Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Invasion and Adherence Assays
2.3. Cytochalasin D Assay
2.4. Transmission Electron Microscopy
2.5. Confocal Microscopy
2.6. Recombinant ClfB Protein
2.7. Gene Silencing by siRNAs
2.8. Overexpression of AnxA2 in MAC-T
2.9. Western Blotting Analysis
2.10. Immunoprecipitation and MS Analysis
2.11. Pull-Down Assay
2.12. Statistical Analysis
3. Results
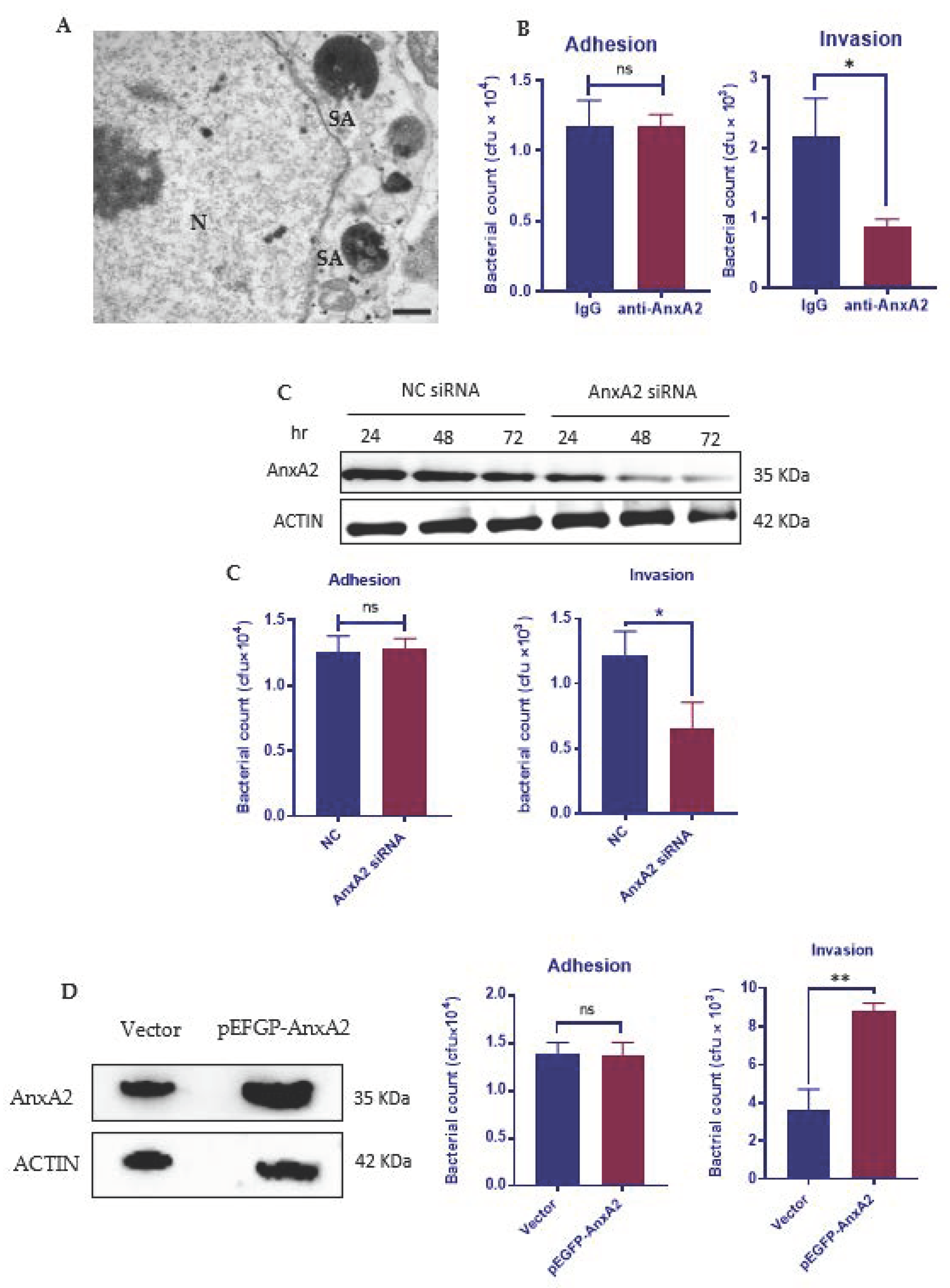
3.1. AnxA2 Is Required for the Internalization of S. aureus by MAC-T Cells
3.2. AnxA2 Contributes to S. aureus-Induced Actin Cytoskeleton Reorganization
3.3. Bacterial Engagement of AnxA2 Activates ILK/p38 MAPK Pathway
3.4. AnxA2 Is a Binding Partner of S. aureus ClfB
3.5. Interaction of ClfB and AnxA2 Regulates the Internalization of S. aureus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Dohoo, I.; Barkema, H.; Descôteaux, L.; Devries, T.; Reyher, K.; Roy, J.-P.; Scholl, D. Manageable risk factors associated with the lactational incidence, elimination, and prevalence of Staphylococcus aureus intramammary infections in dairy cows. J. Dairy Sci. 2012, 95, 1283–1300. [Google Scholar] [CrossRef] [Green Version]
- Cvetnić, L.; Samardžija, M.; Duvnjak, S.; Habrun, B.; Cvetnić, M.; Tkalec, V.J.; Đuričić, D.; Benić, M. Multi locus sequence typing and spa typing of Staphylococcus aureus isolated from the milk of cows with subclinical mastitis in Croatia. Microorganisms 2021, 9, 725. [Google Scholar] [CrossRef]
- Burović, J. Isolation of bovine clinical mastitis bacterial pathogens and their antimicrobial susceptibility in the Zenica region in 2017. Vet. Stanica. 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Z.; Turk, R.; Duričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arhiv. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Algharib, S.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grispoldi, L.; Massetti, L.; Sechi, P.; Iulietto, M.F.; Ceccarelli, M.; Karama, M.; Popescu, P.A.; Pandolfi, F.; Cenci-Goga, B. Short communication: Characterization of enterotoxin-producing Staphylococcus aureus isolated from mastitic cows. J. Dairy Sci. 2019, 102, 1059–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Wu, S.; Huang, J.; Wu, Q.; Zhang, F.; Zhang, J.; Wang, J.; Ding, Y.; Zhang, S.; Yang, X.; et al. Prevalence and characterization of Staphylococcus aureus isolated from pasteurized milk in china. Front. Microbiol. 2019, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.J.; Bacigalupe, R.; Harrison, E.; Weinert, L.A.; Lycett, S.; Vrieling, M.; Robb, K.; Hoskisson, P.A.; Holden, M.T.G.; Feil, E.J.; et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2018, 2, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Spoor, L.E.; McAdam, P.R.; Weinert, L.A.; Rambaut, A.; Hasman, H.; Aarestrup, F.; Kearns, A.M.; Larsen, A.R.; Skov, R.L.; Fitzgerald, J.R. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. mBio 2013, 4, e00356-13. [Google Scholar] [CrossRef] [Green Version]
- Menzies, B.E.; Kourteva, I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 1998, 66, 5994–5998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayles, K.W.; Wesson, C.A.; Liou, L.E.; Fox, L.K.; Bohach, G.A.; Trumble, W.R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 1998, 66, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelzner, K.; Winkler, A.-C.; Liang, C.; Boyny, A.; Ade, C.P.; Dandekar, T.; Fraunholz, M.J.; Rudel, T. Intracellular Staphylococcus aureus perturbs the host cell Ca2+ homeostasis to promote cell death. mBio 2020, 11, e02250-20. [Google Scholar] [CrossRef]
- Edwards, A.M.; Potter, U.; Meenan, N.A.G.; Potts, J.R.; Massey, R.C. Staphylococcus aureus keratinocyte invasion is dependent upon multiple high-affinity fibronectin-binding repeats within FnBPA. PLoS ONE 2011, 6, e18899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouillette, E.; Grondin, G.; Shkreta, L.; Lacasse, P.; Talbot, B.G. In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb. Pathog. 2003, 35, 159–168. [Google Scholar] [CrossRef]
- Bouchard, D.S.; Rault, L.; Berkova, N.; Le Loir, Y.; Even, S. Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl. Environ. Microbiol. 2013, 79, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.A.; Matthews, K.R.; Cifrian, E.; Guidry, A.J.; Oliver, S.P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 1996, 79, 1021–1026. [Google Scholar] [CrossRef]
- Al Kindi, A.; Alkahtani, A.M.; Nalubega, M.; El-Chami, C.; O’Neill, C.; Arkwright, P.D.; Pennock, J.L. Staphylococcus aureus internalized by skin keratinocytes evade antibiotic killing. Front. Microbiol. 2019, 10, 2242. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef] [Green Version]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppée, J.-Y.; Wolz, C.; Tenson, T.; Van Bambeke, F. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; López-Meza, J.E.; Ochoa-Zarzosa, A. Nonprofessional phagocytic cell receptors involved in Staphylococcus aureus internalization. BioMed Res. Int. 2014, 2014, 538546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Genet. 2013, 12, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Geoghegan, J.A.; Foster, T.J. Cell Wall-anchored surface proteins of Staphylococcus aureus: Many proteins, multiple functions. Curr. Top. Microbiol. Immunol. 2015, 409, 95–120. [Google Scholar] [CrossRef]
- Speziale, P.; Arciola, C.R.; Pietrocola, G. Fibronectin and its role in human infective diseases. Cells 2019, 8, 1516. [Google Scholar] [CrossRef] [Green Version]
- Fowler, T.; Wann, E.R.; Joh, D.; Johansson, S.; Foster, T.J.; Hook, M. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 2000, 79, 672–679. [Google Scholar] [CrossRef]
- Agerer, F.; Lux, S.; Michel, A.; Rohde, M.; Ohlsen, K.; Hauck, C.R. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 2005, 118, 2189–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bur, S.; Preissner, K.T.; Herrmann, M.; Bischoff, M. The Staphylococcus aureus extracellular adherence protein promotes bacterial internalization by keratinocytes independent of fibronectin-binding proteins. J. Investig. Dermatol. 2013, 133, 2004–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somarajan, S.R.; Al-Asadi, F.; Ramasamy, K.; Pandranki, L.; Baseman, J.B.; Kannan, T.R. Annexin A2 mediates Mycoplasma pneumoniae community-acquired respiratory distress syndrome toxin binding to eukaryotic cells. mBio 2014, 5, e01497-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschnek, S.; Adams, C.; Gulbins, E. Annexin II is a novel receptor for Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2005, 327, 900–906. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Chang, Q.; Su, Z.; Gong, D.; Zhou, Y.; Xiao, J.; Drelich, A.; Liu, Y.; Popov, V.; et al. A new role for host Annexin A2 in establishing bacterial adhesion to vascular endothelial cells: Lines of evidence from atomic force microscopy and an in vivo study. Lab. Investig. 2019, 99, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Grindheim, A.K.; Saraste, J.; Vedeler, A. Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim. Biophys. Acta BBA Gen. Subj. 2017, 1861, 2515–2529. [Google Scholar] [CrossRef]
- Ashraf, S.; Cheng, J.; Zhao, X. Clumping factor A of Staphylococcus aureus interacts with AnnexinA2 on mammary epithelial cells. Sci. Rep. 2017, 7, 40608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribelli, P.M.; Luqman, A.; Nguyen, M.; Madlung, J.; Fan, S.; Macek, B.; Sass, P.; Bitschar, K.; Schittek, B.; Kretschmer, D.; et al. Staphylococcus aureus Lpl protein triggers human host cell invasion via activation of Hsp90 receptor. Cell. Microbiol. 2019, 22, e13111. [Google Scholar] [CrossRef] [Green Version]
- Sinha, B.; Francois, P.P.; Nusse, O.; Foti, M.; Hartford, O.M.; Vaudaux, P.; Foster, T.J.; Lew, D.P.; Herrmann, M.; Krause, K.H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1999, 1, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Shao, D.-M.; Grieve, A.; Levine, T.; Bailly, M.; Moss, S.E. Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5,-bisphosphate. Biochim. Biophys. Acta BBA Bioenerg. 2009, 1793, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayedyahossein, S.; Xu, S.X.; Rudkouskaya, A.; McGavin, M.J.; McCormick, J.K.; Dagnino, L. Staphylococcus aureus keratinocyte invasion is mediated by integrin-linked kinase and Rac1. FASEB J. 2014, 29, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yurecko, R.S.; Dedhar, S.; Cleary, P.P. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell. Microbiol. 2005, 8, 257–266. [Google Scholar] [CrossRef]
- Okada, T.; Otani, H.; Wu, Y.; Kyoi, S.; Enoki, C.; Fujiwara, H.; Sumida, T.; Hattori, R.; Imamura, H. Role of F-actin organization in p38 MAP kinase-mediated apoptosis and necrosis in neonatal rat cardiomyocytes subjected to simulated ischemia and reoxygenation. Am. J. Physiol. Circ. Physiol. 2005, 289, H2310–H2318. [Google Scholar] [CrossRef] [Green Version]
- Smeeton, J.; Zhang, X.; Bulus, N.; Mernaugh, G.; Lange, A.; Karner, C.; Carroll, T.J.; Fässler, R.; Pozzi, A.; Rosenblum, N.D.; et al. Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development 2010, 137, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.; Song, W.; Xu, S.; Sun, Y.; Wang, Z.-L. Role of ILK/p38 pathway in mediating the enhanced osteogenic differentiation of bone marrow mesenchymal stem cells on amorphous carbon coating. Biomater. Sci. 2018, 7, 975–984. [Google Scholar] [CrossRef]
- O’Brien, L.M.; Walsh, E.J.; Massey, R.C.; Peacock, S.J.; Foster, T.J. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: Implications for nasal colonization. Cell. Microbiol. 2002, 4, 759–770. [Google Scholar] [CrossRef]
- Jolly, C.; Winfree, S.; Hansen, B.; Steele-Mortimer, O. The Annexin A2/p11 complex is required for efficient invasion of Salmonella typhimurium in epithelial cells. Cell. Microbiol. 2013, 16, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, C.; Osborn, M.; Gerke, V. The tight association of the tyrosine kinase substrate Annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J. Cell Sci. 1992, 103, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Moore, G.; Waisman, D. A nonapeptide to the putative F-actin binding site of Annexin-II tetramer inhibits its calcium-dependent activation of actin filament bundling. J. Biol. Chem. 1992, 267, 13993–13997. [Google Scholar] [CrossRef]
- Filipenko, N.R.; Waisman, D. The C terminus of Annexin II mediates binding to F-actin. J. Biol. Chem. 2001, 276, 5310–5315. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin-actin interactions. Traffic 2004, 5, 571–576. [Google Scholar] [CrossRef]
- Grieve, A.G.; Moss, S.E.; Hayes, M.J. Annexin A2 at the interface of actin and membrane dynamics: A focus on its roles in endocytosis and cell polarization. Int. J. Cell Biol. 2012, 2012, 852430. [Google Scholar] [CrossRef] [Green Version]
- Ghatak, S.; Morgner, J.; Wickström, S.A. ILK: A pseudokinase with a unique function in the integrin–actin linkage. Biochem. Soc. Trans. 2013, 41, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Hannigan, G.E.; Mcdonald, P.C.; Walsh, M.P.; Dedhar, S. Integrin-linked kinase: Not so ‘pseudo’ after all. Oncogene 2011, 30, 4375–4385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhou, T.; Chen, Z.; Yan, M.; Li, B.; Lv, H.; Wang, C.; Xiang, S.; Shi, L.; Zhu, Y.; et al. Coupling of integrin α5 to Annexin A2 by flow drives endothelial activation. Circ. Res. 2020, 127, 1074–1090. [Google Scholar] [CrossRef]
- Rankin, C.R.; Hilgarth, R.S.; Leoni, G.; Beste, K.D.; Kwon, M.; Parkos, C.A.; Nusrat, A. AnnexinA2 regulates β1 integrin internalization and intestinal epithelial cell migration. FASEB J. 2012, 26, 56.6. [Google Scholar] [CrossRef]
- Esfandiarei, M.; Yazdi, S.A.; Gray, V.; Dedhar, S.; Van Breemen, C. Integrin-linked kinase functions as a downstream signal of platelet-derived growth factor to regulate actin polymerization and vascular smooth muscle cell migration. BMC Cell Biol. 2010, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Ni, E.D.; Perkins, S.; Francois, P.; Vaudaux, P.; Hook, M.; Foster, T.J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 1998, 30, 245–257. [Google Scholar]
- Mcaleese, F.M.; Walsh, E.J.; Sieprawska, M.; Potempa, J.; Foster, T.J. Loss of clumping factor B fibrinogen binding activity by Staphylococcus aureus involves cessation of transcription, shedding and cleavage by metalloprotease. J. Biol. Chem. 2001, 276, 29969–29978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, K.A.; Mulcahy, M.E.; Towell, A.M.; Geoghegan, J.A.; McLoughlin, R.M. Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target. PLOS Pathog. 2019, 15, e1007713. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; Geoghegan, J.A.; Monk, I.; O’Keeffe, K.M.; Walsh, E.J.; Foster, T.J.; McLoughlin, R.M. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein Loricrin. PLOS Pathog. 2012, 8, e1003092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman-Bausier, P.; Labate, C.; Towell, A.M.; Derclaye, S.; Geoghegan, J.A.; Dufrene, Y.F. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, 5564–5569. [Google Scholar] [CrossRef] [Green Version]
- Kwiecinski, J.; Jin, T.; Josefsson, E. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. APMIS 2014, 122, 1240–1250. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Thompson, C.D.; Park, S.; Park, W.B.; Lee, J.C. Preclinical efficacy of clumping factor A in prevention of Staphylococcus aureus infection. mBio 2016, 7, e02232-15. [Google Scholar] [CrossRef] [Green Version]
- Soltani, E.; Farrokhi, E.; Zamanzad, B.; Abadi, M.S.S.; Deris, F.; Soltani, A.; Gholipour, A. Prevalence and distribution of adhesins and the expression of fibronectin-binding protein (FnbA and FnbB) among Staphylococcus aureus isolates from Shahrekord Hospitals. BMC Res. Notes 2019, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Sharma, S.; Dahiya, D.K.; Khan, A.; Mathur, M.; Sharma, A. Coagulase gene polymorphism, enterotoxigenecity, biofilm production, and antibiotic resistance in Staphylococcus aureus isolated from bovine raw milk in North West India. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Y.-T.; Ren, W.-J.; Tan, X.; Yang, J.; Liu, R.; Du, A.-F. Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B. Microorganisms 2021, 9, 2090. https://doi.org/10.3390/microorganisms9102090
Ying Y-T, Ren W-J, Tan X, Yang J, Liu R, Du A-F. Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B. Microorganisms. 2021; 9(10):2090. https://doi.org/10.3390/microorganisms9102090
Chicago/Turabian StyleYing, Yi-Tian, Wei-Jia Ren, Xun Tan, Jing Yang, Rui Liu, and Ai-Fang Du. 2021. "Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B" Microorganisms 9, no. 10: 2090. https://doi.org/10.3390/microorganisms9102090
APA StyleYing, Y.-T., Ren, W.-J., Tan, X., Yang, J., Liu, R., & Du, A.-F. (2021). Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B. Microorganisms, 9(10), 2090. https://doi.org/10.3390/microorganisms9102090





