Type IA Topoisomerases as Targets for Infectious Disease Treatments
Abstract
1. Introduction
2. Type IA Topoisomerases
2.1. Rationale for Type IA Topoisomerases as Drug Targets
2.2. Validation for Bacterial Topoisomerase IA as a Novel Antibiotics Target
2.3. Eukaryotic Type IA Topoisomerases as Potential Target for Infectious Disease Treatment
3. Screening Approaches
3.1. In Silico Screening
3.2. Biochemical Screening Assays
4. Recent Attempts to Discover Novel Bacterial Topoisomerase I Inhibitors
4.1. Bis-Benzimidazoles
4.2. Tricyclic Antidepressants
4.3. Inhibitors Based on Polyamine Scaffold
4.4. Gold(III) Complexes
4.5. Fluoroquinophenoxazine Derivatives
4.6. Vichem’s Benzo(g)quinoxaline Compound
4.7. Alkaloids SCN and N-SCN
4.8. Additional Small Molecule Inhibitors Identified from Virtual Screening
4.9. DNA Molecules as Bacterial Topoisomerase I Inhibitors
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| WHO | World Health Organization. |
| PDB | Protein Data Bank. |
| ELISA | enzyme-linked immunosorbent assay. |
| MIC | minimum inhibitory concentration: The lowest concentration of an agent that stops microbial growth. |
| CC50 | the 50% cytotoxic concentration: The concentration that reduces the cellular viability by 50%. |
| IC50 | the half maximal inhibitory concentration: The concentration that reduces enzymatic activity by 50%. |
| FDA | Food and Drug Administration |
| NCI | National Cancer Institute |
| SI | selectivity index: A ratio of cytotoxicity to antimicrobial activity. |
| SAR | structure–activity relationship: The relationship between the chemical structure of a molecule and its biological activities. |
| 3D-QSAR | three-dimensional quantitative structure–activity relationship: A quantification of SAR via mathematical relationships between physicochemical properties of a molecule and its biological activities. |
| CoMFA | comparative molecular field analysis: One of the QSAR methods, where mathematical relationships between steric and electrostatic properties of a ligand and the resulting receptor–ligand interactions are determined. |
| CGenFF | CHARMM General Force Field |
| DTP | Developmental Therapeutics Program |
References
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W. Bad Bugs, No Drugs 2002–2020: Progress, Challenges, and Call to Action. Trans. Am. Clin. Climatol. Assoc. 2020, 131, 65–71. [Google Scholar] [PubMed]
- Schrader, S.M.; Vaubourgeix, J.; Nathan, C. Biology of antimicrobial resistance and approaches to combat it. Sci. Transl. Med. 2020, 12, eaaz6992. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of beta-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target protection as a key antibiotic resistance mechanism. Nat. Rev. Microbiol. 2020, 637–648. [Google Scholar] [CrossRef]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Richmond, G.E.; Piddock, L.J.V. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014, 9, 1165–1177. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.J.; Connor, C.; McNally, A. The evolution and transmission of multi-drug resistant Escherichia coli and Klebsiella pneumoniae: The complexity of clones and plasmids. Curr. Opin. Microbiol. 2019, 51, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sugden, R.; Kelly, R.; Davies, S. Combatting antimicrobial resistance globally. Nat. Microbiol. 2016, 1, 16187. [Google Scholar] [CrossRef] [PubMed]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- WHO. Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Resistant Tuberculosis Treatment; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Beiras-Fernandez, A.; Vogt, F.; Sodian, R.; Weis, F. Daptomycin: A novel lipopeptide antibiotic against Gram-positive pathogens. Infect. Drug Resist. 2010, 3, 95–101. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Bush, N.G.; Evans-Roberts, K.; Maxwell, A. DNA Topoisomerases. EcoSal Plus 2015, 6. [Google Scholar] [CrossRef]
- Nagaraja, V.; Godbole, A.A.; Henderson, S.R.; Maxwell, A. DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov. Today 2017, 22, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Tse-Dinh, Y.-C. Exploring DNA Topoisomerases as Targets of Novel Therapeutic Agents in the Treatment of Infectious Diseases. Infect. Disord. Drug Targets 2007, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hiasa, H. DNA Topoisomerases as Targets for Antibacterial Agents. Methods Mol. Biol. 2018, 1703, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Drugging Topoisomerases: Lessons and Challenges. ACS Chem. Biol. 2013, 8, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Zechiedrich, E.L.; Khodursky, A.B.; Bachellier, S.; Schneider, R.; Chen, D.; Lilley, D.M.J.; Cozzarelli, N.R. Roles of Topoisomerases in Maintaining Steady-state DNA Supercoiling in Escherichia coli. J. Biol. Chem. 2000, 275, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Brochu, J.; Breton, É.-V.; Drolet, M. Supercoiling, R-Loops, Replication and the Functions of Bacterial Type 1A Topoisomerases. Genes 2020, 11, 249. [Google Scholar] [CrossRef]
- Liu, L.F.; Wang, J.C. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. USA 1987, 84, 7024–7027. [Google Scholar] [CrossRef]
- Ma, J.; Wang, M.D. DNA supercoiling during transcription. Biophys. Rev. 2016, 8 (Suppl. S1), 75–87. [Google Scholar] [CrossRef]
- Vos, S.M.; Tretter, E.M.; Schmidt, B.H.; Berger, J.M. All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011, 12, 827–841. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.-Y.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Tse, Y.C.; Kirkegaard, K.; Wang, J.C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J. Biol. Chem. 1980, 255, 5560–5565. [Google Scholar] [PubMed]
- Delgado, J.L.; Hsieh, C.-M.; Chan, N.-L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Pommier, Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Baker, N.M.; Rajan, R.; Mondragón, A. Structural studies of type I topoisomerases. Nucleic Acids Res. 2009, 37, 693–701. [Google Scholar] [CrossRef]
- Kirkegaard, K.; Wang, J.C. Bacterial DNA topoisomerase I can relax positively supercoiled DNA containing a single-stranded loop. J. Mol. Biol. 1985, 185, 625–637. [Google Scholar] [CrossRef]
- Lima, C.D.; Wang, J.C.; Mondragón, A. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 1994, 367, 138–146. [Google Scholar] [CrossRef]
- Changela, A.; DiGate, R.J.; Mondragón, A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature 2001, 411, 1077–1081. [Google Scholar] [CrossRef]
- Mills, M.; Tse-Dinh, Y.-C.; Neuman, K.C. Direct observation of topoisomerase IA gate dynamics. Nat. Struct. Mol. Biol. 2018, 25, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Tse-Dinh, Y.C. Uncoupling of the DNA breaking and rejoining steps of Escherichia coli type I DNA topoisomerase. Demonstration of an active covalent protein-DNA complex. J. Biol. Chem. 1986, 261, 10931–10935. [Google Scholar] [PubMed]
- Sorokin, E.P.; Cheng, B.; Rathi, S.; Aedo, S.J.; Abrenica, M.V.; Tse-Dinh, Y.-C. Inhibition of Mg2+ binding and DNA religation by bacterial topoisomerase I via introduction of an additional positive charge into the active site region. Nucleic Acids Res. 2008, 36, 4788–4796. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, A.G.; Leelaram, M.N.; Hegde, S.; Nagaraja, V. Deciphering the Distinct Role for the Metal Coordination Motif in the Catalytic Activity of Mycobacterium smegmatis Topoisomerase I. J. Mol. Biol. 2009, 393, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Tan, K.; Annamalai, T.; Joachimiak, A.; Tse-Dinh, Y.-C. Investigating mycobacterial topoisomerase I mechanism from the analysis of metal and DNA substrate interactions at the active site. Nucleic Acids Res. 2018, 46, 7296–7308. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.D.; Berger, J.M. Structure, Molecular Mechanisms, and Evolutionary Relationships in DNA Topoisomerases. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Xue, Y.; Lee, S.K.; Martindale, J.L.; Shen, W.; Li, W.; Zou, S.; Ciaramella, M.; Debat, H.; Nadal, M.; et al. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Res. 2016, 44, 6335–6349. [Google Scholar] [CrossRef]
- Prasanth, K.R.; Hirano, M.; Fagg, W.S.; McAnarney, E.T.; Shan, C.; Xie, X.; Hage, A.; Pietzsch, C.A.; Bukreyev, A.; Rajsbaum, R.; et al. Topoisomerase III-β is required for efficient replication of positive-sense RNA viruses. Antivir. Res. 2020, 182, 104874. [Google Scholar] [CrossRef]
- Forterre, P.; Gadelle, D. Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res. 2009, 37, 679–692. [Google Scholar] [CrossRef]
- Bjornsti, M.-A.; Kaufmann, S.H. Topoisomerases and cancer chemotherapy: Recent advances and unanswered questions. F1000Research 2019, 8, 1704. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F. DNA Topoisomerase Poisons as Antitumor Drugs. Annu. Rev. Biochem. 1989, 58, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Solary, E.; Bertrand, R.; Pommier, Y. Apoptosis Induced by DNA Topoisomerase I and II Inhibitors in Human Leukemic HL-60 Cells. Leuk. Lymphoma 1994, 15, 21–32. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007, 3, 91. [Google Scholar] [CrossRef]
- Terekhova, K.; Gunn, K.H.; Marko, J.F.; Mondragón, A. Bacterial topoisomerase I and topoisomerase III relax supercoiled DNA via distinct pathways. Nucleic Acids Res. 2012, 40, 10432–10440. [Google Scholar] [CrossRef]
- Terekhova, K.; Marko, J.F.; Mondragón, A. Single-molecule analysis uncovers the difference between the kinetics of DNA decatenation by bacterial topoisomerases I and III. Nucleic Acids Res. 2014, 42, 11657–11667. [Google Scholar] [CrossRef]
- Massé, E.; Drolet, M. Escherichia coliDNA Topoisomerase I Inhibits R-loop Formation by Relaxing Transcription-induced Negative Supercoiling. J. Biol. Chem. 1999, 274, 16659–16664. [Google Scholar] [CrossRef]
- Lee, C.M.; Wang, G.; Pertsinidis, A.; Marians, K.J. Topoisomerase III Acts at the Replication Fork To Remove Precatenanes. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef]
- Raji, A.; Zabel, D.J.; Laufer, C.S.; Depew, R.E. Genetic analysis of mutations that compensate for loss of Escherichia coli DNA topoisomerase I. J. Bacteriol. 1985, 162, 1173–1179. [Google Scholar] [CrossRef]
- Dinardo, S.; Voelkel, K.A.; Sternglanz, R.; Reynolds, A.E.; Wright, A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell 1982, 31, 43–51. [Google Scholar] [CrossRef]
- Pruss, G.J.; Manes, S.H.; Drlica, K. Escherichia coli DNA topoisomerase I mutants: Increased supercoiling is corrected by mutations near gyrase genes. Cell 1982, 31, 35–42. [Google Scholar] [CrossRef]
- Yigit, H.; Reznikoff, W.S. Escherichia coli DNA Topoisomerase I and Suppression of Killing by Tn5 Transposase Overproduction: Topoisomerase I Modulates Tn5 Transposition. J. Bacteriol. 1998, 180, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Reznikoff, W.S. Escherichia coli DNA Topoisomerase I Copurifies with Tn5 Transposase, and Tn5 Transposase Inhibits Topoisomerase I. J. Bacteriol. 1999, 181, 3185–3192. [Google Scholar] [CrossRef]
- Mattenberger, Y.; Silva, F.; Belin, D. 55.2, a Phage T4 ORFan Gene, Encodes an Inhibitor of Escherichia coli Topoisomerase I and Increases Phage Fitness. PLoS ONE 2015, 10, e0124309. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Inouye, M. An endogenous protein inhibitor, YjhX (TopAI), for topoisomerase I from Escherichia coli. Nucleic Acids Res. 2015, 43, 10387–10396. [Google Scholar] [CrossRef]
- Cheng, B.; Shukla, S.; Vasunilashorn, S.; Mukhopadhyay, S.; Tse-Dinh, Y.-C. Bacterial Cell Killing Mediated by Topoisomerase I DNA Cleavage Activity. J. Biol. Chem. 2005, 280, 38489–38495. [Google Scholar] [CrossRef]
- Cheng, B.; Annamalai, T.; Sorokin, E.; Abrenica, M.; Aedo, S.; Tse-Dinh, Y.-C. Asp-to-Asn Substitution at the First Position of the DxD TOPRIM Motif of Recombinant Bacterial Topoisomerase I Is Extremely Lethal to E. coli. J. Mol. Biol. 2009, 385, 558–567. [Google Scholar] [CrossRef]
- Narula, G.; Annamalai, T.; Aedo, S.; Cheng, B.; Sorokin, E.; Wong, A.; Tse-Dinh, Y.-C. The Strictly Conserved Arg-321 Residue in the Active Site of Escherichia coli Topoisomerase I Plays a Critical Role in DNA Rejoining. J. Biol. Chem. 2011, 286, 18673–18680. [Google Scholar] [CrossRef]
- Ravishankar, S.; Ambady, A.; Ramachandran, V.; Eyermann, C.J.; Reck, F.; Rudrapatna, S.; Sambandamurthy, V.K.; Sharma, U.K.; Awasthy, D.; Mudugal, N.V.; et al. Genetic and chemical validation identifies Mycobacterium tuberculosis topoisomerase I as an attractive anti-tubercular target. Tuberculosis 2015, 95, 589–598. [Google Scholar] [CrossRef]
- Ahmed, W.; Menon, S.; Godbole, A.A.; Karthik, P.V.; Nagaraja, V. Conditional silencing of topoisomerase I gene of Mycobacterium tuberculosis validates its essentiality for cell survival. FEMS Microbiol. Lett. 2014, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gallay, C.; Kjos, M.; Domenech, A.; Slager, J.; Van Kessel, S.P.; Knoops, K.; Sorg, R.A.; Zhang, J.-R.; Veening, J.-W. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol. Syst. Biol. 2017, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Hu, S.; Ma, N.; Song, P.; Liang, Q.; Zhang, H.; Li, Y.; Shen, L.; Duan, K.; Chen, L. Regulatory Effect of DNA Topoisomerase I on T3SS Activity, Antibiotic Susceptibility and Quorum- Sensing-Independent Pyocyanin Synthesis in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2019, 20, 1116. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Brauer-Steppkes, T.; Labigne, A.; Cameron, B.; Drlica, K. Topoisomerase I of Helicobacter pylori: Juxtaposition with a flagellin gene (flaB) and functional requirement of a fourth zinc finger motif. Gene 1998, 210, 151–161. [Google Scholar] [CrossRef]
- Goodwin, A.; Wang, S.-W.; Toda, T.; Norbury, C.; Hickso, I.D. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res. 1999, 27, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Maftahi, M.; Han, C.S.; Langston, L.D.; Hope, J.C.; Zigouras, N.; Freyer, G.A. The top3+ gene is essential in Schizosaccharomyces pombe and the lethality associated with its loss is caused by Rad12 helicase activity. Nucleic Acids Res. 1999, 27, 4715–4724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallis, J.W.; Chrebet, G.; Brodsky, G.; Rolfe, M.; Rothstein, R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 1989, 58, 409–419. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.C. Mammalian DNA topoisomerase IIIα is essential in early embryogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 1010–1013. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Wang, J.C. Mice lacking DNA topoisomerase IIIβ develop to maturity but show a reduced mean lifespan. Proc. Natl. Acad. Sci. USA 2001, 98, 5717–5721. [Google Scholar] [CrossRef]
- Stoll, G.; Pietiläinen, O.P.H.; Linder, B.; Suvisaari, J.; Brosi, C.; Hennah, W.; Leppa, V.; Torniainen, M.; Ripatti, S.; Ala-Mello, S.; et al. Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat. Neurosci. 2013, 16, 1228–1237. [Google Scholar] [CrossRef]
- Xu, D.; Shen, W.; Guo, R.; Xue, Y.; Peng, W.; Sima, J.; Yang, J.; Sharov, A.; Srikantan, S.; Yang, J.; et al. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 2013, 16, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wallis, M.; Petrovic, V.; Challis, J.; Kalitsis, P.; Hudson, D.F. Loss of TOP3B leads to increased R-loop formation and genome instability. Open Biol. 2019, 9, 190222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; McBride, K.M.; Hensley, S.; Lu, Y.; Chedin, F.; Bedford, M.T. Arginine Methylation Facilitates the Recruitment of TOP3B to Chromatin to Prevent R Loop Accumulation. Mol. Cell 2014, 53, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Shen, W.; Li, W.; Xue, Y.; Zou, S.; Xu, D.; Wang, W. Topoisomerase 3β is the major topoisomerase for mRNAs and linked to neurodevelopment and mental dysfunction. Nucleic Acids Res. 2016, 45, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Siaw, G.E.-L.; Liu, I.-F.; Lin, P.-Y.; Been, M.D.; Hsieh, T.-S. DNA and RNA topoisomerase activities of Top3β are promoted by mediator protein Tudor domain-containing protein 3. Proc. Natl. Acad. Sci. USA 2016, 113, E5544–E5551. [Google Scholar] [CrossRef]
- Goto-Ito, S.; Yamagata, A.; Takahashi, T.S.; Sato, Y.; Fukai, S. Structural basis of the interaction between Topoisomerase IIIβ and the TDRD3 auxiliary factor. Sci. Rep. 2017, 7, 42123. [Google Scholar] [CrossRef]
- Barrows, N.J.; Anglero-Rodriguez, Y.; Kim, B.; Jamison, S.F.; Le Sommer, C.; McGee, C.E.; Pearson, J.L.; Dimopoulos, G.; Ascano, M.; Bradrick, S.S.; et al. Dual roles for the ER membrane protein complex in flavivirus infection: Viral entry and protein biogenesis. Sci. Rep. 2019, 9, 9711. [Google Scholar] [CrossRef]
- Saha, S.; Chowdhury, S.R.; Majumder, H.K. DNA Topoisomerases of Kinetoplastid Parasites: Brief Overview and Recent Perspectives. Curr. Issues Mol. Biol. 2019, 31, 45–62. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Alvarez-Velilla, R.; Fernandez-Prada, C.; García-Estrada, C.; Reguera, R.M. Trypanosomatids topoisomerase re-visited. New structural findings and role in drug discovery. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 326–337. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cross, G.A.M. TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated VSG Switching in Trypanosoma brucei. PLoS Pathog. 2010, 6, e1000992. [Google Scholar] [CrossRef][Green Version]
- Banerjee, B.; Sen, N.; Majumder, H.K. Identification of a Functional Type IA Topoisomerase, LdTopIIIβ, from Kinetoplastid Parasite Leishmania donovani. Enzym. Res. 2011, 2011, 230542. [Google Scholar] [CrossRef][Green Version]
- Scocca, J.R.; Shapiro, T.A. A mitochondrial topoisomerase IA essential for late theta structure resolution in African trypanosomes. Mol. Microbiol. 2008, 67, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.B.; Chapagain, P.P.; Seddek, A.; Annamalai, T.; Üren, A.; Tse-Dinh, Y. Covalent Complex of DNA and Bacterial Topoisomerase: Implications in Antibacterial Drug Development. ChemMedChem 2020, 15, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Sandhaus, S.; Chapagain, P.P.; Tse-Dinh, Y. Discovery of novel bacterial topoisomerase I inhibitors by use of in silico docking and in vitro assays. Sci. Rep. 2018, 8, 1437. [Google Scholar] [CrossRef]
- Godbole, A.A.; Ahmed, W.; Bhat, R.S.; Bradley, E.K.; Ekins, S.; Nagaraja, V. Targeting Mycobacterium tuberculosis Topoisomerase I by Small-Molecule Inhibitors. Antimicrob. Agents Chemother. 2015, 59, 1549–1557. [Google Scholar] [CrossRef]
- Ekins, S.; Godbole, A.A.; Kéri, G.; Őrfi, L.; Pato, J.; Bhat, R.S.; Verma, R.; Bradley, E.K.; Nagaraja, V. Machine learning and docking models for Mycobacterium tuberculosis topoisomerase I. Tuberculosis 2017, 103, 52–60. [Google Scholar] [CrossRef]
- Temesszentandrási-Ambrus, C.; Tóth, S.; Szakács, G.; Őrfi, L.; Nagaraja, V.; Ekins, S.; Telbisz, Á.; Verma, R.; Bánhegyi, P.; Szabadkai, I.; et al. Characterization of new, efficient Mycobacterium tuberculosis topoisomerase-I inhibitors and their interaction with human ABC multidrug transporters. PLoS ONE 2018, 13, e0202749. [Google Scholar] [CrossRef]
- Levene, S.D. Analysis of DNA Topoisomers, Knots, and Catenanes by Agarose Gel Electrophoresis. Methods Mol. Biol. 2009, 582, 11–25. [Google Scholar] [CrossRef]
- Cheng, B.; Annamalai, T.; Sandhaus, S.; Bansod, P.; Tse-Dinh, Y.-C. Inhibition of Zn(II) Binding Type IA Topoisomerases by Organomercury Compounds and Hg(II). PLoS ONE 2015, 10, e0120022. [Google Scholar] [CrossRef]
- Maxwell, A.; Burton, N.P.; O’Hagan, N. High-throughput assays for DNA gyrase and other topoisomerases. Nucleic Acids Res. 2006, 34, e104. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Jahic, H.; Prasad, S.; Ehmann, D.; Thresher, J.; Gao, N.; Hajec, L. A Homogeneous, High-Throughput Fluorescence Anisotropy-Based DNA Supercoiling Assay. J. Biomol. Screen. 2010, 15, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Jude, K.M.; Hartland, A.; Berger, J.M. Real-time detection of DNA topological changes with a fluorescently labeled cruciform. Nucleic Acids Res. 2013, 41, e133. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Berrido, A.; Gonzalez, W.G.; Miksovska, J.; Chambers, J.W.; Leng, F. Fluorescently labeled circular DNA molecules for DNA topology and topoisomerases. Sci. Rep. 2016, 6, 36006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rakela, S.; Chambers, J.W.; Hua, Z.-C.; Muller, M.T.; Nitiss, J.L.; Tse-Dinh, Y.-C.; Leng, F. Kinetic Study of DNA Topoisomerases by Supercoiling-Dependent Fluorescence Quenching. ACS Omega 2019, 4, 18413–18422. [Google Scholar] [CrossRef]
- Annamalai, T.; Cheng, B.; Keswani, N.; Tse-Dinh, Y.-C. A Fluorescence-Based Assay for Identification of Bacterial Topoisomerase I Poisons. Methods Mol. Biol. 2017, 1703, 259–268. [Google Scholar] [CrossRef]
- Kiianitsa, K.; Maizels, N. A rapid and sensitive assay for DNA–protein covalent complexes in living cells. Nucleic Acids Res. 2013, 41, e104. [Google Scholar] [CrossRef]
- Kiianitsa, K.; Maizels, N. Ultrasensitive isolation, identification and quantification of DNA–protein adducts by ELISA-based RADAR assay. Nucleic Acids Res. 2014, 42, e108. [Google Scholar] [CrossRef]
- Sinha, D.; Kiianitsa, K.; Sherman, D.R.; Maizels, N. Rapid, direct detection of bacterial topoisomerase 1-DNA adducts by RADAR/ELISA. Anal. Biochem. 2020, 608, 113827. [Google Scholar] [CrossRef]
- Ranjan, N.; Fulcrand, G.; King, A.; Brown, J.; Jiang, X.; Leng, F.; Arya, D.P. Selective inhibition of bacterial topoisomerase I by alkynyl-bisbenzimidazoles. MedChemComm 2014, 5, 816–825. [Google Scholar] [CrossRef]
- Nimesh, H.; Sur, S.; Sinha, D.; Yadav, P.; Anand, P.; Bajaj, P.; Virdi, J.S.; Tandon, V. Synthesis and Biological Evaluation of Novel Bisbenzimidazoles as Escherichia coli Topoisomerase IA Inhibitors and Potential Antibacterial Agents. J. Med. Chem. 2014, 57, 5238–5257. [Google Scholar] [CrossRef]
- Sandhaus, S.; Annamalai, T.; Welmaker, G.; Houghten, R.A.; Paz, C.; Garcia, P.K.; Andres, A.; Narula, G.; Felix, C.R.; Geden, S.; et al. Small-Molecule Inhibitors Targeting Topoisomerase I as Novel Antituberculosis Agents. Antimicrob. Agents Chemother. 2016, 60, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Felix, C.R.; Akerman, M.P.; Akerman, K.J.; Slabber, C.A.; Wang, W.; Adams, J.; Shaw, L.N.; Tse-Dinh, Y.-C.; Munro, O.Q.; et al. Evidence for Inhibition of Topoisomerase 1A by Gold(III) Macrocycles and Chelates Targeting Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Yuk-Ching, T.-D.; Zhang, M.; Annamalai, T.; Bansod, P.; Narula, G.; Tse-Dinh, Y.-C.; Sun, D. Synthesis, evaluation, and CoMFA study of fluoroquinophenoxazine derivatives as bacterial topoisomerase IA inhibitors. Eur. J. Med. Chem. 2017, 125, 515–527. [Google Scholar] [CrossRef]
- Garcia, P.K.; Annamalai, T.; Wang, W.; Bell, R.S.; Le, D.; Pancorbo, P.M.; Sikandar, S.; Seddek, A.; Yu, X.; Sun, D.; et al. Mechanism and resistance for antimycobacterial activity of a fluoroquinophenoxazine compound. PLoS ONE 2019, 14, e0207733. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Carreño, D.; Tirado-Vélez, J.M.; Ferrándiz, M.J.; Rodrigues, L.; Gracia, B.; Amblar, M.; Aínsa, J.A.; De La Campa, A.G. Boldine-Derived Alkaloids Inhibit the Activity of DNA Topoisomerase I and Growth of Mycobacterium tuberculosis. Front. Microbiol. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Yu, C.; Gatto, B.; Liu, L.F. DNA minor groove-binding ligands: A different class of mammalian DNA topoisomerase I inhibitors. Proc. Natl. Acad. Sci. USA 1993, 90, 8131–8135. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Yu, C.; Bodley, A.; Peng, L.F.; Liu, L.F. A new mammalian DNA topoisomerase I poison Hoechst 33342: Cytotoxicity and drug resistance in human cell cultures. Cancer Res. 1993, 53, 1332–1337. [Google Scholar]
- Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Preti, D.; Tabrizi, M.A.; Pavani, M.G.; Romagnoli, R. DNA minor groove binders as potential antitumor and antimicrobial agents. Med. Res. Rev. 2004, 24, 475–528. [Google Scholar] [CrossRef]
- Bansal, S.; Sinha, D.; Singh, M.; Cheng, B.; Tse-Dinh, Y.-C.; Tandon, V. 3,4-Dimethoxyphenyl bis-benzimidazole, a novel DNA topoisomerase inhibitor that preferentially targets Escherichia coli topoisomerase I. J. Antimicrob. Chemother. 2012, 67, 2882–2891. [Google Scholar] [CrossRef]
- Ranjan, N.; Story, S.; Fulcrand, G.; Leng, F.; Ahmad, M.; King, A.; Sur, S.; Wang, W.; Tse-Dinh, Y.; Arya, D.P. Selective Inhibition of Escherichia coli RNA and DNA Topoisomerase I by Hoechst 33258 Derived Mono- and Bisbenzimidazoles. J. Med. Chem. 2017, 60, 4904–4922. [Google Scholar] [CrossRef]
- Chamberlin, J.; Story, S.; Ranjan, N.; Chesser, G.; Arya, D.P. Gram-negative synergy and mechanism of action of alkynyl bisbenzimidazoles. Sci. Rep. 2019, 9, 14171. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pandey, S.; Sur, S.; Tandon, V. PPEF: A bisbenzimdazole potent antimicrobial agent interacts at acidic triad of catalytic domain of E. coli topoisomerase IA. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-X.; Tse-Dinh, Y.-C. The Acidic Triad Conserved in Type IA DNA Topoisomerases Is Required for Binding of Mg(II) and Subsequent Conformational Change. J. Biol. Chem. 2000, 275, 5318–5322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, B.; Tse-Dinh, Y.-C. Crystal structure of a covalent intermediate in DNA cleavage and rejoining by Escherichia coli DNA topoisomerase I. Proc. Natl. Acad. Sci. USA 2011, 108, 6939–6944. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef]
- Houghten, R.A.; Pinilla, C.; Giulianotti, M.A.; Appel, J.R.; Dooley, C.T.; Nefzi, A.; Ostresh, J.M.; Yu, Y.; Maggiora, G.; Medina-Franco, J.L.; et al. Strategies for the Use of Mixture-Based Synthetic Combinatorial Libraries: Scaffold Ranking, Direct Testing In Vivo, and Enhanced Deconvolution by Computational Methods. J. Comb. Chem. 2008, 10, 3–19. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Maida, L.E.; Santos, R.G.; Welmaker, G.S.; LaVoi, T.M.; Nefzi, A.; Yu, Y.; Houghten, R.A.; Toll, L.; et al. Scaffold Ranking and Positional Scanning Utilized in the Discovery of nAChR-Selective Compounds Suitable for Optimization Studies. J. Med. Chem. 2013, 56, 10103–10117. [Google Scholar] [CrossRef]
- Al-Ali, H.; Debevec, G.; Santos, R.G.; Houghten, R.A.; Davis, J.C.; Nefzi, A.; Lemmon, V.P.; Bixby, J.L.; Giulianotti, M.A. Scaffold Ranking and Positional Scanning Identify Novel Neurite Outgrowth Promoters with Nanomolar Potency. ACS Med. Chem. Lett. 2018, 9, 1057–1062. [Google Scholar] [CrossRef]
- Rideout, M.C.; Boldt, J.L.; Vahi-Ferguson, G.; Salamon, P.; Nefzi, A.; Ostresh, J.M.; Giulianotti, M.; Pinilla, C.; Segall, A.M. Potent antimicrobial small molecules screened as inhibitors of tyrosine recombinases and Holliday junction-resolving enzymes. Mol. Divers. 2011, 15, 989–1005. [Google Scholar] [CrossRef]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef]
- Chaffman, M.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Auranofin. A preliminary review of its pharmacological properties and therapeutic use in rheumatoid arthritis. Drugs 1984, 27, 378–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.W.-Y.; Li, C.K.-L.; Ma, D.-L.; Yan, J.J.; Lok, C.-N.; Leung, C.-H.; Zhu, N.; Che, C.-M. Stable Anticancer Gold(III)-Porphyrin Complexes: Effects of Porphyrin Structure. Chemistry 2010, 16, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Akerman, K.J.; Fagenson, A.M.; Cyril, V.; Taylor, M.; Muller, M.T.; Akerman, M.P.; Munro, O.Q. Gold(III) Macrocycles: Nucleotide-Specific Unconventional Catalytic Inhibitors of Human Topoisomerase I. J. Am. Chem. Soc. 2014, 136, 5670–5682. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Pató, J.; Nagaraja, V.; Godbole, A.A.; Bush, N.; Collin, F.; Maxwell, A.; Cole, S.T.; Kéri, G.; Székely, R.; et al. Lead selection and characterization of antitubercular compounds using the Nested Chemical Library. Tuberculosis 2015, 95 (Suppl. S1), S200–S206. [Google Scholar] [CrossRef]
- García, M.T.; Blázquez, M.A.; Ferrándiz, M.-J.; Sanz, M.-J.; Silva-Martín, N.; Hermoso, J.A.; De La Campa, A.G. New Alkaloid Antibiotics That Target the DNA Topoisomerase I of Streptococcus pneumoniae. J. Biol. Chem. 2011, 286, 6402–6413. [Google Scholar] [CrossRef]
- De La Campa, A.G.; Ferrándiz, M.J.; Martín-Galiano, A.J.; García, M.T.; Tirado-Vélez, J.M. The Transcriptome of Streptococcus pneumoniae Induced by Local and Global Changes in Supercoiling. Front. Microbiol. 2017, 8, 1447. [Google Scholar] [CrossRef]
- Ferrándiz, M.-J.; Martín-Galiano, A.J.; Arnanz, C.; Camacho-Soguero, I.; Tirado-Vélez, J.-M.; De La Campa, A.G. An increase in negative supercoiling in bacteria reveals topology-reacting gene clusters and a homeostatic response mediated by the DNA topoisomerase I gene. Nucleic Acids Res. 2016, 44, 7292–7303. [Google Scholar] [CrossRef]
- Dasgupta, T.; Ferdous, S.; Tse-Dinh, Y.-C. Mechanism of Type IA Topoisomerases. Molecules 2020, 25, 4769. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, T.; Zhong, H.; Kang, Y. Bulge oligonucleotide as an inhibitory agent of bacterial topoisomerase I. J. Enzym. Inhib. Med. Chem. 2018, 33, 319–323. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Zhou, B.; Zhuge, Q.; Lv, B. Small DNA circles as bacterial topoisomerase I inhibitors. RSC Adv. 2019, 9, 18415–18419. [Google Scholar] [CrossRef]

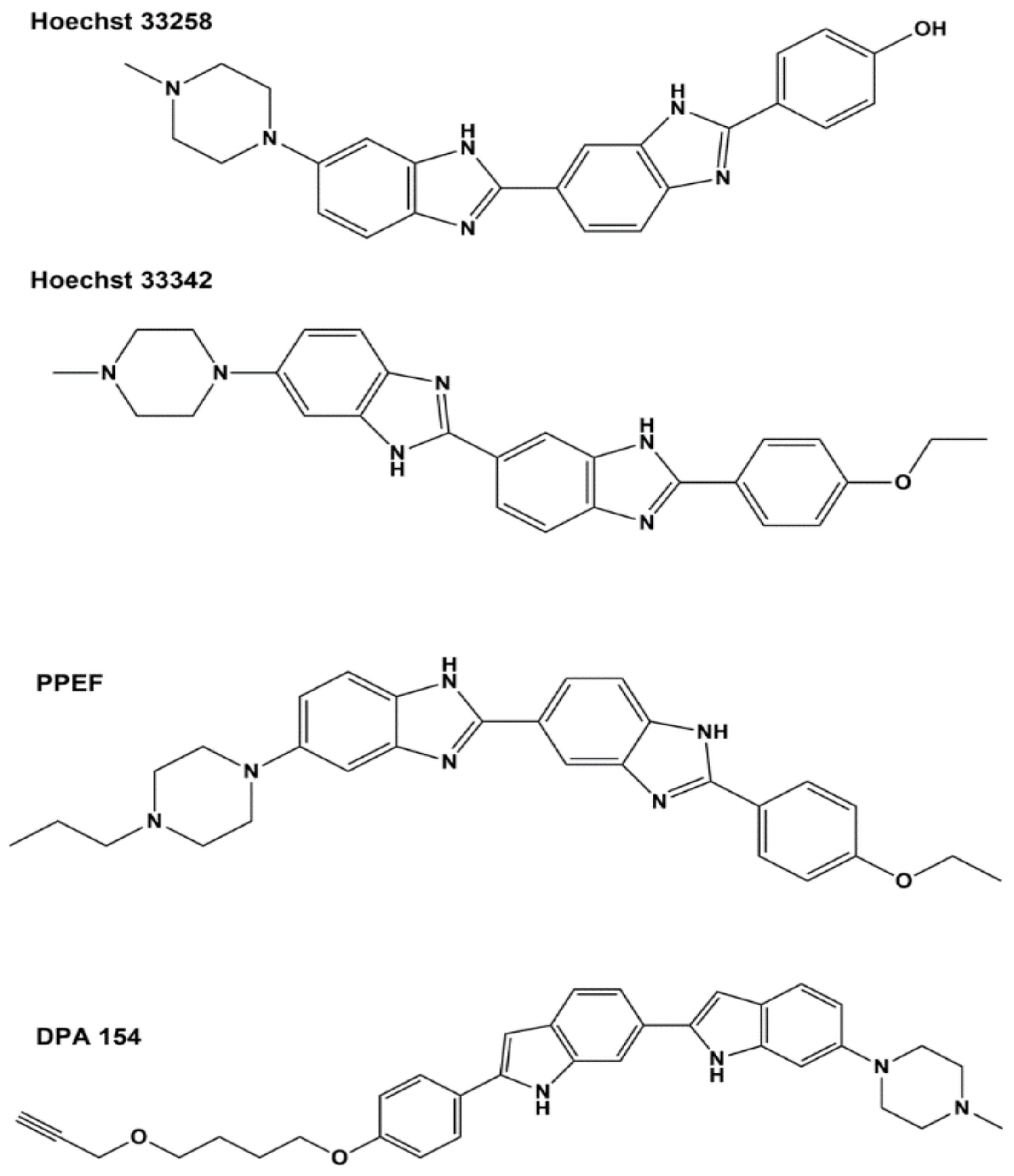




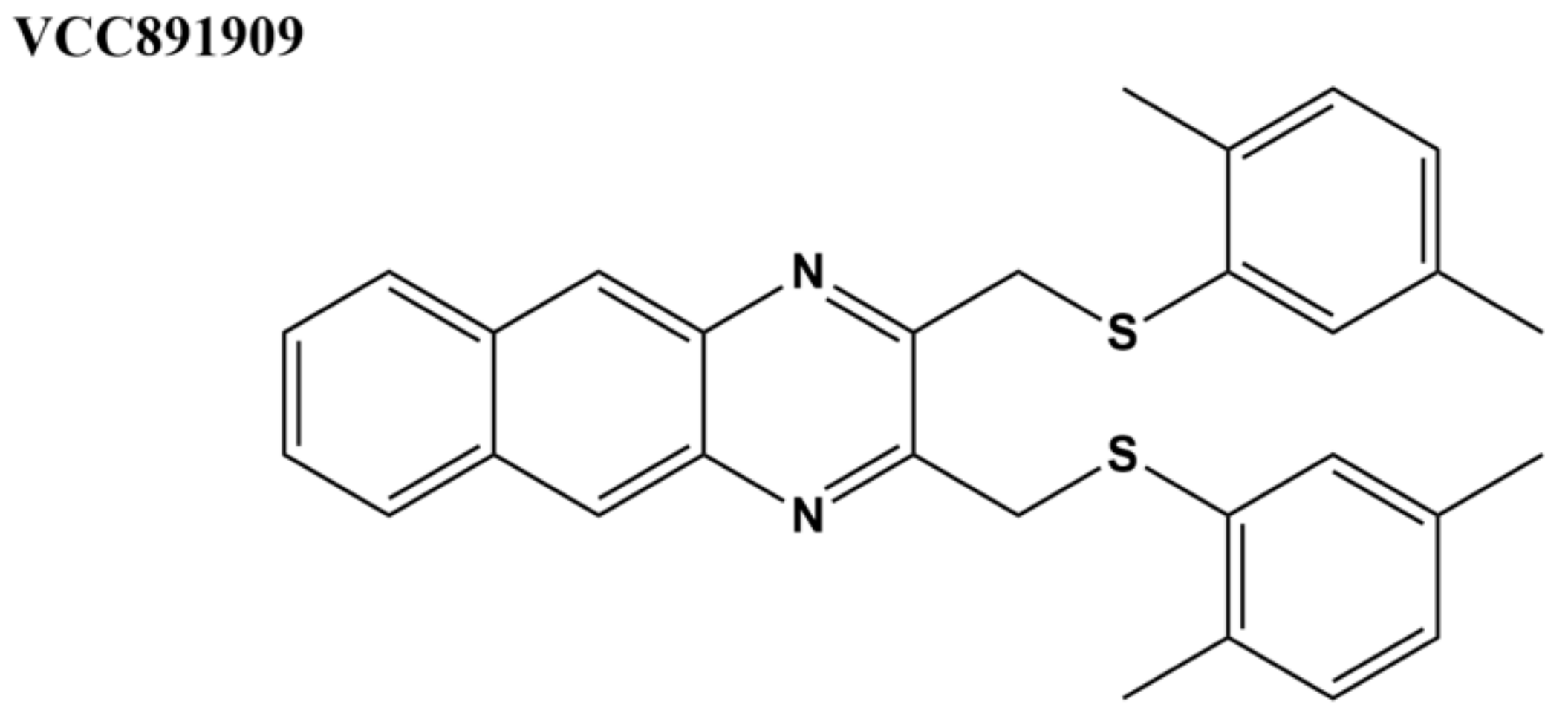


| Inhibitor | IC50 E. coli Topo I | IC50 M. tuberculosis Topo I |
|---|---|---|
| Bis-benzimidazole DPA 154 [110] | 6.6 µM | n.d. 1 |
| Bis-benzimidazole PPEF [111] | 9.4 µM | n.d. |
| Imipramine [96] | No inhibition at 25 µM | <0.1 µM |
| Norclomipramine [96] | No inhibition at 25 µM | <0.1 µM |
| Polyamine 2471-12 [112] | 7.5 µM | 7.5 µM |
| Polyamine 2471-24 [112] | 10 µM | 7.5 µM |
| Gold(III) macrocycle 10 [113] | 5 µM | 10 µM |
| Gold(III) chelate 14 [113] | 1.3 µM | 1.3 µM |
| Fluoroquinophenoxazine 11a [114] | 0.48 µM | 0.98 µM |
| Fluoroquinophenoxazine 11g [114,115] | 0.48 µM | 0.24 µM |
| VCC891909 [98] | n.d. | <7.5 µM |
| Seconeolitsine (SCN) [116] | n.d. | 5.6 µM |
| N-methyl-seconeolitsine (N-SCN) [116] | n.d. | 8.4 µM |
| Piperidine amide 7 [95] | 15.6–31.3 µM | 2 µM |
| NSC76027 [94] | 2.2 µM | 4 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seddek, A.; Annamalai, T.; Tse-Dinh, Y.-C. Type IA Topoisomerases as Targets for Infectious Disease Treatments. Microorganisms 2021, 9, 86. https://doi.org/10.3390/microorganisms9010086
Seddek A, Annamalai T, Tse-Dinh Y-C. Type IA Topoisomerases as Targets for Infectious Disease Treatments. Microorganisms. 2021; 9(1):86. https://doi.org/10.3390/microorganisms9010086
Chicago/Turabian StyleSeddek, Ahmed, Thirunavukkarasu Annamalai, and Yuk-Ching Tse-Dinh. 2021. "Type IA Topoisomerases as Targets for Infectious Disease Treatments" Microorganisms 9, no. 1: 86. https://doi.org/10.3390/microorganisms9010086
APA StyleSeddek, A., Annamalai, T., & Tse-Dinh, Y.-C. (2021). Type IA Topoisomerases as Targets for Infectious Disease Treatments. Microorganisms, 9(1), 86. https://doi.org/10.3390/microorganisms9010086





