Characterization of the Eukaryotic Virome of Mice from Different Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Animals
2.3. Tissue Collection
2.4. RNA, DNA Extraction and cDNA Synthesis
2.5. Primer Information
2.6. Library Preparation
2.7. Virome Sequencing
2.8. Statistical Analysis
3. Results
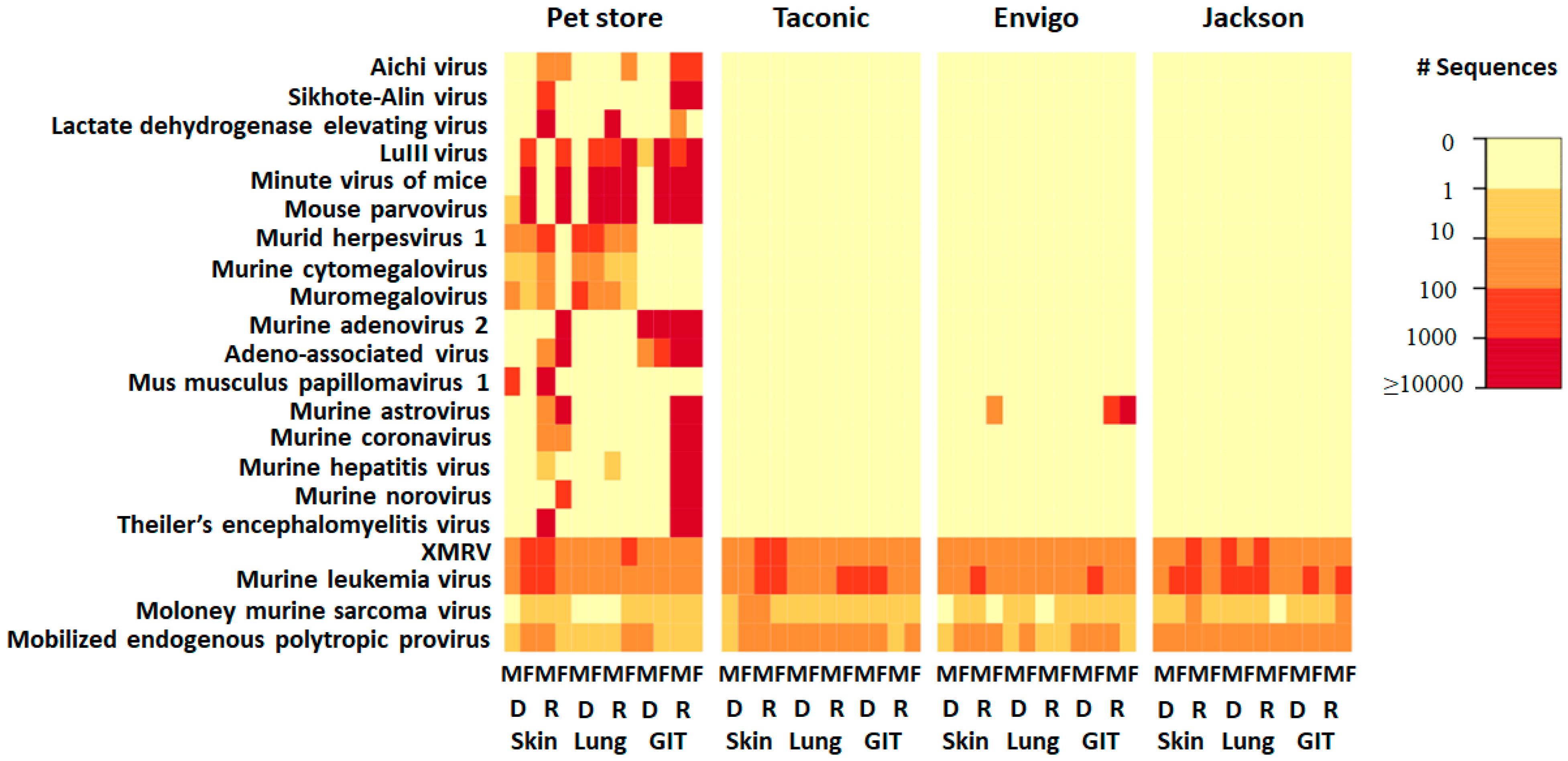
3.1. Comparison of the Eukaryotic Virome of Mice from Laboratories and Pet Stores
3.2. Comparison of the Eukaryotic Virome within Laboratory Mice
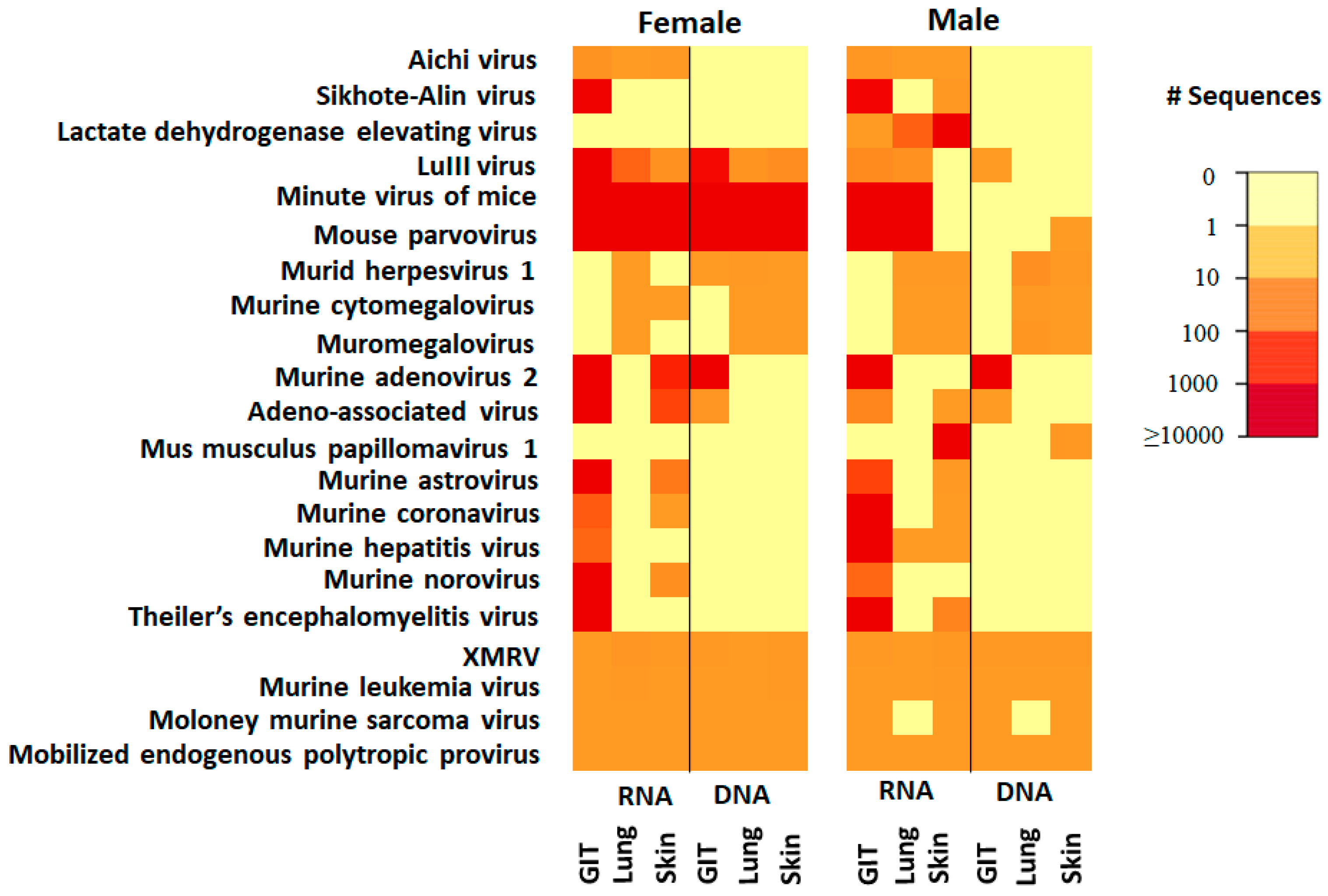
3.3. Comparison of the Viruses in Specific Tissues/Tropisms
3.4. Potential for Novel Virus Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model. Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S. Preclinical research: Make mouse studies work. Nature 2014, 507, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Tabak, L.A. Policy: NIH plans to enhance reproducibility. Nature 2014, 505, 612–613. [Google Scholar] [CrossRef] [PubMed]
- Wegorzewska, I.; Bell, S.; Cairns, N.J.; Miller, T.M.; Baloh, R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 18809–18814. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Kidd, J.; Vieira, F.; Thompson, K.; Perrin, S. No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALS. PLoS ONE 2009, 4, e6489. [Google Scholar] [CrossRef][Green Version]
- Pizzasegola, C.; Caron, I.; Daleno, C.; Ronchi, A.; Minoia, C.; Carri, M.T.; Bendotti, C. Treatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant mice. Amyotroph. Lateral Scler. 2009, 10, 221–228. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Tlaskalova-Hogenova, H.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Vannucci, L.; Tuckova, L.; Rossmann, P.; Hrncir, T.; Kverka, M.; Zakostelska, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Nunez, G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 2014, 146, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Gut bacteria in health and disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- de Vos, W.M.; de Vos, E.A. Role of the intestinal microbiome in health and disease: From correlation to causation. Nutr. Rev. 2012, 70 (Suppl. 1), S45–S56. [Google Scholar] [CrossRef]
- Virgin, H.W. The virome in mammalian physiology and disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef]
- Karlsson, O.E.; Larsson, J.; Hayer, J.; Berg, M.; Jacobson, M. The Intestinal Eukaryotic Virome in Healthy and Diarrhoeic Neonatal Piglets. PLoS ONE 2016, 11, e0151481. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Davis, J.W.; Spollen, W.; Bivens, N.; Givan, S.; Hagan, C.E.; McIntosh, M.; Franklin, C.L. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS ONE 2015, 10, e0116704. [Google Scholar] [CrossRef]
- Ericsson, A.C.; Montonye, D.R.; Smith, C.R.; Franklin, C.L. Modeling a Superorganism-Considerations Regarding the Use of “Dirty” Mice in Biomedical Research. Yale J. Biol. Med. 2017, 90, 361–371. [Google Scholar]
- Zhang, C.; Franklin, C.L.; Ericsson, A.C. Consideration of Gut Microbiome in Murine Models of Diseases. Microorganisms 2021, 9, 1062. [Google Scholar] [CrossRef]
- Wylie, T.N.; Wylie, K.M.; Herter, B.N.; Storch, G.A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015, 25, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.C.; Yiu, S.M.; Chin, F.Y. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Milne, I.; Stephen, G.; Bayer, M.; Cock, P.J.; Pritchard, L.; Cardle, L.; Shaw, P.D.; Marshall, D. Using Tablet for visual exploration of second-generation sequencing data. Brief. Bioinform. 2013, 14, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- Briese, T.; Kapoor, A.; Mishra, N.; Jain, K.; Kumar, A.; Jabado, O.J.; Lipkin, W.I. Virome Capture Sequencing Enables Sensitive Viral Diagnosis and Comprehensive Virome Analysis. MBio 2015, 6, e01491-15. [Google Scholar] [CrossRef]
- Prussin, A.J., 2nd; Marr, L.C.; Bibby, K.J. Challenges of studying viral aerosol metagenomics and communities in comparison with bacterial and fungal aerosols. FEMS Microbiol. Lett. 2014, 357, 1–9. [Google Scholar] [CrossRef]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016, 532, 512–516. [Google Scholar] [CrossRef]
- Dammann, P.; Hilken, G.; Hueber, B.; Kohl, W.; Bappert, M.T.; Mahler, M. Infectious microorganisms in mice (Mus musculus) purchased from commercial pet shops in Germany. Lab. Anim. 2011, 45, 271–275. [Google Scholar] [CrossRef]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Hickman, H.D.; McCulloch, J.A.; Badger, J.H.; Ajami, N.J.; et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 2017, 171, 1015–1028.e1013. [Google Scholar] [CrossRef]
- Farkas, T.; Fey, B.; Keller, G.; Martella, V.; Egyed, L. Molecular detection of novel astroviruses in wild and laboratory mice. Virus Genes 2012, 45, 518–525. [Google Scholar] [CrossRef]
- Tao, L.; Reese, T.A. Making Mouse Models That Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L. 14—The Microbiota in Immunity and Inflammation. In Clinical Immunology, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Content Repository Only: London, UK, 2019; pp. 207–219.e201. [Google Scholar]
- Ungaro, F.; Massimino, L.; Furfaro, F.; Rimoldi, V.; Peyrin-Biroulet, L.; D’Alessio, S.; Danese, S. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes 2018, 10, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, P.; Pullaiah, T. Chapter 7—Pathogen Identification Using Novel Sequencing Methods. In Advances in Cell and Molecular Diagnostics; Raghavendra, P., Pullaiah, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 161–202. [Google Scholar]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Shi, Z.; Gewirtz, A.T. Together Forever: Bacterial-Viral Interactions in Infection and Immunity. Viruses 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.M.; Handley, S.A.; Virgin, H.W. Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities. Gastroenterology 2014, 146, 1459–1469. [Google Scholar] [CrossRef]
- Wylie, K.M.; Weinstock, G.M.; Storch, G.A. Emerging view of the human virome. Transl. Res. 2012, 160, 283–290. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lim, D.; Greenhalgh, D.; Cho, K. Highly Variable Genomic Landscape of Endogenous Retroviruses in the C57BL/6J Inbred Strain, Depending on Individual Mouse, Gender, Organ Type, and Organ Location. Int. J. Genom. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Phan, T.G.; Kapusinszky, B.; Wang, C.; Rose, R.K.; Lipton, H.L.; Delwart, E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011, 7, e1002218. [Google Scholar] [CrossRef]
- Compton, S.R.; Booth, C.J.; Macy, J.D. Murine Astrovirus Infection and Transmission in Neonatal CD1 Mice. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 402–411. [Google Scholar]
- Yokoyama, C.C.; Loh, J.; Zhao, G.; Stappenbeck, T.S.; Wang, D.; Huang, H.V.; Virgin, H.W.; Thackray, L.B. Adaptive immunity restricts replication of novel murine astroviruses. J. Virol. 2012, 86, 12262–12270. [Google Scholar] [CrossRef]
- Ng, T.F.; Kondov, N.O.; Hayashimoto, N.; Uchida, R.; Cha, Y.; Beyer, A.I.; Wong, W.; Pesavento, P.A.; Suemizu, H.; Muench, M.O.; et al. Identification of an astrovirus commonly infecting laboratory mice in the US and Japan. PLoS ONE 2013, 8, e66937. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W.t. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef]
- Cadwell, K. Expanding the role of the virome: Commensalism in the gut. J. Virol. 2015, 89, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Kernbauer, E.; Ding, Y.; Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014, 516, 94–98. [Google Scholar] [CrossRef]


| DNA/RNA Virus | Family | Virus Species |
|---|---|---|
| RNA | Picornaviridae | Aichi Virus |
| RNA | Picornaviridae | Skihote alin virus |
| RNA | Picornaviridae | Theilers encephalomyelitis virus |
| RNA | Arteriviridae | Lactate dehydrogenase elevating virus |
| RNA | Astroviridae * | Murine astrovirus |
| RNA | Coronaviridae | Murine coronavirus |
| RNA | Coronaviridae | Murine hepatitis virus |
| RNA | Caliciviridae | Murine norovirus |
| DNA | Parvoviridae | Lull virus |
| DNA | Parvoviridae | Minute virus of mice |
| DNA | Parvoviridae | Mouse parvovirus |
| DNA | Parvoviridae | Adeno associated virus |
| DNA | Herpesviridae | Murid herpesvirus 1 |
| DNA | Herpesviridae | Murine cytomegalovirus |
| DNA | Herpesviridae | Muromegalovirus |
| DNA | Adenoviridae | Murine adenovirus 2 |
| DNA | Papillomaviridae | Mus musculus papillomavirus. Type 1 |
| Tissue Specificity Vertebrate Virus in Pet Store Mice | ||||
|---|---|---|---|---|
| Viral Family | Viral Species | GI | Lung | Skin |
| Retroviridae | XMRV | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) |
| Retroviridae | Murine leukemia viruses | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) |
| Retroviridae | Moloney murine sarcoma virus | 1/4 (25%) | 1/4 (25%) | 2/4 (50%) |
| Retroviridae | Mus musculus mobilized endogenous polytropic provirus | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) |
| Astroviridae | Murine astrovirus | 2/4 (50%) | 0/4 None | 2/4 (50%) |
| Picornaviridae | Aichi Virus | 2/4 (50%) | 1/4 (25%) | 2/4 (50%) |
| Picornaviridae | Skihote alin virus | 2/4 (50%) | 0/4 None | 1/4 (25%) |
| Arteriviridae | Lactate dehydrogenase elevating virus | 1/4 (25%) | 1/4 (25%) | 1/4 (25%) |
| Parvoviridae | LuIII virus | 3/4 (75%) | 3/4 (75%) | 2/4 (50%) |
| Parvoviridae | Minute virus of mice | 3/4 (75%) | 3/4 (75%) | 2/4 (50%) |
| Parvoviridae | Mouse parvovirus | 3/4 (75%) | 3/4 (75%) | 2/4 (50%) |
| Herpesviridae | Murid herpesvirus 1 | 0/4 None | 4/4 (100%) | 3/4 (75%) |
| Adenoviridae | Murine adenovirus 2 | 4/4 (100%) | 0/4 None | 1/4 (25%) |
| Parvoviridae | Adeno associated virus | 4/4 (100%) | 0/4 None | 2/4 (50%) |
| Papillomaviridae | Mus musculus papillomavirus. Type 1 | 0/4 None | 0/4 None | 2/4 (50%) |
| Coronaviridae | Murine coronavirus | 2/4 (50%) | 0/4 None | 2/4 (50%) |
| Coronaviridae | Murine hepatitis virus | 2/4 (50%) | 0/4 None | 1/4 (25%) |
| Caliciviridae | Murine norovirus | 2/4 (50%) | 0/4 None | 1/4 (25%) |
| Picornaviridae | Theilers encephalomyelitis virus | 2/4 (50%) | 0/4 None | 1/4 (25%) |
| Tissue Specific Vertebrate Viruses in Laboratory Mice | ||||
| Retroviridae | XMRV | 12/12 (100%) | 12/12 (100%) | 12/12 (100%) |
| Retroviridae | Murine leukemia viruses | 12/12 (100%) | 12/12 (100%) | 12/12 (100%) |
| Retroviridae | Moloney murine sarcoma virus | 6/12 (50%) | 8/12 (67%) | 8/12 (67%) |
| Retroviridae | Mus musculus mobilized endogenous polytropic provirus | 12/12 (100%) | 11/12 (92%) | 12/12 (100%) |
| Astroviridae | Murine astrovirus | 2/12 (17%) | 0/12 None | 1/12 (8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Burch, M.; Wylie, K.; Herter, B.; Franklin, C.L.; Ericsson, A.C. Characterization of the Eukaryotic Virome of Mice from Different Sources. Microorganisms 2021, 9, 2064. https://doi.org/10.3390/microorganisms9102064
Zhang C, Burch M, Wylie K, Herter B, Franklin CL, Ericsson AC. Characterization of the Eukaryotic Virome of Mice from Different Sources. Microorganisms. 2021; 9(10):2064. https://doi.org/10.3390/microorganisms9102064
Chicago/Turabian StyleZhang, Chunye, Matt Burch, Kristine Wylie, Brandi Herter, Craig L. Franklin, and Aaron C. Ericsson. 2021. "Characterization of the Eukaryotic Virome of Mice from Different Sources" Microorganisms 9, no. 10: 2064. https://doi.org/10.3390/microorganisms9102064
APA StyleZhang, C., Burch, M., Wylie, K., Herter, B., Franklin, C. L., & Ericsson, A. C. (2021). Characterization of the Eukaryotic Virome of Mice from Different Sources. Microorganisms, 9(10), 2064. https://doi.org/10.3390/microorganisms9102064






