Effects of Heat-Killed Lactococcus lactis Strain Plasma on Skin Homeostasis-Related Genes and the Skin Microbiome among Healthy Adults: A Randomized Controlled Double-Blind Study
Abstract
:1. Introduction
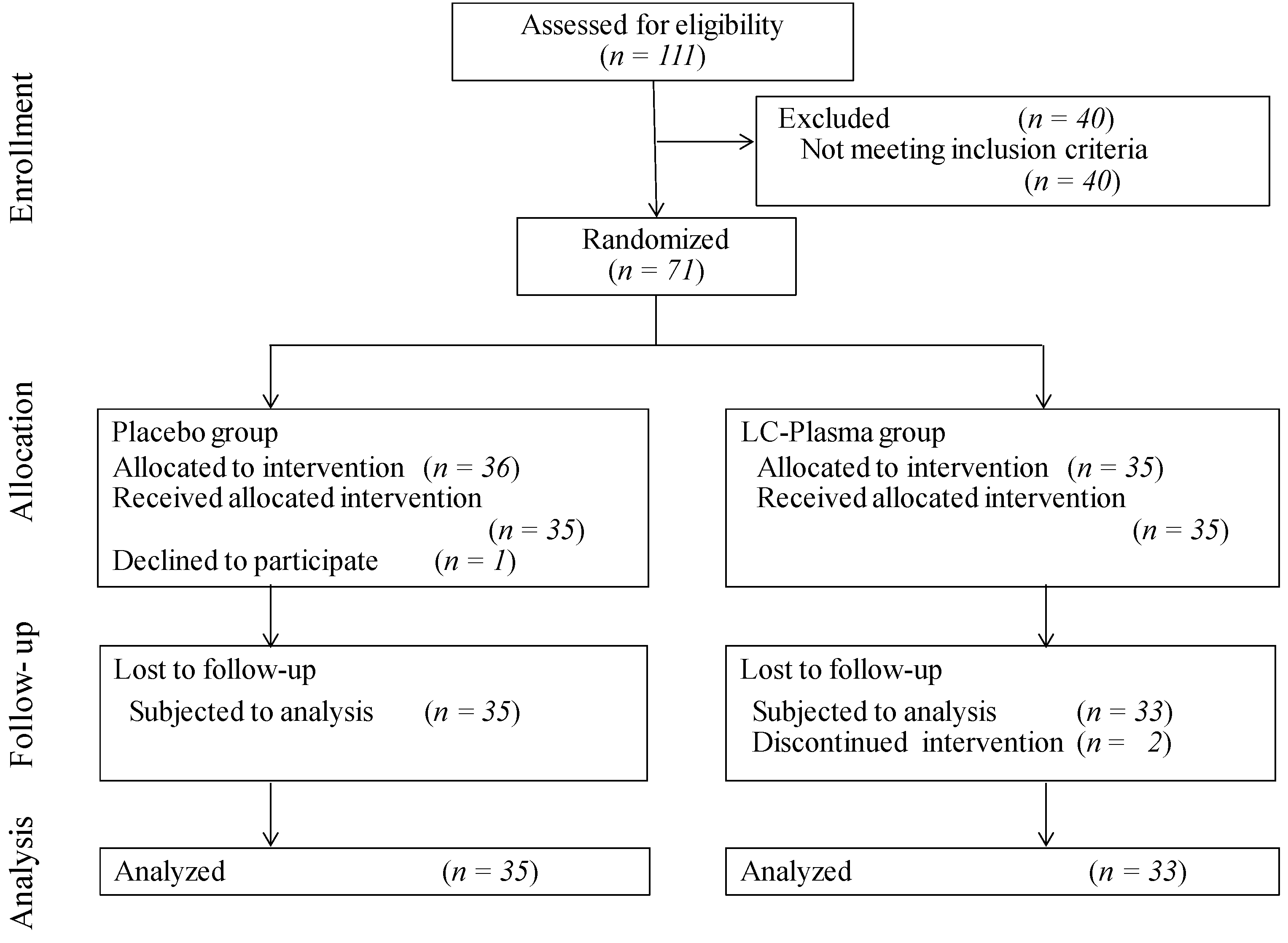
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Study Outcomes
2.4. Preparation of Total RNA from Hair-Root
2.5. Quantitative PCR Analysis
2.6. Skin Moisture, TEWL, and Pigmentation Assessment
2.7. Skin Microbiome Sample Collection, DNA Extraction, and Metagenomics Shotgun Sequencing
2.8. Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Alpha Diversity of the Skin Microbiome
3.3. Change in the Relative Quantity of Microbiota
3.4. LEfSe Analysis of Skin Micoroibota
3.5. Quantitative PCR Analysis of Skin Barrier-Related Genes
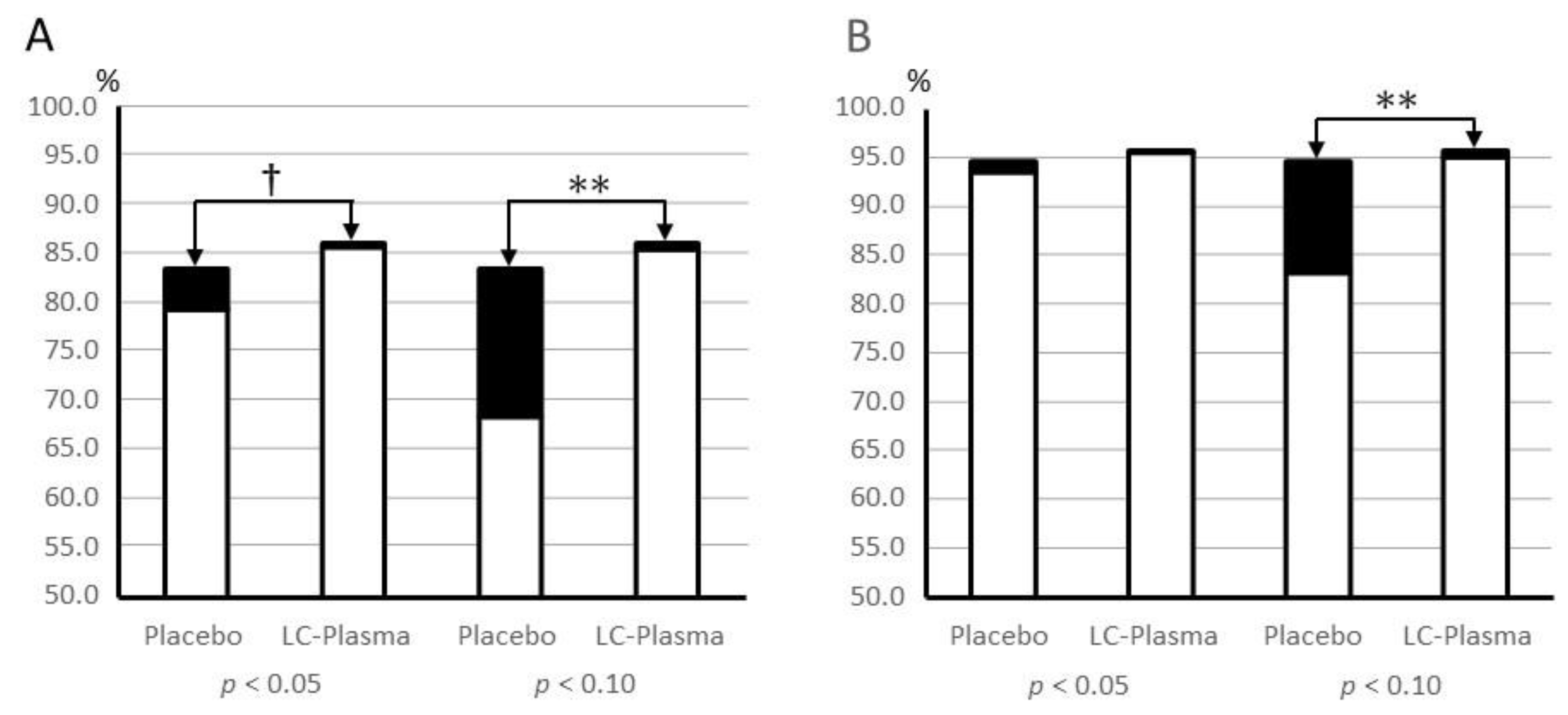
3.6. Skin Condition Assessments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shigwedha, N.; Sichel, L.; Jia, L.; Al-Shura, A.N.; Zhang, L. Probiotics, Paraprobiotics, and Probiotical Cell Fragments (PCFs) as Crisis Management Tools for Important Health Problems. AASCIT J. Med. 2015, 1, 1–9. [Google Scholar]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for Preterm Neonates-The Next Frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Park, J.H.; Jung, H.K. Potential Health-Promoting Benefits of Paraprobiotics, Inactivated Probiotic Cells. J. Microbiol. Biotechnol. 2020, 30, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef]
- Horie, A.; Tomita, Y.; Ohshio, K.; Fujiwara, D.; Fujii, T. Characterization of genomic DNA of lactic acid bacteria for activation of plasmacytoid dendritic cells. BMC Microbiol. 2019, 19, 88. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef]
- Fujii, T.; Jounai, K.; Horie, A.; Takahashi, H.; Suzuki, H.; Ohshio, K.; Fujiwara, D.; Yamamoto, N. Effects of heat-killed Lactococcus lactis subsp. lactis JCM 5805 on mucosal and systemic immune parameters, and antiviral reactions to influenza virus in healthy adults; a randomized controlled double-blind study. J. Funct. Foods 2017, 35, 513–521. [Google Scholar] [CrossRef]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Suzuki, H.; Kirisako, T.; Sugihara, Y.; Fujiwara, D. Long-term administration of pDC-Stimulative Lactococcus lactis strain decelerates senescence and prolongs the lifespan of mice. Int. Immunopharmacol. 2018, 58, 166–172. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simanski, M.; Erkens, A.-S.; Rademacher, F.; Harder, J. Staphylococcus epidermidis-induced Interleukin-1 Beta and Human Beta-defensin-2 Expression in Human Keratinocytes is Regulated by the Host Molecule A20 (TNFAIP3). Acta Derm. Venereol. 2019, 99, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, R.; Fujii, T.; Nakamura, Y.; Yazawa, K.; Kanauchi, O. Staphylococcus aureus Epicutaneous Infection Is Suppressed by Lactococcus lactis Strain Plasma via Interleukin 17A Elicitation. J. Infect. Dis. 2019, 220, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P.P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062. [Google Scholar] [CrossRef]
- Patra, V.; Sérézal, I.G.; Wolf, P. Potential of Skin Microbiome, Pro- and/or Pre-Biotics to Affect Local Cutaneous Responses to UV Exposure. Nutrients 2020, 12, 1795. [Google Scholar] [CrossRef]
- Rogerson, C.; Bergamaschi, D.; O’Shaughnessy, R.F.L. Uncovering mechanisms of nuclear degradation in keratinocytes: A paradigm for nuclear degradation in other tissues. Nucleus 2018, 9, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Yamada, K.; Toyoshima, M.; Ohnishi, T.; Iwayama, Y.; Shimamoto, C.; Toyota, T.; Nozaki, Y.; Balan, S.; Matsuzaki, H.; et al. Utility of Scalp Hair Follicles as a Novel Source of Biomarker Genes for Psychiatric Illnesses. Biol. Psychiatry 2015, 78, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Byrd, A.L.; Park, M.; Program, N.C.S.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Jeong, S.E.; Lee, S.; Kim, S.; Han, H.; Jeon, C.O. Effects of cosmetics on the skin microbiome of facial cheeks with different hydration levels. MicrobiologyOpen 2018, 7, e00557. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [Green Version]
- Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.-M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019, 20, 4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marito, S.; Keshari, S.; Traisaeng, S.; My, D.T.T.; Balasubramaniam, A.; Adi, P.; Hsieh, M.-F.; Herr, D.R.; Huang, C.-M. Electricity-producing Staphylococcus epidermidis counteracts Cutibacterium acnes. Sci. Rep. 2021, 11, 12001. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Martens, K.; Claes, J.; Jorissen, M.; Steelant, B.; van den Broek, M.F.L.; Seys, S.F.; Hellings, P.W.; Vanderveken, O.M.; et al. Anterior Nares Diversity and Pathobionts Represent Sinus Microbiome in Chronic Rhinosinusitis. mSphere 2019, 4, e00532-19. [Google Scholar] [CrossRef] [Green Version]
- Jetté, M.E.; Dill-McFarland, K.A.; Hanshew, A.S.; Suen, G.; Thibeault, S.L. The human laryngeal microbiome: Effects of cigarette smoke and reflux. Sci. Rep. 2016, 6, 35882. [Google Scholar] [CrossRef] [Green Version]
- Marazzato, M.; Zicari, A.M.; Aleandri, M.; Conte, A.L.; Longhi, C.; Vitanza, L.; Bolognino, V.; Zagaglia, C.; De Castro, G.; Brindisi, G.; et al. 16S Metagenomics Reveals Dysbiosis of Nasal Core Microbiota in Children With Chronic Nasal Inflammation: Role of Adenoid Hypertrophy and Allergic Rhinitis. Front. Cell. Infect. Microbiol. 2020, 10, 458. [Google Scholar] [CrossRef]
- Alekseyenko, A.V.; Perez-Perez, G.I.; De Souza, A.; Strober, B.; Gao, Z.; Bihan, M.; Li, K.; Methé, B.A.; Blaser, M.J. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K. Regulation of keratinocyte function by growth factors. J. Dermatol. Sci. 2000, 24 (Suppl. S1), S46–S50. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pummi, K.; Malminen, M.; Aho, H.; Karvonen, S.-L.; Peltonen, J.; Peltonen, S. Epidermal Tight Junctions: ZO-1 and Occludin are Expressed in Mature, Developing, and Affected Skin and In Vitro Differentiating Keratinocytes. J. Investig. Dermatol. 2001, 117, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Kilsdonk, J.W.J.; Jansen, P.A.M.; van den Bogaard, E.H.; Bos, C.; Bergers, M.; Zeeuwen, P.L.J.M.; Schalkwijk, J. The Effects of Human Beta-Defensins on Skin Cells In Vitro. Dermatology 2017, 233, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Casal, D.; Iria, I.; Ramalho, J.S.; Alves, S.; Mota-Silva, E.; Mascarenhas-Lemos, L.; Pontinha, C.; Guadalupe-Cabral, M.; Ferreira-Silva, J.; Ferraz-Oliveira, M.; et al. BD-2 and BD-3 increase skin flap survival in a model of ischemia and Pseudomonas aeruginosa infection. Sci. Rep. 2019, 9, 7854. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boguniewicz, M.; Leung, D.Y.M. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Lynn, K.S.; Peterson, R.J.; Koval, M. Ruffles and spikes: Control of tight junction morphology and permeability by claudins. Biochim. Et Biophys. Acta-Biomembr. 2020, 1862, 183339. [Google Scholar] [CrossRef]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef] [Green Version]
- Gröne, J.; Weber, B.; Staub, E.; Heinze, M.; Klaman, I.; Pilarsky, C.; Hermann, K.; Castanos-Velez, E.; Röpcke, S.; Mann, B.; et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: Frequent dysregulation of claudin-1, -8 and -12. Int. J. Colorectal Dis. 2007, 22, 651–659. [Google Scholar] [CrossRef]
- Ishii, Y.; Okada, Y.; Matsuoka, S.; Adachi, T.; Yui, K.; Fujita, Y.; Ichihashi, M. Effect of beauty drink containing vitamin C, glucosyul hesperidin, artichoke leaf extract, olive leaf estract, niacin, L-cystine and pineapple extract on skin condition in Japanese women. a randomized, placebo-controlled, double-blind, parallel group trial. Pharmacometrics 2016, 91, 77–84. [Google Scholar]
- Fukuda, Y.; Soga, H.; Satoh, H.; Kitahara, T.; Yoshizuka, N.; Takema, Y. Spectroscopic characterization of color polymorphism in the orbital skin. J. Soc. Cosmet. Jpn. 2005, 39, 195–200. [Google Scholar] [CrossRef]
- Si, J.; Lee, S.; Park, J.M.; Sung, J.; Ko, G. Genetic associations and shared environmental effects on the skin microbiome of Korean twins. BMC Genom. 2015, 16, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| (1) | Individuals who have diseases with medications. |
| (2) | Individuals who have continuously received medications within one month before the examination. |
| (3) | Individuals who have medical histories of serious disease of their liver, kidney, heart, lung, and blood. |
| (4) | Individuals who have comorbidity or medication history in their digestive organs. |
| (5) | Individuals whose systolic blood pressure is over 160 mmHg, or whose diastolic blood pressure is over 100 mmHg. |
| (6) | Individuals who have sever skin disorder. |
| (7) | Individuals who have severe anemia. |
| (8) | Individuals who might be allergic to test foods, or who might be seriously allergic to other foods, or medicaments. |
| (9) | Individuals who are pregnant, breastfeeding, or planning to be pregnant. |
| (10) | Individuals who are alcoholic or have mental disorder. |
| (11) | Individuals who will change their life style during the test period. |
| (12) | Individuals who have skin disease and need the application of medicine containing steroids or antibiotics to their face. |
| (13) | Individuals who have severe menopausal symptoms. |
| (14) | Individuals who cannot stop eating foods containing lactic acid bacteria. |
| (15) | Individuals who continuously took medicines for skin-condition-improvement within the last three month. |
| (16) | Individuals who use cosmetics that have strong effects on skin moisture or wrinkles. |
| (17) | Individuals who cannot keep from outdoor activities with the risk of getting sunburned during the test. |
| (18) | Individuals who had a surgery in their face within the past 6 months. |
| (19) | Individuals who are participating or participated in another clinical trial within the last 3 months. |
| (20) | Individuals who and whose family work for a company manufacturing or selling healthy foods, functional foods, and cosmetics. |
| (21) | Individuals who have smoking habitat. |
| (22) | Individuals who are judged unsuitable for this study by the investigator for other reasons. |
| Item | Placebo | LC-Plasma | p Value *1 |
|---|---|---|---|
| Number of subjects | 35 | 33 | |
| Gender | Male 10 | Male 9 | |
| Female 25 | Female 26 | ||
| Age | 41.7 ± 8.6 | 40.2 ± 7.0 | 0.442 |
| Weight (kg) | 58.4 ± 10.9 | 56.2 ± 7.9 | 0.347 |
| BMI *2 (kg/m2) | 21.6 ± 2.9 | 21.1 ± 2.2 | 0.488 |
| RBC *3 (×103/mL) | 4.72 ± 0.39 | 4.63 ± 0.37 | 0.331 |
| WBC *4 (×103/mL) | 6.51 ± 1.82 | 6.13 ± 2.00 | 0.407 |
| CD86 *5 (M.F.I.) *6 | 1378.7 ± 136.0 | 1402.3 ± 210.8 | 0.583 |
| Skin moisture (A.U.) *7 | 50.7 ± 11.2 | 50.2 ± 14.0 | 0.871 |
| Genes | Group | 0 W | 8 W | p Value (0 W vs 8 W) | p Value (Placebo vs LC-Plasma) *1 |
|---|---|---|---|---|---|
| Cytokine genes | |||||
| IL1A | Placebo | 1.31 ± 0.91 | 1.59 ± 1.46 | 0.26 | N.T. |
| LC-Plasma | 1.16 ± 0.64 | 1.59 ± 1.77 | 0.16 | ||
| TGFB1 | Placebo | 1.07 ± 0.44 | 1.01 ± 0.51 | 0.62 | 0.36 |
| LC-Plasma | 0.95 ± 0.55 | 0.75 ± 0.27 | 0.03 * | ||
| TJ genes | |||||
| CLDN1 | Placebo | 1.01 ± 0.32 | 1.02 ± 0.32 | 0.83 | 0.24 |
| LC-Plasma | 0.91 ± 0.25 | 1.02 ± 0.28 | 0.05 * | ||
| CLDN4 | Placebo | 1.05 ± 0.54 | 1.66 ± 3.00 | 0.25 | N.T. |
| LC-Plasma | 1.02 ± 0.36 | 1.02 ± 0.34 | 0.99 | ||
| CLDN12 | Placebo | 3.10 ± 2.33 | 3.63 ± 1.85 | 0.23 | 0.67 |
| LC-Plasma | 2.46 ± 1.95 | 3.24 ± 1.59 | 0.00 ** | ||
| OCLN | Placebo | 1.24 ± 0.59 | 1.26 ± 0.52 | 0.87 | N.T. |
| LC-Plasma | 1.18 ± 0.42 | 1.31 ± 0.62 | 0.27 | ||
| ZO1 | Placebo | 0.86 ± 0.39 | 1.04 ± 0.49 | 0.06 † | 0.83 |
| LC-Plasma | 0.72 ± 0.23 | 0.88 ± 0.44 | 0.03 * | ||
| ZO2 | Placebo | 1.12 ± 0.55 | 1.17 ± 0.48 | 0.23 | N.T. |
| LC-Plasma | 1.03 ± 0.33 | 1.11 ± 0.46 | 0.00 ** | ||
| AMP genes | |||||
| BD1 | Placebo | 1.20 ± 0.67 | 1.55 ± 0.80 | 0.01 ** | 0.34 |
| LC-Plasma | 1.08 ± 0.56 | 1.30 ± 0.57 | 0.02 * | ||
| BD2 | Placebo | 0.74 ± 2.26 | 0.79 ± 1.50 | 0.90 | N.T. |
| LC-Plasma | 1.17 ± 1.89 | 1.57 ± 3.03 | 0.45 | ||
| BD3 | Placebo | 1.07 ± 0.66 | 1.19 ± 0.70 | 0.28 | 0.78 |
| LC-Plasma | 0.98 ± 0.49 | 1.14 ± 0.54 | 0.05 * |
| Indices | Group | 0 W | 8 W | p Value (0 W vs 8 W) | p Value (Placebo vs LC-Plasma) *1 |
|---|---|---|---|---|---|
| Melanin | Placebo | 0.90 ± 0.20 | 0.88 ± 0.18 | 0.04 * | 0.92 |
| LC-Plasma | 0.96 ± 0.19 | 0.93 ± 0.19 | 0.02 * | ||
| Hb | Placebo | 1.01 ± 0.28 | 0.97 ± 0.22 | 0.20 | 0.32 |
| LC-Plasma | 1.03 ± 0.23 | 0.95 ± 0.25 | 0.00 ** | ||
| HbSO2 | Placebo | 58.04 ± 5.52 | 56.92 ± 7.15 | 0.58 | N.T. |
| LC-Plasma | 57.77 ± 7.21 | 58.40 ± 11.23 | 0.86 | ||
| L* | Placebo | 64.79 ± 2.89 | 64.93 ± 2.51 | 0.51 | 0.47 |
| LC-Plasma | 64.46 ± 3.46 | 64.81 ± 2.93 | 0.08 † | ||
| a* | Placebo | 7.00 ± 1.71 | 6.64 ± 1.29 | 0.06 † | 0.46 |
| LC-Plasma | 7.38 ± 1.46 | 6.83 ± 1.44 | 0.00 ** | ||
| b* | Placebo | 16.52 ± 2.55 | 16.22 ± 2.44 | 0.08 † | 0.57 |
| LC-Plasma | 17.20 ± 2.28 | 17.03 ± 2.33 | 0.29 | ||
| TEWL (gm−2h−1) | Placebo | 15.66 ± 5.60 | 15.99 ± 5.81 | 0.60 | N.T. |
| LC-Plasma | 17.87 ± 5.95 | 18.71 ± 6.12 | 0.33 | ||
| Skin Moisture (A.U.) *2 | Placebo | 47.95 ± 12.69 | 49.90 ± 12.25 | 0.20 | N.T. |
| LC-Plasma | 48.22 ± 15.43 | 50.33 ± 15.48 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, T.; Fujitomo, T.; Tsuji, R.; Kubo, R.; Kato, Y.; Kanauchi, O. Effects of Heat-Killed Lactococcus lactis Strain Plasma on Skin Homeostasis-Related Genes and the Skin Microbiome among Healthy Adults: A Randomized Controlled Double-Blind Study. Microorganisms 2021, 9, 2029. https://doi.org/10.3390/microorganisms9102029
Fujii T, Fujitomo T, Tsuji R, Kubo R, Kato Y, Kanauchi O. Effects of Heat-Killed Lactococcus lactis Strain Plasma on Skin Homeostasis-Related Genes and the Skin Microbiome among Healthy Adults: A Randomized Controlled Double-Blind Study. Microorganisms. 2021; 9(10):2029. https://doi.org/10.3390/microorganisms9102029
Chicago/Turabian StyleFujii, Toshio, Takashi Fujitomo, Ryohei Tsuji, Ryuichi Kubo, Yukiko Kato, and Osamu Kanauchi. 2021. "Effects of Heat-Killed Lactococcus lactis Strain Plasma on Skin Homeostasis-Related Genes and the Skin Microbiome among Healthy Adults: A Randomized Controlled Double-Blind Study" Microorganisms 9, no. 10: 2029. https://doi.org/10.3390/microorganisms9102029
APA StyleFujii, T., Fujitomo, T., Tsuji, R., Kubo, R., Kato, Y., & Kanauchi, O. (2021). Effects of Heat-Killed Lactococcus lactis Strain Plasma on Skin Homeostasis-Related Genes and the Skin Microbiome among Healthy Adults: A Randomized Controlled Double-Blind Study. Microorganisms, 9(10), 2029. https://doi.org/10.3390/microorganisms9102029






