Conjunctival Sac Microbiome in Infectious Conjunctivitis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Sample Collection and Testing
2.3. Polymerase Chain Reaction (PCR) Amplification and Preparation for 16S rRNA Gene Sequencing
2.4. Sequence Analysis
2.5. Statistical Analysis
3. Results
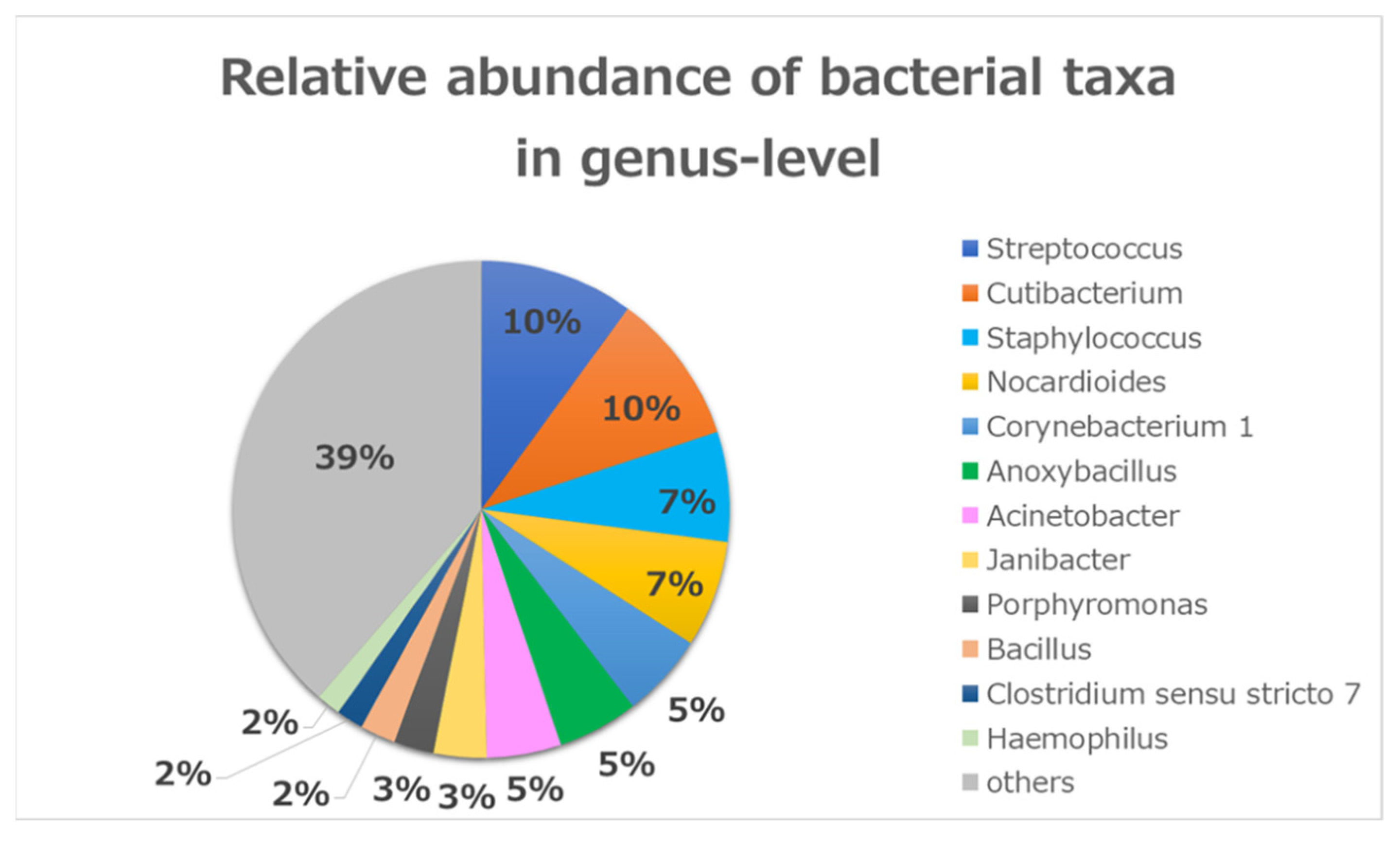
3.1. Bacterial Community Composition in Conjunctivitis
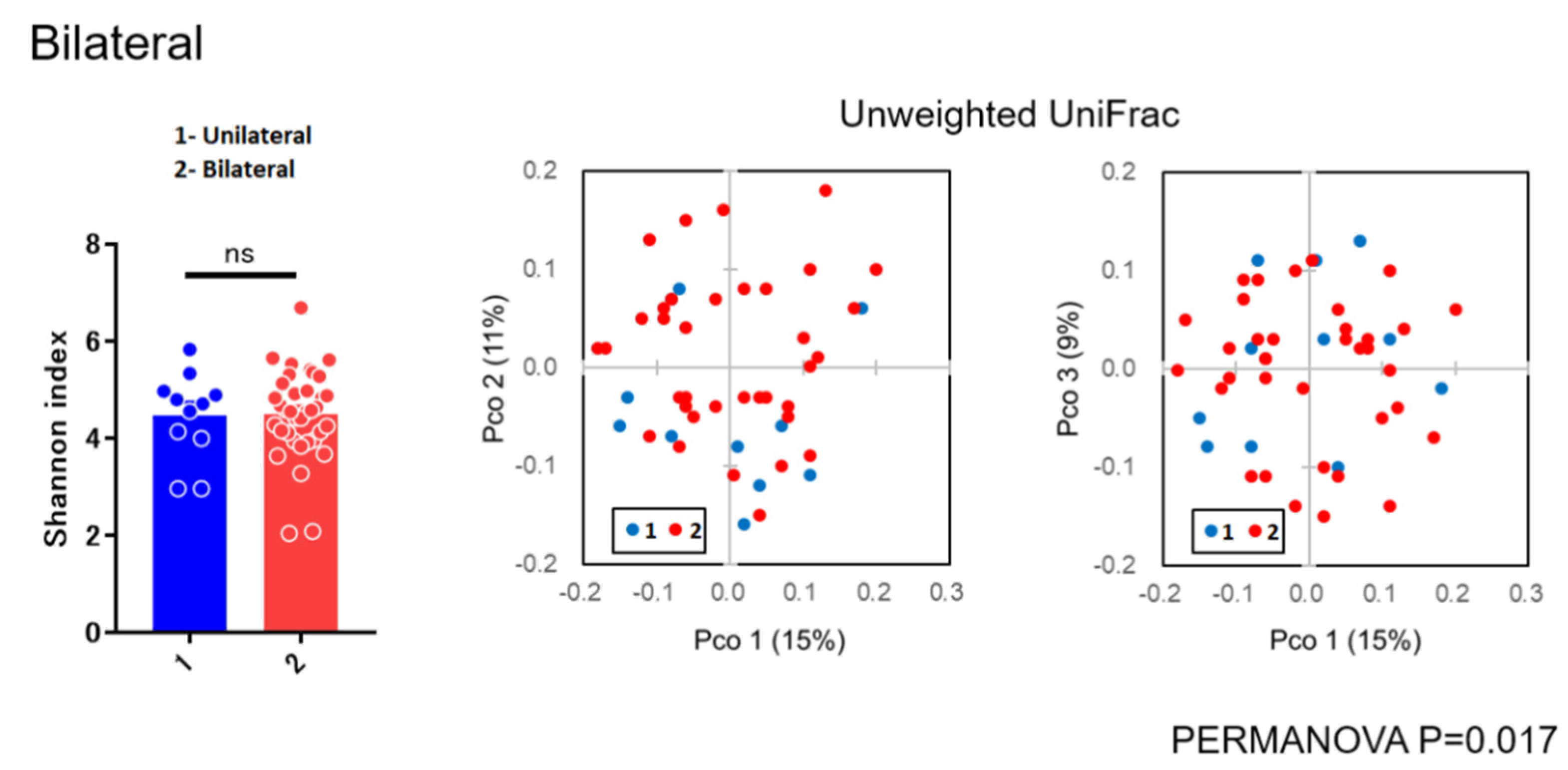
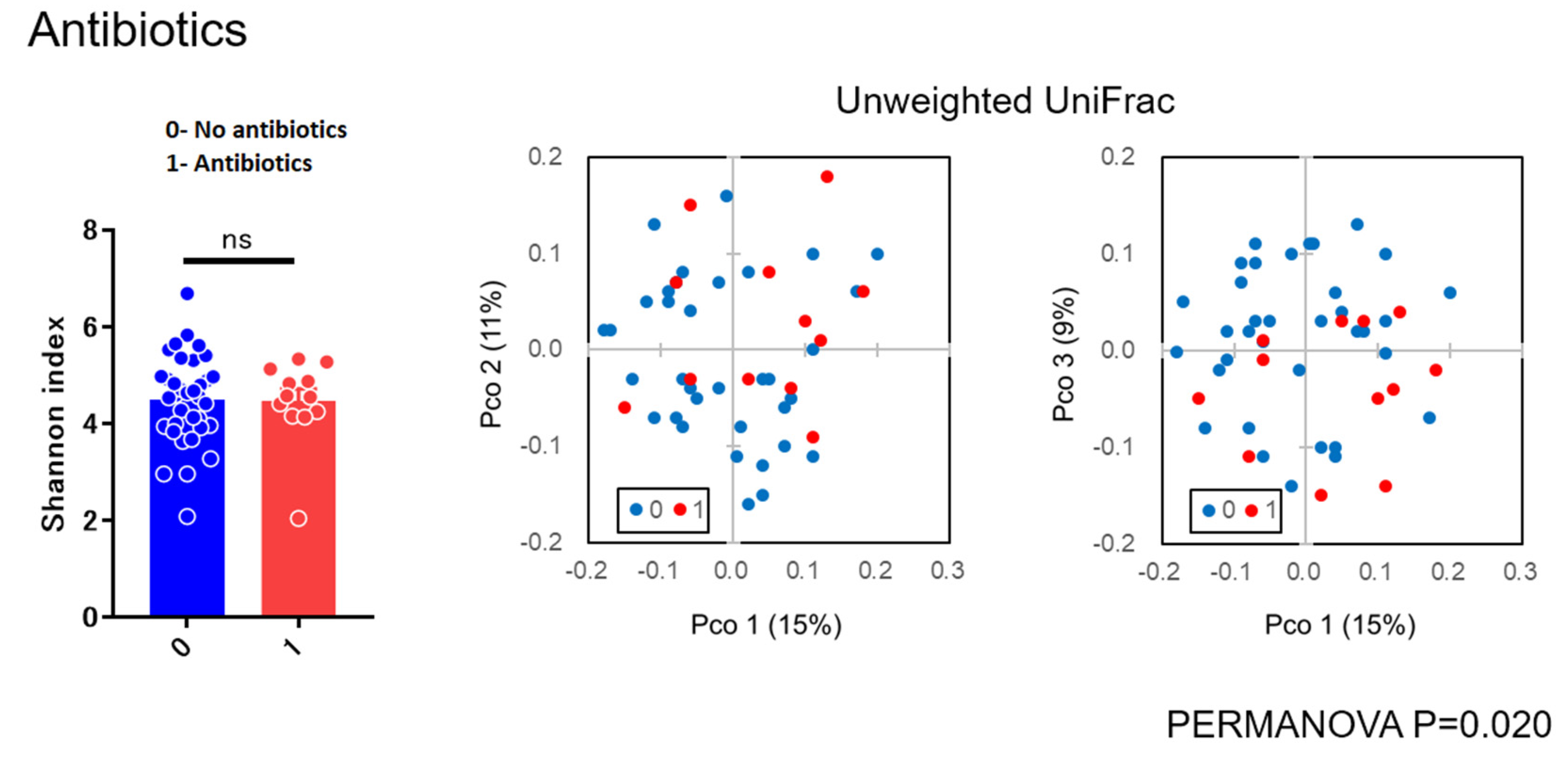
3.2. Diversity Analysis
3.3. Relative Abundance of Each Sample
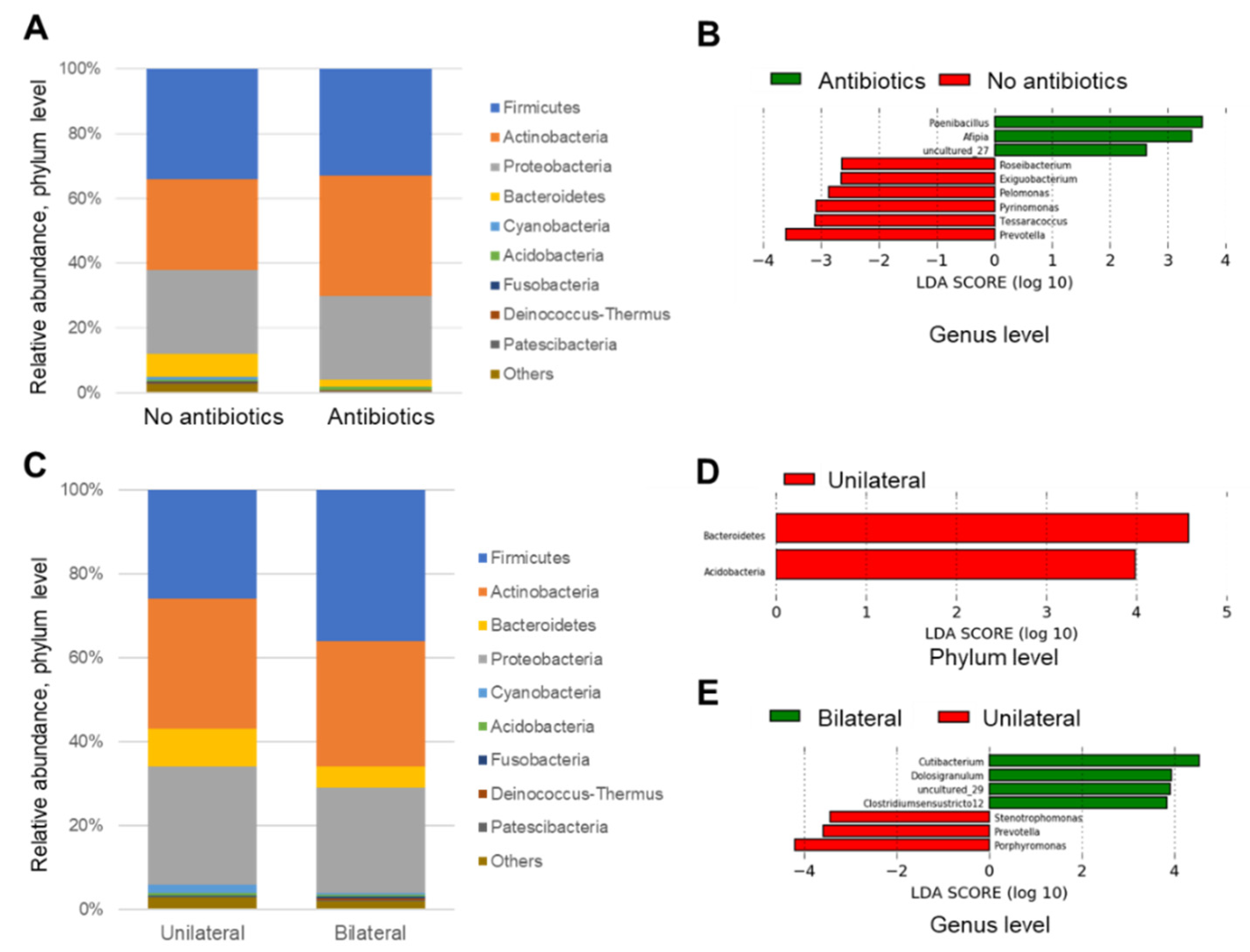
3.4. Linear Discriminant Analysis
3.5. Comparison of Cultivation and Metagenome
4. Discussion
4.1. Overview of the Microbiome in All Patients
4.2. Microbial Diversity
4.3. Linear Discrimination Analysis
4.4. Individual Metagenomics
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dart, J.K.G. Eye disease at a community health centre. Br. Med. J. 1986, 293, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Everitt, H.; Little, P. How do GPs diagnose and manage acute infective conjunctivitis? A GP survey. Fam. Pract. 2002, 19, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, R.P.; ter Riet, G.; Bindels, P.J.E.; Bink, D.; Sloos, J.H.; van Weert, H.C.P.M. The treatment of acute infectious conjunctivitis with fusidic acid: A randomised controlled trial. Br. J. Gen Pract. 2005, 55, 924–930. [Google Scholar] [PubMed]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.S.; Hayes, V.E.A.; Dartt, D.A.; Downes, C.S.; Moore, T.C.B. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Hotta, F.; Kuwahara, T.; Imaohji, H.; Miyazaki, C.; Hirose, M.; Kusaka, S.; Fukuda, M.; Shimomura, Y. Diagnostic Approach to Ocular Infections Using Various Techniques From Conventional Culture to Next-Generation Sequencing Analysis. Cornea 2017, 36, S46–S52. [Google Scholar] [CrossRef]
- Perkins, R.E.; Kundsin, R.B.; Pratt, M.V.; Abrahamsen, I.; Leibowitz, H.M. Bacteriology of normal and infected conjunctiva. J. Clin. Microbiol. 1975, 1, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Yasuda, F.; Higashitsutsumi, M. Preoperative disinfection of the conjunctival sac in cataract surgery. Ophthalmologica 1997, 211, 62–67. [Google Scholar] [CrossRef]
- Michael, E.Z.; Russell, N.V. Considerations in Understanding the Ocular Surface Microbiome. Am. J. Ophthalmol. 2014, 158, 420–422. [Google Scholar]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Revanna, K.V.; Gao, X.; Antonopoulos, D.A.; Slepak, V.Z.; et al. Diversity of bacteria at healthy human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5408–5413. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, e7–e643. [Google Scholar] [CrossRef]
- Morinaga, Y.; Take, Y.; Sasaki, D.; Ota, K.; Kaku, N.; Uno, N.; Sakamoto, K.; Kosai, K.; Miyazaki, T.; Hasegawa, H.; et al. Exploring the microbiota of upper respiratory tract during the development of pneumonia in a mouse model. PLoS ONE 2019, 14, e0222589. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef]
- Gallon, P.; Parekh, M.; Ferrari, S.; Fasolo, A.; Ponzin, D.; Borroni, D. Metagenomics in ophthalmology: Hypothesis or real prospective? Biotechnol. Rep. 2019, 23, e00355. [Google Scholar] [CrossRef]
- Lu, L.J.; Liu, J. Human Microbiota and Ophthalmic Disease. Yale J. Biol. Med. 2016, 89, 325–330. [Google Scholar] [PubMed]
- Dave, S.B.; Toma, H.S.; Kim, S.J. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Ophthalmology 2013, 120, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Boutite, F.; Tristan, A.; Bes, M.; Etienne, J.; Vandenesch, F. Bacterial competition for human nasal cavity colonization: Role of staphylococcal agr alleles. Appl. Environ. Microbiol. 2003, 69, 18–23. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative ocular microbial communities in humans with and without blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Maca, S.; Rölleke, S.; Nigl, K.; Lukas, J.; Hirschl, A.; Lubitz, W.; Barisani-Asenbauer, T. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1164–1171. [Google Scholar]
- Aoki, R.; Fukuda, K.; Ogawa, M.; Ikeno, T.; Kondo, H.; Tawara, A.; Taniguchi, H. Identification of causative pathogens in eyes with bacterial conjunctivitis by bacterial cell count and microbiota analysis. Ophthalmology 2013, 120, 668–676. [Google Scholar] [CrossRef]
- De Kaspar, H.M.; Kreutzer, T.C.; Aguirre-Romo, I.; Ta, C.N.; Dudichum, J.; Bayrhof, M.; Klauss, V.; Kampik, A. A Prospective Randomized Study to Determine the Efficacy of Preoperative Topical Levofloxacin in Reducing Conjunctival Bacterial Flora. Am. J. Ophthalmol. 2008, 145, 136–142.e2. [Google Scholar] [CrossRef]
- Matysiak, A.; Kabza, M.; Karolak, J.A.; Jaworska, M.M.; Rydzanicz, M.; PLoSki, R.; Szaflik, J.P.; Gajecka, M. Characterization of Ocular Surface Microbial Profiles Revealed Discrepancies between Conjunctival and Corneal Microbiota. Pathogens 2021, 10, 405. [Google Scholar] [CrossRef]
- Ozkan, J.; Willcox, M.; Wemheuer, B.; Wilcsek, G.; Coroneo, M.; Thomas, T. Biogeography of the human ocular microbiota. Ocul Surf. 2019, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; LeyTurnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Singer, T.R.; Isenberg, S.J.; Apt, L. Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br. J. Ophthalmol. 1988, 72, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tse, H.; Yuen, K.Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Romano, V.; Kaye, S.B.; Somerville, T.; Napoli, L.; Fasolo, A.; Gallon, P.; Ponzin, D.; Esposito, A.; Ferrari, S. Metagenomics in ophthalmology: Current findings and future prospectives. BMJ Open Ophthalmol. 2019, 4, e000248. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Adegbola, R.A.; Kinteh, F.; Ikumapayi, U.N.; Foster, A.; Mabey, D.C.; Bailey, R.L. Bacterial infection and trachoma in the Gambia: A case control study. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4440–4444. [Google Scholar] [CrossRef][Green Version]
- Elnifro, E.M.; Cooper, R.J.; Klapper, P.E.; Yeo, A.C.; Tullo, A.B. Multiplex polymerase chain reaction for diagnosis of viral and chlamydial keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1818–1822. [Google Scholar]
- Lietman, T.M.; Dhital, S.P.; Dean, D. Conjunctival impression cytology for vitamin A deficiency in the presence of infectious trachoma. Br. J. Ophthalmol. 1998, 82, 1139–1142. [Google Scholar] [CrossRef][Green Version]
- Rudolph, T.; Welinder-Olsson, C.; Lind-Brandberg, L.; Stenevi, U. 16S rDNA PCR analysis of infectious keratitis: A case series. Acta Ophthalmol. Scand. 2004, 82, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, C.; Cornut, P.L.; Benito, Y.; Thuret, G.; Maurin, M.; Lafontaine, P.O.; Pechinot, A.; Palombi, K.; Lina, G.; Bron, A.; et al. Eubacterial PCR for bacterial detection and identification in 100 acute postcataract surgery endophthalmitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Daroy, M.L.; Lopez, J.S.; Torres, B.C.; Loy, M.J.; Tuano, P.M.; Matias, R.R. Identification of unknown ocular pathogens in clinically suspected eye infections using ribosomal RNA gene sequence analysis. Clin Microbiol. Infect. 2011, 17, 776–779. [Google Scholar] [CrossRef] [PubMed]


| ID | Age | Sex | Uni/Bilateral | Antibiotics Drops | Sample Collection Date | Underlying Medical Condition | Culture |
|---|---|---|---|---|---|---|---|
| 12 | 67 | Male | Bilateral | - | 10/25/2016 | ||
| 17 | 20 | Male | Bilateral | + | 10/25/2016 | ||
| 19 | 1 | Female | Bilateral | - | 10/25/2016 | ||
| 51 | 40 | Female | Unilateral | + | 11/7/2016 | ||
| 60 | 57 | Female | Unilateral | - | 11/11/2016 | ||
| 64 | 53 | Male | Bilateral | - | 11/17/2016 | Post-cataract surgery and IOL | |
| 67 | 68 | Female | Bilateral | + | 11/17/2016 | ||
| 75 | 33 | Male | Bilateral | - | 11/25/2016 | ||
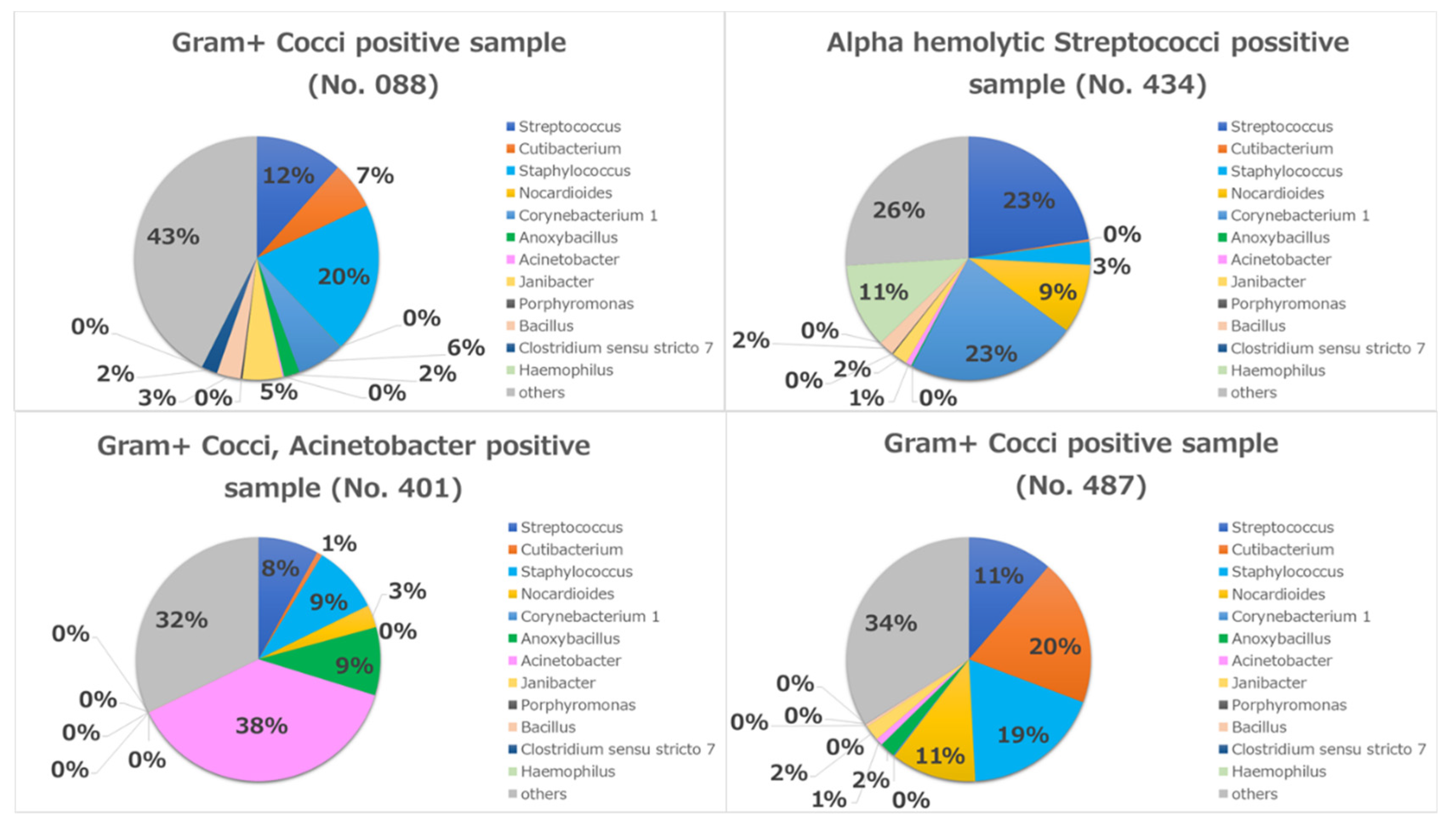
| 88 | 46 | Female | Bilateral | - | 12/2/2016 | Gram+ Cocci | |
| 92 | 1 | Female | Unilateral | + | 12/5/2016 | ||
| 94 | 26 | Female | Bilateral | - | 12/6/2016 | ||
| 111 | 71 | Female | Bilateral | - | 12/20/2016 | Hypertension | |
| 117 | 49 | Female | Bilateral | + | 12/20/2016 | Hypertension | |
| 123 | 8 | Female | Bilateral | - | 12/21/2016 | ||
| 149 | 40 | Male | Bilateral | + | 1/12/2017 | ||
| 163 | 19 | Male | Bilateral | + | 2/13/2017 | ||
| 165 | 33 | Male | Bilateral | + | 2/13/2017 | ||
| 173 | 56 | Female | Bilateral | - | 2/23/2017 | Diabetes mellitus | |
| 174 | 22 | Male | Bilateral | + | 2/23/2017 | ||
| 177 | 26 | Male | Bilateral | + | 2/28/2017 | Dyslipidemia | |
| 194 | 20 | Male | Bilateral | - | 3/16/2017 | ||
| 195 | 23 | Female | Unilateral | - | 3/17/2017 | ||
| 236 | 48 | Male | Bilateral | - | 3/31/2017 | Smoking | |
| 255 | 59 | Female | Bilateral | - | 4/14/2017 | Hypertension | |
| 278 | 35 | Female | Bilateral | - | 4/28/2017 | ||
| 284 | 43 | Female | Bilateral | - | 5/8/2017 | ||
| 317 | 8 | Female | Bilateral | - | 5/15/2017 | ||
| 339 | 34 | Female | Bilateral | - | 5/23/2017 | ||
| 347 | 6 | Female | Bilateral | - | 5/29/2017 | ||
| 362 | 73 | Male | Unilateral | - | 6/13/2017 | Cataract | |
| 367 | 29 | Female | Unilateral | - | 6/13/2017 | ||
| 373 | 49 | Male | Bilateral | - | 6/22/2017 | Hypertension | |
| 376 | 46 | Female | Bilateral | - | 6/23/2017 | ||
| 401 | 65 | Male | Bilateral | - | 7/17/2017 | Smoking | Gram+ Cocci, Acinetobacter |
| 410 | 55 | Female | Unilateral | - | 7/28/2017 | ||
| 422 | 45 | Male | Bilateral | - | 8/7/2017 | ||
| 424 | 16 | Male | Unilateral | - | 8/8/2017 | ||
| 434 | 71 | Female | Unilateral | - | 8/24/2017 | Hypertension | Alfa hemolytic Streptococci |
| 446 | 35 | Female | Bilateral | - | 8/28/2017 | ||
| 455 | 45 | Female | Unilateral | - | 9/7/2017 | ||
| 461 | 40 | Female | Bilateral | - | 9/11/2017 | ||
| 463 | 17 | Female | Bilateral | + | 9/11/2017 | ||
| 479 | 3 | Male | Unilateral | - | 9/14/2017 | ||
| 484 | 28 | Male | Bilateral | + | 9/21/2017 | ||
| 487 | 35 | Female | Bilateral | - | 9/22/2017 | Gram+ Cocci | |
| 507 | 74 | Male | Bilateral | - | 9/29/2017 | ||
| 530 | 66 | Female | Bilateral | - | 10/10/2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, Y.H.; Uematsu, M.; Morinaga, Y.; Nguyen, H.-A.T.; Toizumi, M.; Sasaki, D.; Yanagihara, K.; Dang, D.-A.; Kitaoka, T.; Yoshida, L.-M. Conjunctival Sac Microbiome in Infectious Conjunctivitis. Microorganisms 2021, 9, 2095. https://doi.org/10.3390/microorganisms9102095
Mohamed YH, Uematsu M, Morinaga Y, Nguyen H-AT, Toizumi M, Sasaki D, Yanagihara K, Dang D-A, Kitaoka T, Yoshida L-M. Conjunctival Sac Microbiome in Infectious Conjunctivitis. Microorganisms. 2021; 9(10):2095. https://doi.org/10.3390/microorganisms9102095
Chicago/Turabian StyleMohamed, Yasser Helmy, Masafumi Uematsu, Yoshitomo Morinaga, Hien-Anh Thi Nguyen, Michiko Toizumi, Daisuke Sasaki, Katsunori Yanagihara, Duc-Anh Dang, Takashi Kitaoka, and Lay-Myint Yoshida. 2021. "Conjunctival Sac Microbiome in Infectious Conjunctivitis" Microorganisms 9, no. 10: 2095. https://doi.org/10.3390/microorganisms9102095
APA StyleMohamed, Y. H., Uematsu, M., Morinaga, Y., Nguyen, H.-A. T., Toizumi, M., Sasaki, D., Yanagihara, K., Dang, D.-A., Kitaoka, T., & Yoshida, L.-M. (2021). Conjunctival Sac Microbiome in Infectious Conjunctivitis. Microorganisms, 9(10), 2095. https://doi.org/10.3390/microorganisms9102095







