Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato
Abstract
:1. Introduction
2. Materials and Methods
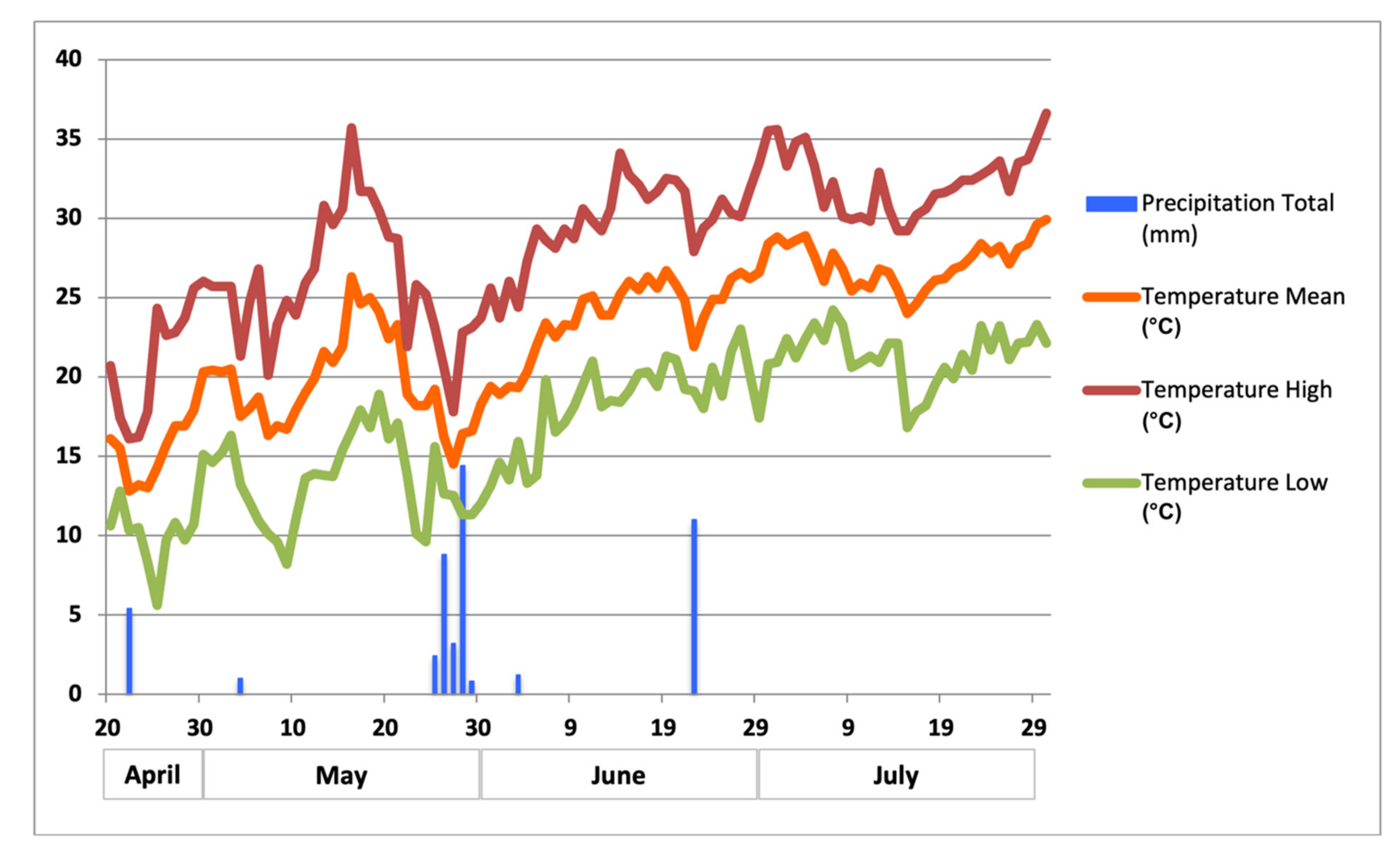
2.1. Experimental Site and Design
2.2. Cultivation of Bacteria
2.3. Measurements
2.4. Evaluation of Quality Parameters of Tomatoes
2.4.1. Physicochemical Parameters
2.4.2. Determination of Endogenous Enzymes Activity of Tomatoes
2.4.3. Determination of Intracellular Bioactive Compounds of Tomatoes
2.4.4. Extraction and Quantification of Total Carotenoids
2.4.5. Lycopene Extraction and Quantification by HPLC
2.4.6. Extraction and Quantification of Total Phenolic Compounds
2.4.7. Antioxidant Capacity
2.5. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Physiology Measurements
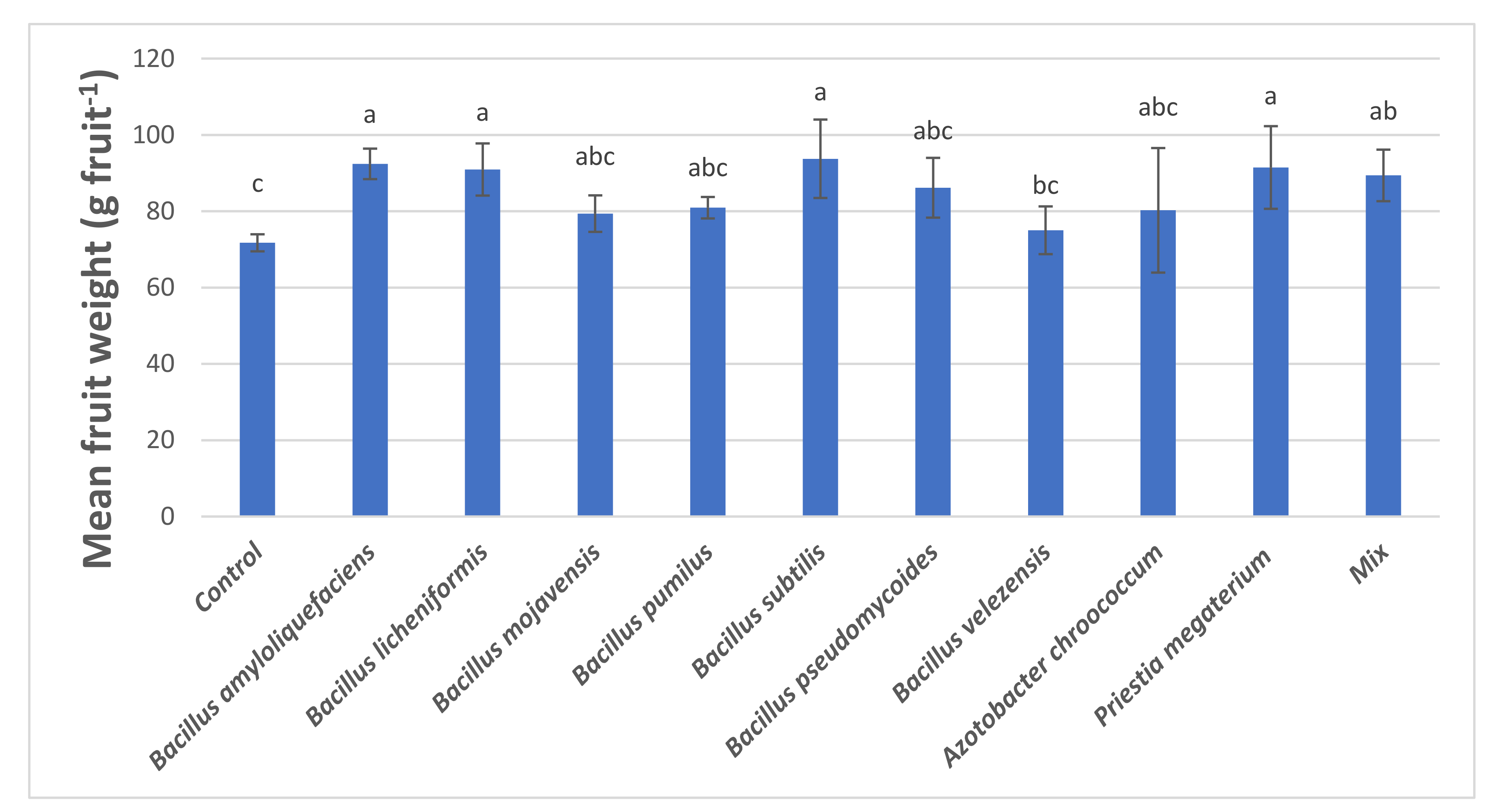
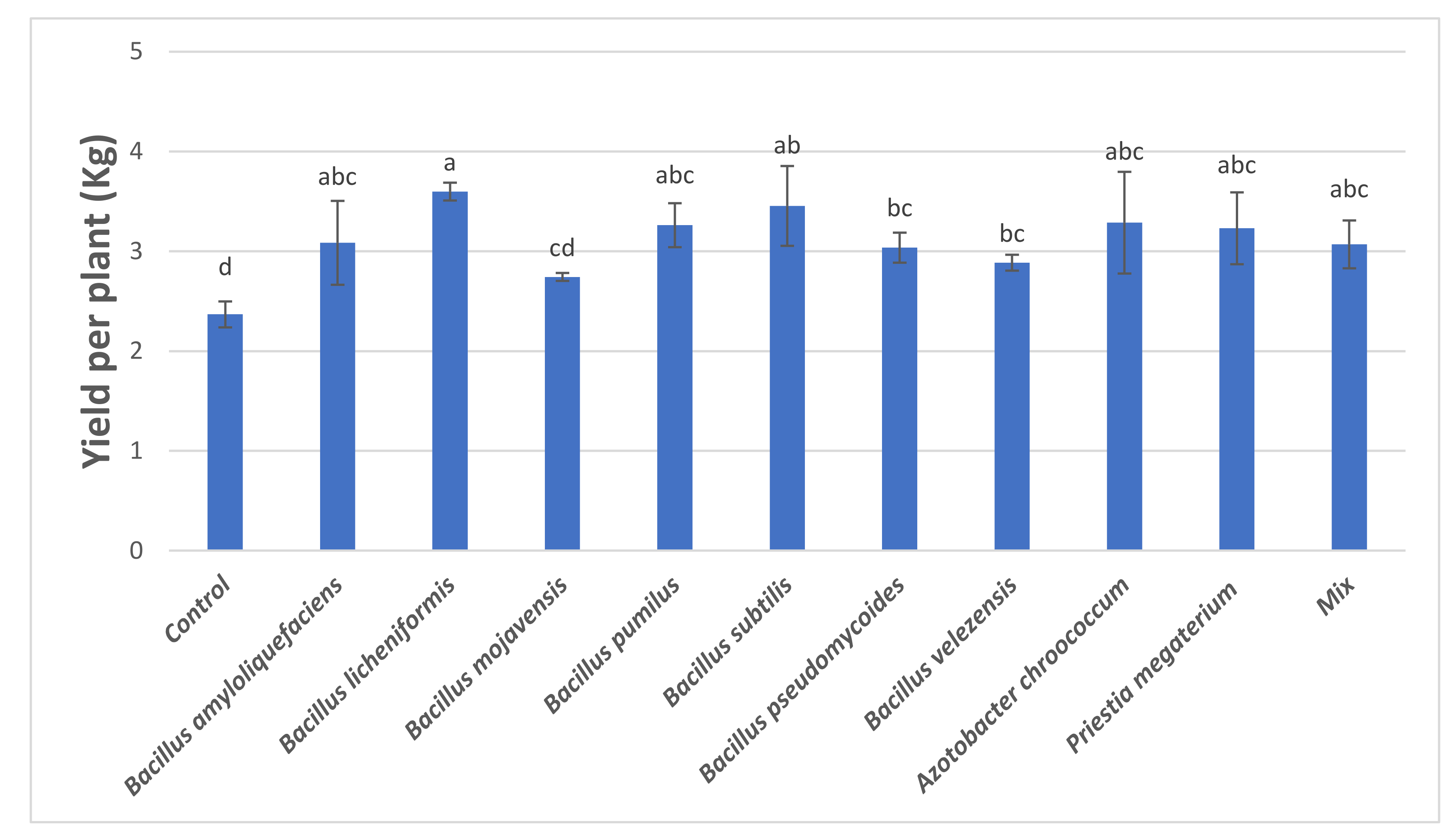
3.3. Yield
3.4. Quality Characteristics of the Harvested Tomatoes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Zhao, H.; Zhao, D.; Zhu, X.; Wang, Y.; Duan, Y.; Xuan, Y.; Chen, L. Isolation and Identification of Bacteria from Rhizosphere Soil and Their Effect on Plant Growth Promotion and Root-Knot Nematode Disease. Biol. Control 2018, 119, 12–19. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Efthimiadou, A.; Katsenios, N.; Chanioti, S.; Giannoglou, M.; Djordjevic, N.; Katsaros, G. Effect of Foliar and Soil Application of Plant Growth Promoting Bacteria on Growth, Physiology, Yield and Seed Quality of Maize under Mediterranean Conditions. Sci. Rep. 2020, 10, 21060. [Google Scholar] [CrossRef] [PubMed]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Figen Donmez, M.; Turan, M.; Gunes, A. Effects of Plant Growth Promoting Bacteria (PGPB) on Yield, Growth and Nutrient Contents of Organically Grown Strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Do Rosário Rosa, V.; Farias dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased Soybean Tolerance to Water Deficiency through Biostimulant Based on Fulvic Acids and Ascophyllum nodosum (L.) Seaweed Extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant Growth Promoting Rhizobacteria (PGPR) Confer Drought Resistance and Stimulate Biosynthesis of Secondary Metabolites in Pennyroyal (Mentha pulegium L.) under Water Shortage Condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Ahmadi-Rad, S.; Gholamhoseini, M.; Ghalavand, A.; Asgharzadeh, A.; Dolatabadian, A. Foliar Application of Nitrogen Fixing Bacteria Increases Growth and Yield of Canola Grown under Different Nitrogen Regimes. Rhizosphere 2016, 2, 34–37. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of Inoculation with Plant Growth-Promoting Bacteria (PGPB) on Amelioration of Saline Stress in Maize (Zea Mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Nieto, K.F.; Frankenberger, W.T. Biosynthesis of Cytokinins by Azotobacter Chroococcum. Soil Biol. Biochem. 1989, 21, 967–972. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of Free-Living Rhizospheric Bacteria for Their Multiple Plant Growth Promoting Activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Bahadur, A.; Singh, U.P.; Sarma, B.K.; Singh, D.P.; Singh, K.P.; Singh, A. Foliar Application of Plant Growth-Promoting Rhizobacteria Increases Antifungal Compounds in Pea (Pisum Sativum) Against Erysiphe Pisi. Mycobiology 2007, 35, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Romero-Perdomo, F.; Abril, J.; Camelo, M.; Moreno-Galván, A.; Pastrana, I.; Rojas-Tapias, D.; Bonilla, R. Azotobacter Chroococcum as a Potentially Useful Bacterial Biofertilizer for Cotton (Gossypium Hirsutum): Effect in Reducing N Fertilization. Rev. Argent. Microbiol. 2017, 49, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar Behl, R.; Narula, N. Establishment of Phosphate-Solubilizing Strains of Azotobacter Chroococcum in the Rhizosphere and Their Effect on Wheat Cultivars under Green House Conditions. Microbiol. Res. 2001, 156, 87–93. [Google Scholar] [CrossRef]
- Pirlak, L.; Turan, M.; Sahin, F.; Esitken, A. Floral and Foliar Application of Plant Growth Promoting Rhizobacteria (PGPR) to Apples Increases Yield, Growth, and Nutrient Element Contents of Leaves. J. Sustain. Agric. 2007, 30, 145–155. [Google Scholar] [CrossRef]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.C.; Façanha, A.R.; Olivares, F.L. A Combination of Humic Substances and Herbaspirillum Seropedicae Inoculation Enhances the Growth of Maize (Zea mays L.). Plant Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- Dudás, A. Sporeforming bacillus bioeffectors for healthier fruit quality of tomato in pots and field. Appl. Ecol. Environ. Res. 2017, 15, 1399–1418. [Google Scholar] [CrossRef]
- Berger, B.; Baldermann, S.; Ruppel, S. The Plant Growth-Promoting Bacterium Kosakonia Radicincitans Improves Fruit Yield and Quality of Solanum Lycopersicum: Kosakonia Radicincitans Improves Tomato Fruit Yield and Quality. J. Sci. Food Agric. 2017, 97, 4865–4871. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Pseudomonads Improve Yield, Quality and Nutritional Value of Tomato: A Field Study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Lee, K.-H.; Koh, R.-H.; Song, H.-G. Enhancement of Growth and Yield of Tomato by Rhodopseudomonas sp. under Greenhouse Conditions. J. Microbiol. 2008, 46, 641–646. [Google Scholar] [CrossRef]
- Lagouvardos, K.; Kotroni, V.; Bezes, A.; Koletsis, I.; Kopania, T.; Lykoudis, S.; Mazarakis, N.; Papagiannaki, K.; Vougioukas, S. The Automatic Weather Stations NOANN Network of the National Observatory of Athens: Operation and Database. Geosci. Data J. 2017, 4, 4–16. [Google Scholar] [CrossRef]
- International Standard Organisation (ISO). ISO 11260:1994. Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- International Standard Organisation (ISO). ISO 14870:2001. Soil Quality—Extraction of Trace Elements by Buffered DTPA Solution; ISO: Geneva, Switzerland, 2001. [Google Scholar]
- Bingham, F.T. Boron. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 431–447. ISBN 978-0-89118-977-0. [Google Scholar]
- International Standard Organisation (ISO). ISO 11261:1995. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- International Standard Organisation (ISO). ISO 14235:1998. Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- International Standard Organisation (ISO). ISO 11263:1994. Soil Quality—Determination of Phosphorus—Spectrometric Determination of Phosphorus Soluble in Sodium Hydrogen Carbonate Solution; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- International Standard Organisation (ISO). ISO 11265:1994. Soil Quality—Determination of the Specific Electrical Conductivity; ISO: Geneva, Switzerland, 1994. [Google Scholar]
- Galarza-Seeber, R.; Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, A.D.; Bielke, L.R.; Menconi, A.; Hargis, B.M.; Tellez, G. Isolation, Screening and Identification of Bacillus spp. as Direct-Fed Microbial Candidates for Aflatoxin B1 Biodegradation. Asian Pac. J. Trop. Biomed. 2015, 5, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Lοper, J.E.; Schroth, M.N. Influence of Bacterial Sources of Indole-3-Acetic Acid on Root Elongation of Sugar Beet. Phytopathology 1986, 76, 386–389. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Katsaros, G.; Taoukis, P. Comparison of the Application of High Pressure and Pulsed Electric Fields Technologies on the Selective Inactivation of Endogenous Enzymes in Tomato Products. Innov. Food Sci. Emerg. Technol. 2016, 38, 349–355. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of Pulsed Electric Fields to Improve Product Yield and Waste Valorization in Industrial Tomato Processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Dermesonlouoglou, E.K.; Andreou, V.; Alexandrakis, Z.; Katsaros, G.J.; Giannakourou, M.C.; Taoukis, P.S. The Hurdle Effect of Osmotic Pretreatment and High-Pressure Cold Pasteurisation on the Shelf-Life Extension of Fresh-Cut Tomatoes. Int. J. Food Sci. Technol. 2017, 52, 916–926. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of Ultrasound-Assisted Extraction of Oil from Olive Pomace Using Response Surface Technology: Oil Recovery, Unsaponifiable Matter, Total Phenol Content and Antioxidant Activity. LWT Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus Pumilus Promotes the Growth and Nitrogen Uptake of Tomato Plants under Nitrogen Fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Sirajuddin; Khan, A.; Ali, L.; Chaudhary, H.J.; Hussain Munis, M.F.; Bano, A.; Masood, S. Bacillus Pumilus Alleviates Boron Toxicity in Tomato (Lycopersicum Esculentum L.) Due to Enhanced Antioxidant Enzymatic Activity. Sci. Hortic. 2016, 200, 178–185. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, S.-H.; Balaraju, K.; Park, K.-S.; Nam, K.-W.; Park, J.-W.; Park, K. Growth Promotion and Induced Disease Suppression of Four Vegetable Crops by a Selected Plant Growth-Promoting Rhizobacteria (PGPR) Strain Bacillus Subtilis 21-1 under Two Different Soil Conditions. Acta Physiol. Plant. 2014, 36, 1353–1362. [Google Scholar] [CrossRef]
- García, J.A.L.; Probanza, A.; Ramos, B.; Palomino, M.R.; Gutiérrez Mañero, F.J. Effect of Inoculation of Bacillus Licheniformis on Tomato and Pepper. Agronomie 2004, 24, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant Growth-Promoting Bacteria Confer Resistance in Tomato Plants to Salt Stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of Biostimulants Improves Yield and Fruit Quality in Tomato. Int. J. Veg. Sci. 2020, 1–6. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Schwartz, S.J. Lycopene: Chemical Chemical and Biological Properties: Developing Nutraceuticals for the New Millenium. Food Technol. Chic. 1999, 53, 38–45. [Google Scholar]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- D’Souza, M.C.; Singha, S.; Ingle, M. Lycopene Concentration of Tomato Fruit Can Be Estimated from Chromaticity Values. HortScience 1992, 27, 465–466. [Google Scholar] [CrossRef] [Green Version]
- Bona, E.; Todeschini, V.; Cantamessa, S.; Cesaro, P.; Copetta, A.; Lingua, G.; Gamalero, E.; Berta, G.; Massa, N. Combined Bacterial and Mycorrhizal Inocula Improve Tomato Quality at Reduced Fertilization. Sci. Hortic. 2018, 234, 160–165. [Google Scholar] [CrossRef]
- Le, T.; Pék, Z.; Takács, S.; Neményi, A.; Helyes, L. The Effect of Plant Growth-Promoting Rhizobacteria on Yield, Water Use Efficiency and Brix Degree of Processing Tomato. Plant Soil Environ. 2018, 64, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Zaidi, A.; Khan, M.S.; Rizvi, A.; Saif, S.; Shahid, M. Perspectives of Plant Growth Promoting Rhizobacteria in Growth Enhancement and Sustainable Production of Tomato. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 125–149. ISBN 978-3-319-54401-4. [Google Scholar]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of Nitrogen Fertilization and Bacillus Licheniformis Biofertilizer Addition on the Antioxidants Compounds and Antioxidant Activity of Greenhouse Cultivated Tomato Fruits (Solanum lycopersicum L. Var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C.; Oh, J.W.; Paramasivan, M.; Saini, R.K.; Sahayarayan, J.J. Bacillus Subtilis CBR05 for Tomato (Solanum lycopersicum) Fruits in South Korea as a Novel Plant Probiotic Bacterium (PPB): Implications from Total Phenolics, Flavonoids, and Carotenoids Content for Fruit Quality. Agronomy 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]

| Identification | Strain | NCBI Accession Number | pH | CFU/mL | Concentration of Auxin (ppm) |
|---|---|---|---|---|---|
| Bacillus amyloliquefaciens | B002 | MW562326 | 6.70 | 6.5 × 109 | 38.45 |
| Bacillus licheniformis | B017 | MW562833 | 6.15 | 6.0 × 109 | 45.00 |
| Bacillus mojavensis | B010 | MW562828 | 5.95 | 4.1 × 109 | 40.52 |
| Bacillus pumilus | W27-4 | MW562832 | 6.01 | 2.6 × 109 | 58.10 |
| Bacillus subtilis | Z3 | MW396734 | 5.99 | 3.0 × 109 | 43.97 |
| Bacillus pseudomycoides | S3 | MW687620 | 5.92 | 6.0 × 109 | 39.14 |
| Bacillus velezensis | B006 | MW562831 | 6.08 | 5.2 × 108 | 46.03 |
| Azotobacter chroococcum | A004 | - | 7.20 | 6.4 × 109 | 24.00 |
| Priestia megaterium | B004 | MW562819 | 6.40 | 6.2 × 109 | 57.76 |
| Mix |
| Parameters | Values |
|---|---|
| Sand (%) | 34 |
| Silt (%) | 28 |
| Clay (%) | 38 |
| Soil Texture | Clay Loam |
| pH | 7.6 |
| Saturation percentage (%) | 55 |
| Electrical Conductivity (mS cm−1) | 1.41 |
| Total salts (%) | 0.05 |
| Organic Matter (%) | 4.9 |
| Total Nitrogen (mg g−1) | 2.2 |
| Available K (cmoℓ+ kg−1) | 1.2 |
| Available Ca (cmoℓ+ kg−1) | 22 |
| Available Mg (cmoℓ+ kg−1) | 6.4 |
| Available P (mg kg−1) | 87 |
| Fe-DTPA (mg kg−1) | 34 |
| Cu-DTPA (mg kg−1) | 3.7 |
| Zn-DTPA (mg kg−1) | 8.2 |
| Mn-DTPA (mg kg−1) | 15.7 |
| Available B (mg kg−1) | 1.5 |
| Treatment | Dry Weight (g per Plant) | ||
|---|---|---|---|
| 66 DAT | 80 DAT | 94 DAT | |
| Control | 243 ± 5 de | 361 ± 16 d | 546v24 d |
| B. amyloliquefaciens | 289 ± 6 bc | 403 ± 21 bc | 659 ± 65 bc |
| B. licheniformis | 328 ± 10 a | 442 ± 19 a | 761 ± 11 a |
| B. mojavensis | 301 ± 21 b | 402 ± 24 bc | 624 ± 18 cd |
| B. pumilus | 305 ± 18 b | 389 ± 6 cd | 697 ± 45 abc |
| B. subtilis | 304 ± 13 b | 381 ± 24 cd | 722 ± 68 ab |
| B. pseudomycoides | 336 ± 23 a | 388 ± 24 cd | 683 ± 24 abc |
| B. velezensis | 268 ± 5 cd | 371 ± 21 cd | 627 ± 15 cd |
| A. chroococcum | 256 ± 10 de | 361 ± 16 d | 699 ± 88 abc |
| P. megaterium | 275 ± 10 cd | 379 ± 20 cd | 695 ± 43 abc |
| Mix | 334 ± 13 a | 436 ± 19 ab | 702 ± 45 abc |
| Ftreat | 16.513 *** | 5.597 *** | 4.572 *** |
| Treatment | Photosynthetic Rate (μmol CO2 m−2 s−1) | ||
|---|---|---|---|
| 66 DAT | 80 DAT | 94 DAT | |
| Control | 14.34 ± 0.20 d | 15.38 ± 0.25 e | 16.62 ± 0.23 de |
| B. amyloliquefaciens | 17.19 ± 0.41 b | 18.00 ± 0.19 ab | 17.38 ± 0.58 abcd |
| B. licheniformis | 17.41 ± 0.51 ab | 17.09 ± 0.48 cd | 17.01 ± 0.32 cde |
| B. mojavensis | 16.07 ± 0.44 c | 16.72 ± 0.47 d | 16.38 ± 0.45 e |
| B. pumilus | 16.75 ± 0.47 bc | 15.87 ± 0.28 e | 17.32 ± 0.40 abcde |
| B. subtilis | 17.96 ± 0.44 a | 17.75 ± 0.19 b | 16.94 ± 0.61 cde |
| B. pseudomycoides | 17.29 ± 0.39 ab | 17.43 ± 0.08 bc | 17.22 ± 0.81 bcde |
| B. velezensis | 16.13 ± 0.27 c | 16.82 ± 0.34 cd | 18.02 ± 0.35 ab |
| A. chroococcum | 16.93 ± 0.47 b | 16.79 ± 0.54 cd | 17.82 ± 0.64 abc |
| P. megaterium | 18.03 ± 0.38 a | 18.48 ± 0.15 a | 18.27 ± 0.41 a |
| Mix | 17.38 ± 0.36 ab | 17.81 ± 0.60 b | 17.54 ± 0.64 abcd |
| Ftreat | 20.161 *** | 19.264 *** | 3.680 *** |
| Treatment | Transpiration Rate (mmol H2O m−2 s−1) | ||
|---|---|---|---|
| 66 DAT | 80 DAT | 94 DAT | |
| Control | 2.46 ± 0.08 f | 2.51 ± 0.22 c | 3.23 ± 0.05 |
| B. amyloliquefaciens | 3.38 ± 0.21 abc | 2.75 ± 0.09 bc | 3.44 ± 0.15 |
| B. licheniformis | 3.29 ± 0.21 bc | 2.98 ± 0.20 ab | 3.68 ± 0.41 |
| B. mojavensis | 3.35 ± 0.12 bc | 3.09 ± 0.18 ab | 3.60 ± 0.29 |
| B. pumilus | 2.93 ± 0.27 de | 3.23 ± 0.42 a | 3.52 ± 0.37 |
| B. subtilis | 3.71 ± 0.13 a | 3.05 ± 0.20 ab | 3.39 ± 0.25 |
| B. pseudomycoides | 3.04 ± 0.31 cde | 2.94 ± 0.24 ab | 3.78 ± 0.12 |
| B. velezensis | 3.23 ± 0.05 bcd | 3.25 ± 04 a | 3.59 ± 0.12 |
| A. chroococcum | 2.77 ± 0.12 ef | 2.74 ± 0.10 bc | 3.64 ± 0.21 |
| P. megaterium | 3.55 ± 0.26 ab | 2.88 ± 0.05 abc | 3.45 ± 0.38 |
| Mix | 3.40 ± 0.10 ab | 3.02 ± 0.25 ab | 3.36 ± 0.23 |
| Ftreat | 11.119 *** | 3.404 *** | 1.152 ns |
| Samples | °Brix | PME Activity (units/mL) | PG Activity (units/mL) | Total Carotenoids (mg /g d.m) | Total Phenolic Compounds (mg of CAE/g d.m) | Lycopene (μg/g d.m) | Antioxidant Activity (mg Trolox/g d.m) |
|---|---|---|---|---|---|---|---|
| Control | 4.00 ± 0.05 d | 15.17 ± 1.95 d | 19.01 ± 1.60 c | 5.82 ± 0.35 c | 4.12 ± 0.01 | 13.75 ± 3.35 cd | 2.88 ± 0.07 bc |
| B. amyloliquefaciens | 4.60 ± 0.00 ab | 19.58 ± 2.50 bc | 16.28 ± 0.81 cd | 7.15 ± 0.86 ab | 4.23 ± 0.18 | 19.05 ± 2.52 ab | 3.78 ± 0.64 a |
| B. licheniformis | 4.70 ± 0.13 a | 19.66 ± 2.74 bc | 14.46 ± 4.05 d | 6.42 ± 0.91 abc | 4.35 ± 0.41 | 18.12 ± 3.12 ab | 3.71 ± 0.35 a |
| B. mojavensis | 4.60 ± 0.15 ab | 22.83 ± 1.63 ab | 30.09 ± 1.40 a | 6.76 ± 0.77 abc | 4.43 ± 0.32 | 17.19 ± 2.82 abc | 3.45 ± 0.36 ab |
| B. pumilus | 4.75 ± 0.05 a | 20.52 ± 2.61 bc | 16.92 ± 4.31 cd | 6.27 ± 0.45 abc | 4.53 ± 0.10 | 15.32 ± 4.35 acd | 3.22 ± 0.25 abc |
| B. subtilis | 4.50 ± 0.10 bc | 22.34 ± 3.40 abc | 16.35 ± 0.52 cd | 7.36 ± 0.28 a | 4.85 ± 0.10 | 18.75 ± 0.10 ab | 3.77 ± 0.32 a |
| B. pseudomycoides | 4.50 ± 0.04 bc | 24.94 ± 3.27 a | 24.80 ± 1.99 b | 6.13 ± 0.52 bc | 4.12 ± 0.31 | 11.34 ± 3.54 d | 3.23 ± 0.43 abc |
| B. velezensis | 4.40 ± 0.02 c | 19.64 ± 1.34 cd | 28.97 ± 3.01 ab | 7.04 ± 0.44 ab | 4.48 ± 0.36 | 16.42 ± 4.25 abc | 3.37 ± 0.16 a |
| A. chroococcum | 4.60 ± 0.07 ab | 18.24 ± 2.08 ad | 25.04 ± 1.96 b | 7.38 ± 0.95 a | 4.73 ± 0.26 | 16.84 ± 2.19 abc | 2.62 ± 0.35 c |
| Mix | 4.70 ± 0.13 a | 21.23 ± 0.94 abc | 32.53 ± 0.79 a | 7.21 ± 0.29 ab | 4.48 ± 0.20 | 18.70 ± 4.35 ab | 3.56 ± 0.57 ab |
| P. megaterium | 4.60 ± 0.03 ab | 20.53 ± 1.06 bc | 24.84 ± 1.62 b | 7.28 ± 0.44 a | 4.19 ± 0.27 | 20.64 ± 3.81 a | 3.45 ± 0.27 ab |
| Ftreat | 19.560 *** | 3.841 ** | 21.179 *** | 3.384 ** | 2.496 ns | 3.650 ** | 3.824 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Stergiou, P.; Xanthou, M.-Z.; Kakabouki, I.; Vlachakis, D.; et al. Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato. Microorganisms 2021, 9, 2099. https://doi.org/10.3390/microorganisms9102099
Katsenios N, Andreou V, Sparangis P, Djordjevic N, Giannoglou M, Chanioti S, Stergiou P, Xanthou M-Z, Kakabouki I, Vlachakis D, et al. Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato. Microorganisms. 2021; 9(10):2099. https://doi.org/10.3390/microorganisms9102099
Chicago/Turabian StyleKatsenios, Nikolaos, Varvara Andreou, Panagiotis Sparangis, Nikola Djordjevic, Marianna Giannoglou, Sofia Chanioti, Panagiota Stergiou, Maria-Zacharoula Xanthou, Ioanna Kakabouki, Dimitrios Vlachakis, and et al. 2021. "Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato" Microorganisms 9, no. 10: 2099. https://doi.org/10.3390/microorganisms9102099
APA StyleKatsenios, N., Andreou, V., Sparangis, P., Djordjevic, N., Giannoglou, M., Chanioti, S., Stergiou, P., Xanthou, M.-Z., Kakabouki, I., Vlachakis, D., Djordjevic, S., Katsaros, G., & Efthimiadou, A. (2021). Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato. Microorganisms, 9(10), 2099. https://doi.org/10.3390/microorganisms9102099











