosicki, R.; Viegas, S. Bioburden contamination and Staphylococcus aureus colonization associated with firefighter’s ambulances. Environ. Res. 2021, 197. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Carolino, E.; Sabino, R. Culture Media and Sampling Collection Method for Aspergillus spp. Assessment: Tackling the Gap between Recommendations and the Scientific Evidence. Atmosphere 2021, 12, 23. [Google Scholar] [CrossRef]
- EPA. United States Environmental Protection Agency EPA Observational Economy Series Volume1: Composite Sampling. Available online: https://www.epa.gov/environmentaljustice/epa-environmental-justice-strategy-1995 (accessed on 1 September 2021).
- Viegas, C.; Dias, M.; Almeida, B.; Vicente, E.; Candeias, C.; Aranha Caetano, L.; Carolino, E.; Alves, C. Loading Rates of Dust and Bioburden in Dwellings in an Inland City of Southern Europe. Atmosphere 2021, 12, 378. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Routine and Extended Internal Quality Control for MIC Determination and Agar Dilution for Yeasts, Moulds and Dermatophytes as Recommended by EUCAST. Available online: http://www.eucast.org (accessed on 1 September 2021).
- De Hoog, D.; Guarro, J.; Gene, G.; Figueras, M. Atlas of Clinical Fungi—The Ultimate Benchtool for Diagnosis; Utr Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2016; Volume 4, Issue 1. [Google Scholar]
- Cruz-Perez, P.; Buttner, M.P.; Stetzenbach, L.D. Detection and quantitation of Aspergillus fumigatus in pure culture using polymerase chain reaction. Mol. Cell. Probes 2001, 15, 81–88. [Google Scholar] [CrossRef]
- WHO. World Health Organisation Guidelines for Indoor Air Quality: Dampness and Mould. Available online: https://www.who.int/publications/i/item/9789289041683 (accessed on 1 September 2021).
- APA. Qualidade do Ar em Espaços Interiores Um Guia Técnico Agência Portuguesa do Ambiente. Available online: https://www.voltimum.pt/artigos/biblioteca-de-flipbooks/qualidade-do-ar-em (accessed on 1 September 2021).
- Strachan, D.P. Damp housing and childhood asthma: Validation of reporting of symptoms. BMJ 1988, 297, 1223–1226. [Google Scholar] [CrossRef]
- Platt, S.D.; Martin, C.J.; Hunt, S.M.; Lewis, C.W. Damp housing, mould growth, and symptomatic health state. BMJ 1989, 298, 1673–1678. [Google Scholar] [CrossRef]
- Dales, R.E.; Burnett, R.; Zwanenburg, H. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 1991, 143, 505–509. [Google Scholar] [CrossRef]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health. Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef]
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20. [Google Scholar] [CrossRef]
- Adams, R.; Leppänen, H.; Karvonen, A.; Jacobs, J.; Borràs-Santos, A.; Valkonen, M.; Krop, E.; Haverinen-Shaughnessy, U.; Huttunen, K.; Zock, J.-P.; et al. Microbial exposures in moisture-damaged schools and associations with respiratory symptoms in students: A multi-country environmental exposure study. Indoor Air 2021. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A.; Balasubramanian, R. Assessment of airborne bacteria and fungi in food courts. Build. Environ. 2011, 46, 2081–2087. [Google Scholar] [CrossRef]
- Alberti, C.; Bouakline, A.; Ribaud, P.; Lacroix, C.; Rousselot, P.; Leblanc, T.; Derouin, F.; Aspergillus group. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J. Hosp. Infect. 2001, 48, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Viegas, C.; Verde, S.C.; Wolterbeek, H.T.; Almeida, S.M. Characterizing the fungal and bacterial microflora and concentrations in fitness centres. Indoor Built Environ. 2016, 25, 872–882. [Google Scholar] [CrossRef]
- Reponen, T. Sampling for Microbial Determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 85–96. [Google Scholar]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-resistant Aspergillus fumigatus harboring the tr34/l98h mutation: First report in Portugal in environmental samples. Microorganisms 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Cebola de Oliveira, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S. A new approach to assess fungal contamination and mycotoxins occupational exposure in forklifts drivers from waste sorting. Mycotoxin Res. 2017, 33, 285–295. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Mao, Y.; Ding, P.; Wang, Y.; Ding, C.; Wu, L.; Zheng, P.; Zhang, X.; Lia, X.; Wang, L.; Suna, Z. Comparison of culturable antibiotic-resistant bacteria in polluted and non-polluted air in Beijing, China. Environ. Int. 2019, 131, 104936. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Bowyer, P.; Sabino, R. The human lung and Aspergillus: You are what you breathe in? Med. Mycol. 2019, 57, S145–S154. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal burden in waste industry: An occupational risk to be solved. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Garland, J.L.; Bolster, C.H.; Mills, A.L. Impact of dilution on microbial community structure and functional potential: Comparison of numerical simulations and batch culture experiments. Appl. Environ. Microbiol. 2001, 67, 702–712. [Google Scholar] [CrossRef]
- Black, W.D. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne. Australia. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Nagano, Y.; Millar, B.C.; Goldsmith, C.E.; Walker, J.M.; Elborn, J.S.; Rendall, J.; Moore, J.E. Development of selective media for the isolation of yeasts and filamentous fungi from the sputum of adult patients with cystic fibrosis (CF). J. Cyst Fibros. 2008, 7, 566–572. [Google Scholar] [CrossRef][Green Version]
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The medical effects of mold exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for bioaerosol characterization: Limits and perspectives for human health risk assessment in organicwaste treatment. Atmosphere 2020, 11, 452. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Lees, P.S.J.; Merz, W.G.; Schwab, K.J. Inhibition of quantitative PCR analysis of fungal conidia associated with indoor air particulate matter. Aerobiologia 2007, 23, 35–45. [Google Scholar] [CrossRef]
- Mensah-Attipoe, J.; Taubel, M. Analysis Approaches for Fungi in Indoor Environmental Assessments. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 109–126. [Google Scholar]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2016, 51, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.L.; Miller, J.D.; Dillon, K.H. Field Guide for the Determination of Biological Contaminants in Environmental Samples, 2nd ed.; AIHA: Fairfax, VA, USA, 2005. [Google Scholar]
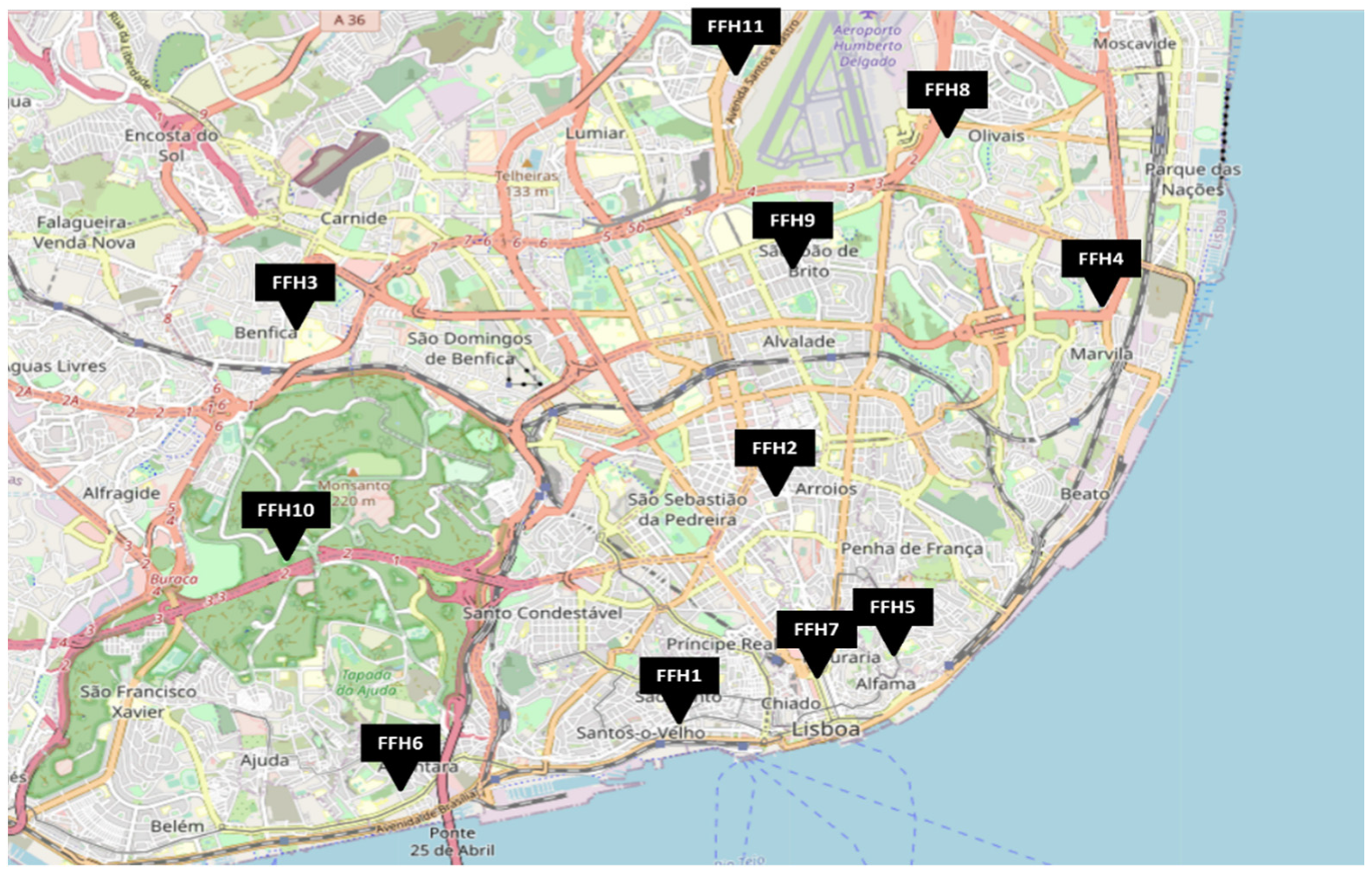
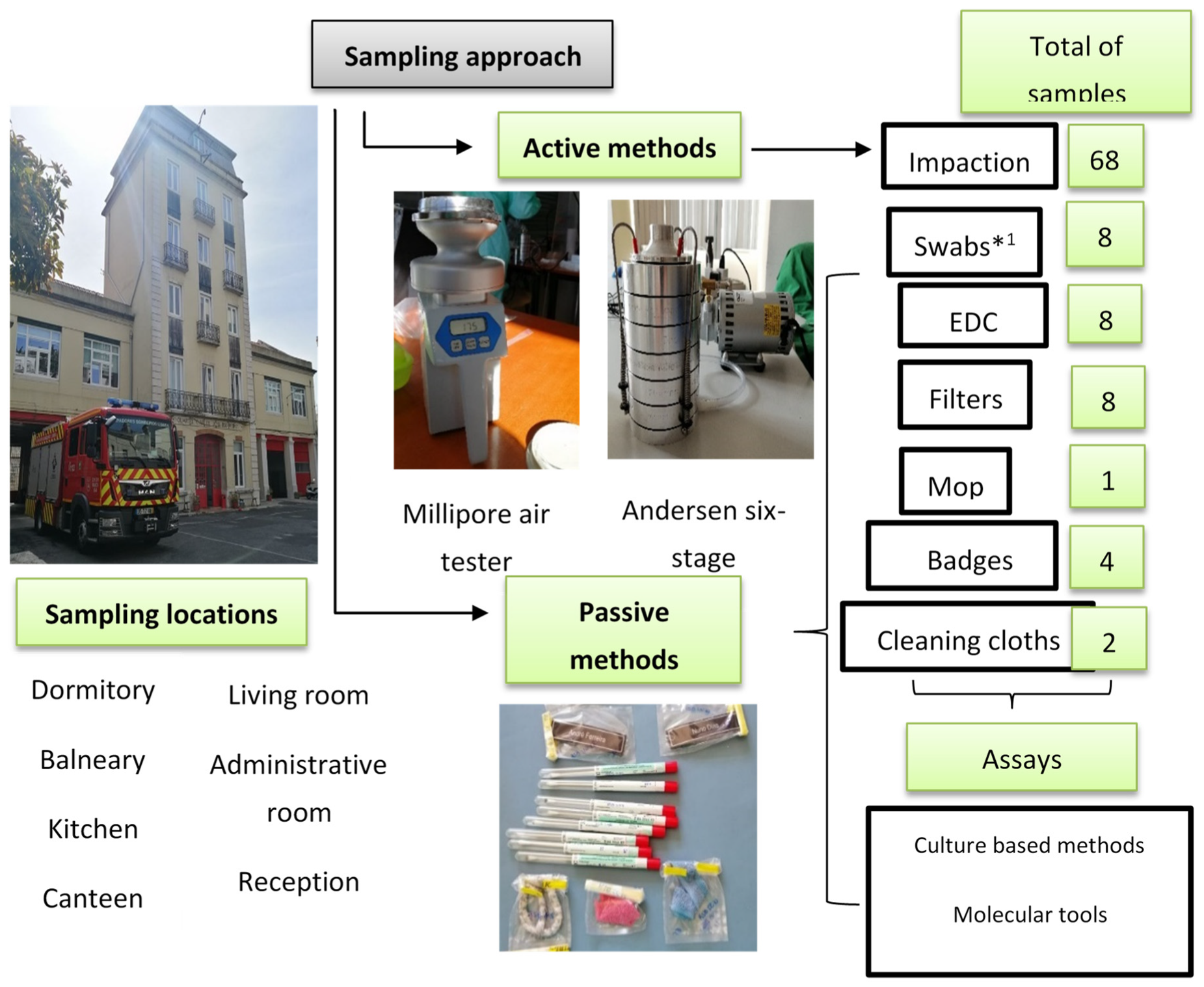
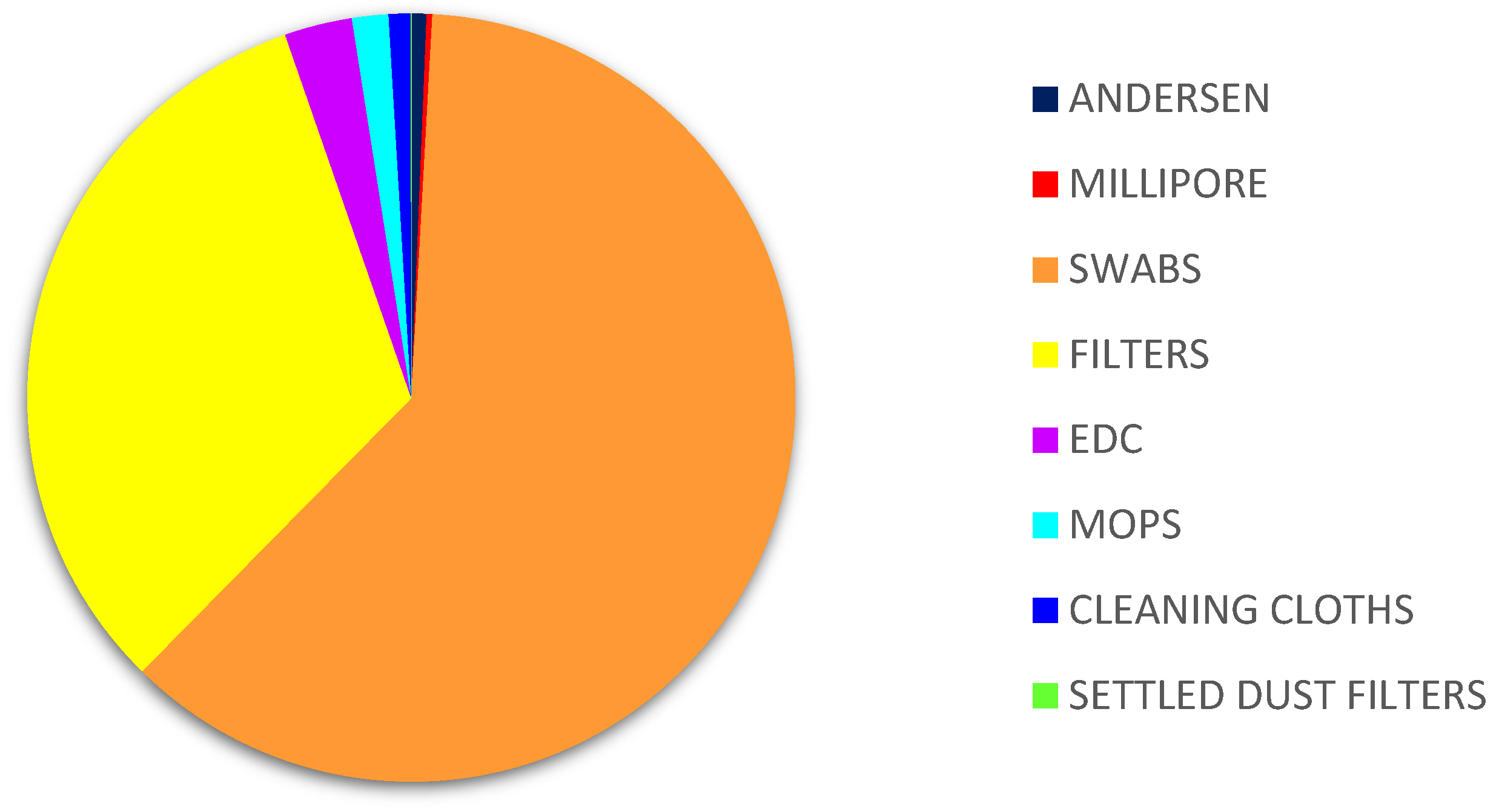
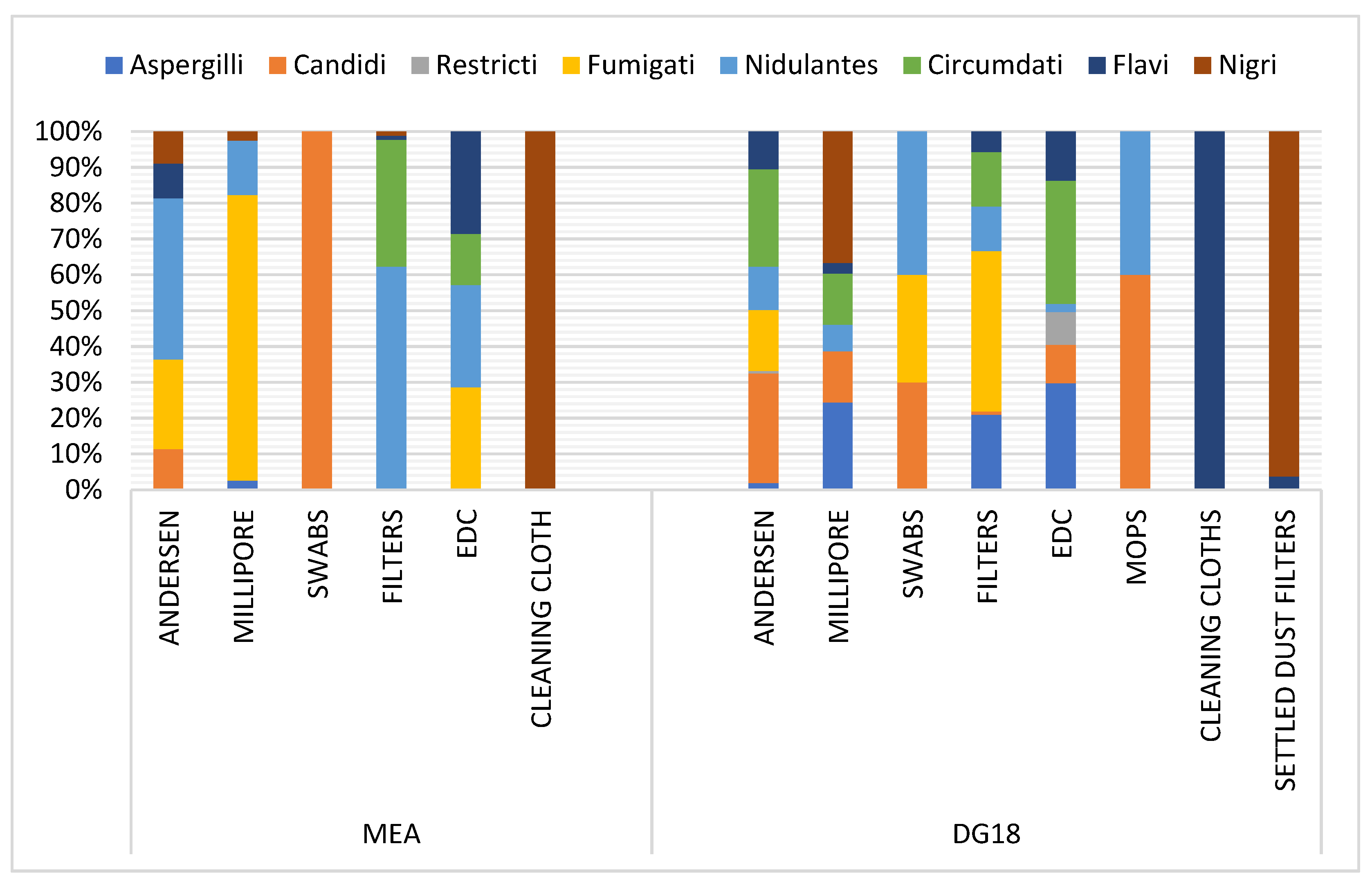
| Headquarter | Building Age | Number of Occupants | Cracks in the Walls /Floor | Mould Growth | Visible Problems |
|---|---|---|---|---|---|
| FFH1 FFH2 FFH3 FFH4 FFH5 FFH6 FFH7 FFH8 FFH9 FFH10 FFH11 | 100 * 100 * 37 20 100 * 100 * 2 100 * 62 30 4 | 50 12 30 11 20 16 7 16 20 20 7 | Frequently Frequently Often - - - - - - - - | Frequently Frequently Often Often Frequently Often - - Often - - | Wall cracks Wall cracks Infiltrations - Infiltrations Infiltrations - - Infiltrations Infiltrations - |
| Primers and Probes | Sequences | Reference |
|---|---|---|
| Forward Primer | 5‘-CGCGTCCGGTCCTCG-3‘ | |
| Reverse Primer | 5‘-TTAGAAAAATAAAGTTGGGTGTCGG -3‘ | [24] |
| Probe | 5‘-TGTCACCTGCTCTGTAGGCCCG -3‘ |
Sample | MEA | DG18 | ||||
|---|---|---|---|---|---|---|
| Fungi | CFU. m−3/m−2/g | % | Fungi | CFU. m3 3/m2/g | % | |
Andersen | Other species Aspergillus sp. | 405,281.8 486 | 99.9 0.1 | Other species Aspergillus sp. | 67,915.2 1040 | 98.5 1.5 |
Millipore | Other species Aspergillus sp. | 147,399.8 158.2 | 99.9 0.1 | Other species Aspergillus sp | 50,624.64 393 | 99.2 0.8 |
| EDC * | Other species Aspergillus sp. | 391,345 743.1 | 99.8 0.2 | Other species Aspergillus sp. | 210,245.9 4640.126 | 97.8 2.2 |
Cleaning cloths | Other species Aspergillus sp. | 29,500 500 | 98.3 1.7 | Other species Aspergillus sp. | 19,700 1500 | 92.9 7.1 |
Mops | Other species Aspergillus sp. | 2600 - | 100 - | Other species Aspergillus sp. | 13,500 2500 | 84.4 15.6 |
Identification badges | Other species Aspergillus sp. | 45,500 - | 100 - | Other species Aspergillus sp. | 32,500 - | 100 - |
Filters | Other species Aspergillus sp. | 3,679,700 128,500 | 96.6 3.4 | Other species Aspergillus sp. | 2,699,000 52,505 | 98.1 1.9 |
Settled dust | Other species Aspergillus sp. | 6496.5 - | 100 - | Other species Aspergillus sp. | 2983.9 27 | 99.1 0.9 |
Swabs | Other species Aspergillus sp. | 4,562,500 10,000 | 99.8 0.2 | Other species Aspergillus sp. | 4,131,556 100,000 | 97.6 2.4 |
Matrices | SDA | ITR | VOR | POS | ||||
|---|---|---|---|---|---|---|---|---|
| CFU.m² | % | CFU.m² | % | CFU.m² | % | CFU.m² | % | |
| Mops | 2500 | 100% | - | - | - | - | - | - |
| EDC * | 106 | 4.4% | 1062 | 100% | 3503 | 97.1% | - | - |
| Settled dust filters | 1500 | 3.7% | 1062 | 100% | - | - | - | - |
| Total | 4106 | 8.9% | 1562 | 100% | 3503 | 85.3% | - | - |
| Sampling Method | Ranks | Test Statistics a,b | ||||
|---|---|---|---|---|---|---|
| N | Mean Rank | Kruskal–Wallis H | df | p | ||
| Fungal Contamination (CFUs) | Millipore | 33 | 107.21 | 115.897 | 5 | 0.000 * |
| Andersen | 112 | 77.39 | ||||
| Filter | 30 | 204.05 | ||||
| EDC | 34 | 165.87 | ||||
| Settled dust | 12 | 112.67 | ||||
| Others | 15 | 176.47 | ||||
| Total | 236 | |||||
| Aspergillus sp. (CFUs) | Millipore | 33 | 104.05 | 149.849 | 5 | 0.000 * |
| Andersen | 112 | 73.04 | ||||
| Settled dust Filters | 30 | 208.37 | ||||
| EDC | 34 | 178.04 | ||||
| Settled dust | 12 | 97.13 | ||||
| Others | 15 | 192.17 | ||||
| Total | 236 | |||||
| Aspergillus section Fumigati | Millipore | 33 | 106.64 | 6.787 | 5 | 0.237 |
| Andersen | 112 | 119.06 | ||||
| Filter | 30 | 125.27 | ||||
| EDC | 34 | 125.35 | ||||
| Settled dust | 12 | 99.50 | ||||
| Others | 15 | 126.53 | ||||
| Total | 236 | |||||
| Media | Ranks | Test Statistics a,b | ||||
|---|---|---|---|---|---|---|
| N | Mean Rank | Kruskal–Wallis H | df | p | ||
| Fungal contamination (CFUs) | MEA | 71 | 121.16 | 20.273 | 3 | 0.000 * |
| DG18 | 140 | 107.38 | ||||
| SDA | 16 | 179.69 | ||||
| ITR+VOR | 9 | 161.67 | ||||
| Total | 236 | |||||
| Aspergillus sp. (CFUs) | MEA | 71 | 102.49 | 27.499 | 3 | 0.000 * |
| DG18 | 140 | 114.99 | ||||
| SDA | 16 | 181.16 | ||||
| ITR+VOR | 9 | 188.06 | ||||
| Total | 236 | |||||
| Aspergillus section Fumigati | MEA | 71 | 124.52 | 39.411 | 3 | 0.000 * |
| DG18 | 140 | 108.86 | ||||
| SDA | 16 | 131.38 | ||||
| ITR+VOR | 9 | 198.06 | ||||
| Total | 236 | |||||
| FFH | Ranks | Test Statistics a,b | ||||
|---|---|---|---|---|---|---|
| N | Mean Rank | Kruskal–Wallis H | df | p | ||
| Fungal contamination (CFUs) | 1 | 31 | 115.48 | 22.200 | 10 | 0.014 * |
| 2 | 23 | 108.41 | ||||
| 3 | 20 | 139.53 | ||||
| 4 | 7 | 103.14 | ||||
| 5 | 40 | 78.63 | ||||
| 6 | 27 | 128.44 | ||||
| 7 | 13 | 140.00 | ||||
| 8 | 26 | 137.04 | ||||
| 9 | 14 | 136.14 | ||||
| 10 | 22 | 122.66 | ||||
| 11 | 13 | 136.88 | ||||
| Total | 236 | |||||
| Aspergillus sp. (CFUs) | 1 | 31 | 115.90 | 22.227 | 10 | 0.014 * |
| 2 | 23 | 116.57 | ||||
| 3 | 20 | 140.30 | ||||
| 4 | 7 | 127.07 | ||||
| 5 | 40 | 86.88 | ||||
| 6 | 27 | 136.17 | ||||
| 7 | 13 | 168.92 | ||||
| 8 | 26 | 117.31 | ||||
| 9 | 14 | 132.29 | ||||
| 10 | 22 | 99.95 | ||||
| 11 | 13 | 119.08 | ||||
| Total | 236 | |||||
| Aspergillus section Fumigati | 1 | 31 | 112.00 | 22.481 | 10 | 0.013 * |
| 2 | 23 | 109.20 | ||||
| 3 | 20 | 119.83 | ||||
| 4 | 7 | 99.50 | ||||
| 5 | 40 | 117.89 | ||||
| 6 | 27 | 144.22 | ||||
| 7 | 13 | 137.35 | ||||
| 8 | 26 | 130.62 | ||||
| 9 | 14 | 108.00 | ||||
| 10 | 22 | 104.23 | ||||
| 11 | 13 | 99.50 | ||||
| Total | 236 | |||||
| Aspergillus Section Fumigati | Fungal Contamination (CFUs) | |
|---|---|---|
| Aspergillus sp. (CFUs) | 0.137 * | 0.705 ** |
| Aspergillus section Fumigati | 0.130 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Aranha Caetano, L. Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms 2021, 9, 2112. https://doi.org/10.3390/microorganisms9102112
Viegas C, Gomes B, Dias M, Carolino E, Aranha Caetano L. Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms. 2021; 9(10):2112. https://doi.org/10.3390/microorganisms9102112
Chicago/Turabian StyleViegas, Carla, Bianca Gomes, Marta Dias, Elisabete Carolino, and Liliana Aranha Caetano. 2021. "Aspergillus Section Fumigati in Firefighter Headquarters" Microorganisms 9, no. 10: 2112. https://doi.org/10.3390/microorganisms9102112
APA StyleViegas, C., Gomes, B., Dias, M., Carolino, E., & Aranha Caetano, L. (2021). Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms, 9(10), 2112. https://doi.org/10.3390/microorganisms9102112










