A Gnotobiotic Model to Examine Plant and Microbiome Contributions to Survival under Arsenic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds Selection and Plants Collection
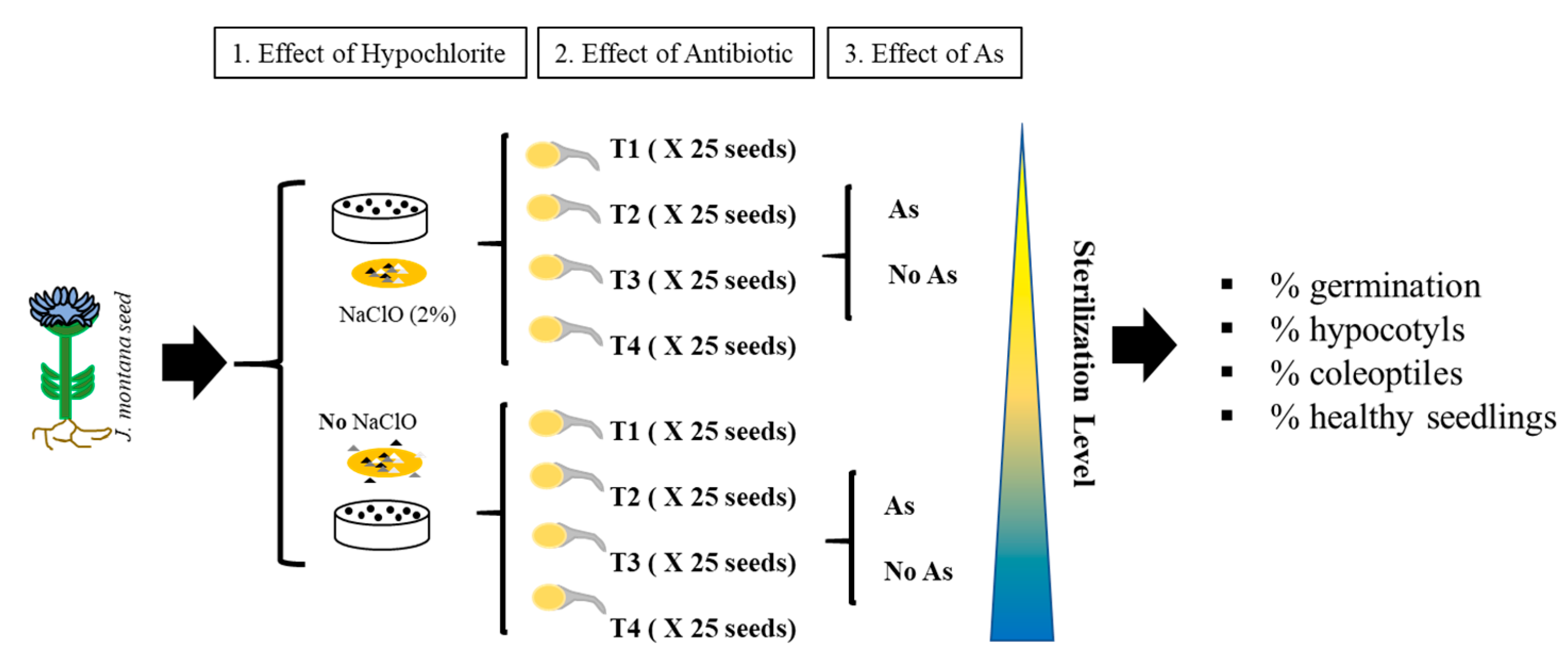
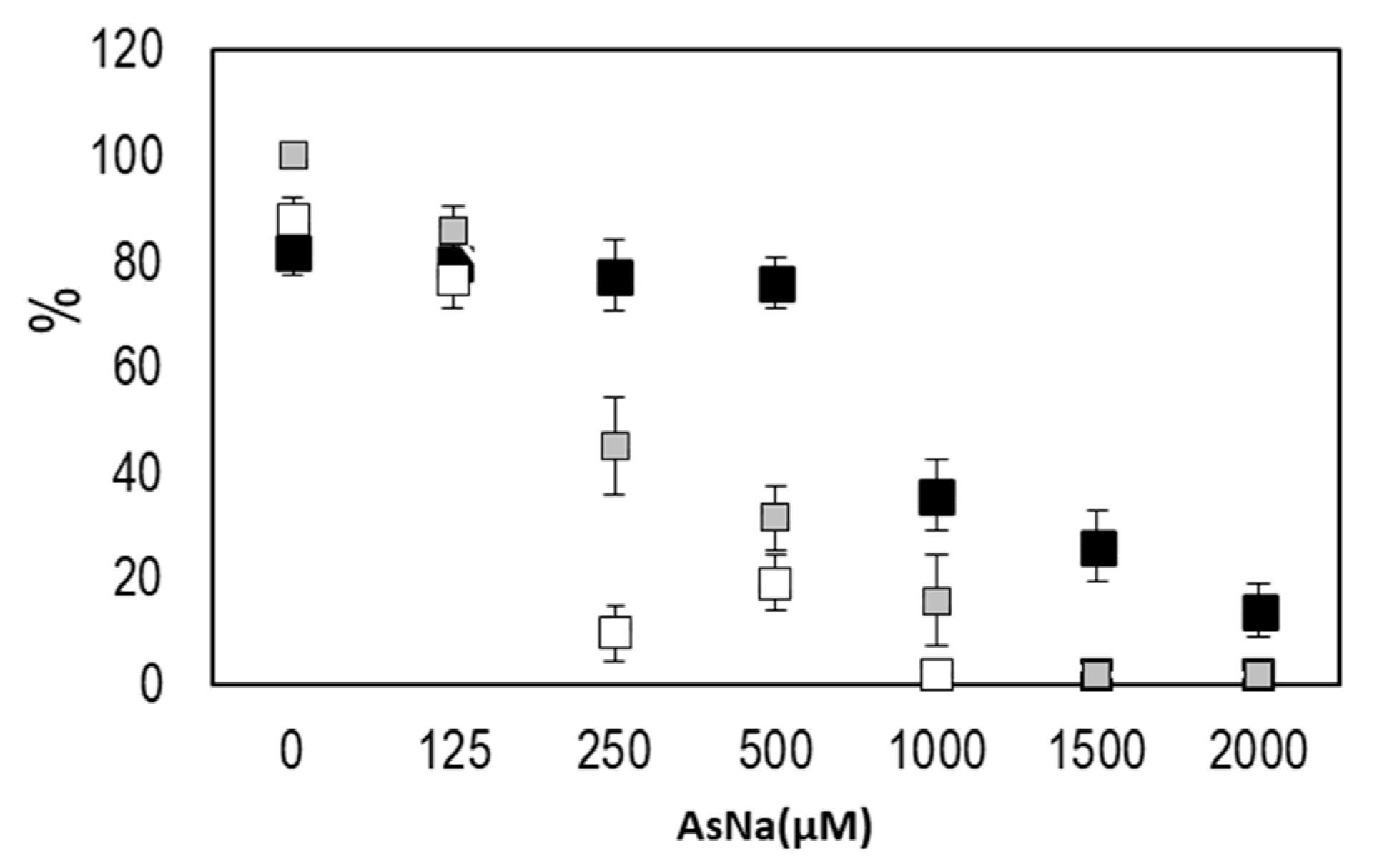
2.2. Screening of Arsenic Effect on the Germination and Development of Seedlings
2.3. Seed Sterilization Effects on Plant Development
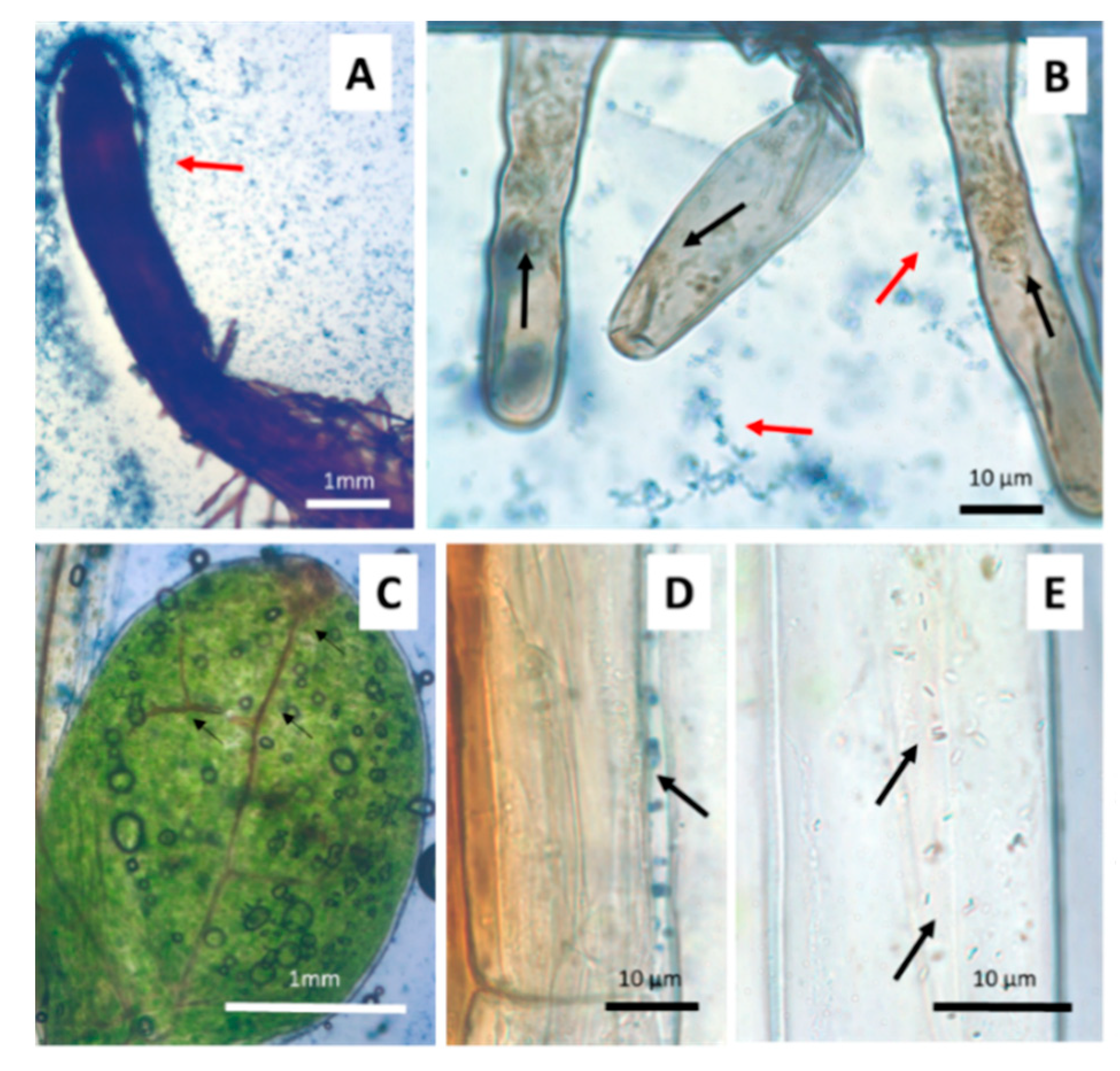
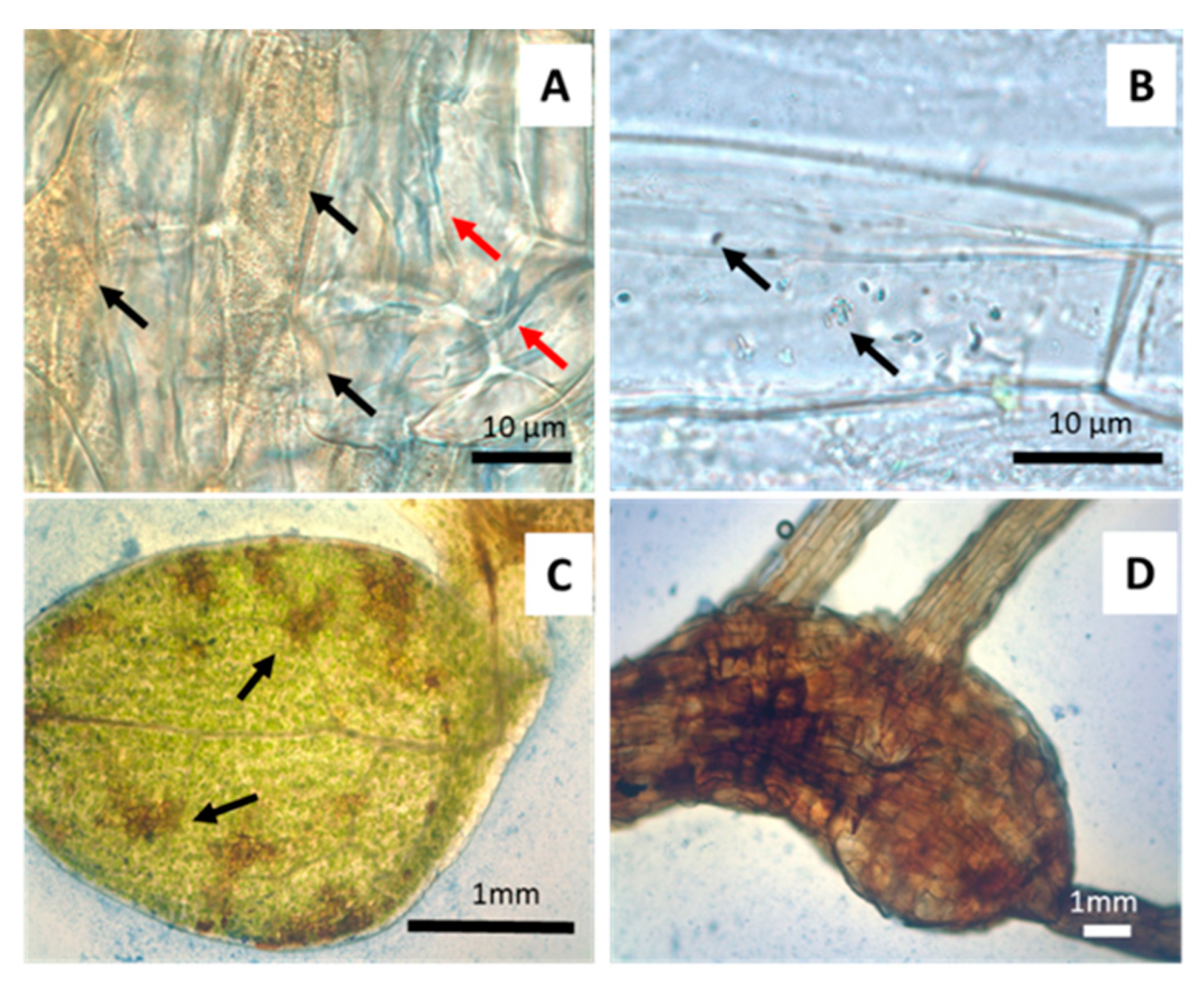
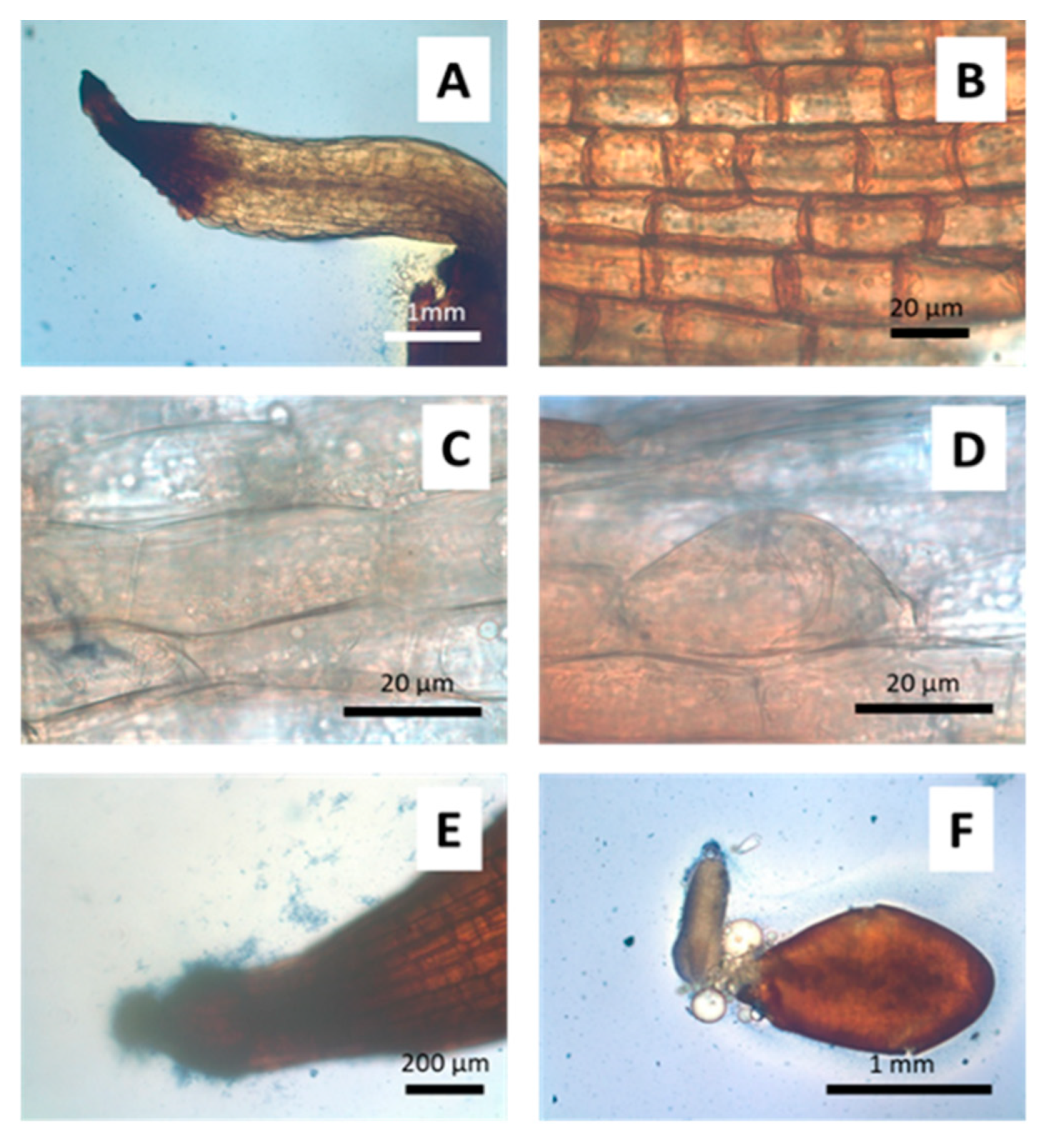
2.4. Microscopic Microbiome Detection
2.5. Isolation of Endophytic Bacteria Resistant to As(V)
2.6. Molecular Identification and Characterization of Endophytic Bacteria
2.7. Inoculation of Single Bacteria and Bacterial Mixture onto Gnotobiotic Seeds
2.8. Statistical Analysis
3. Results
3.1. Molecular Identification and Characterization of Bacteria
3.2. Anatomical and Physiological Effects of Arsenic on Seedlings
3.3. Anatomical and Physiological Effects on Gnotobiotic Jasione (1 mM Arsenate)
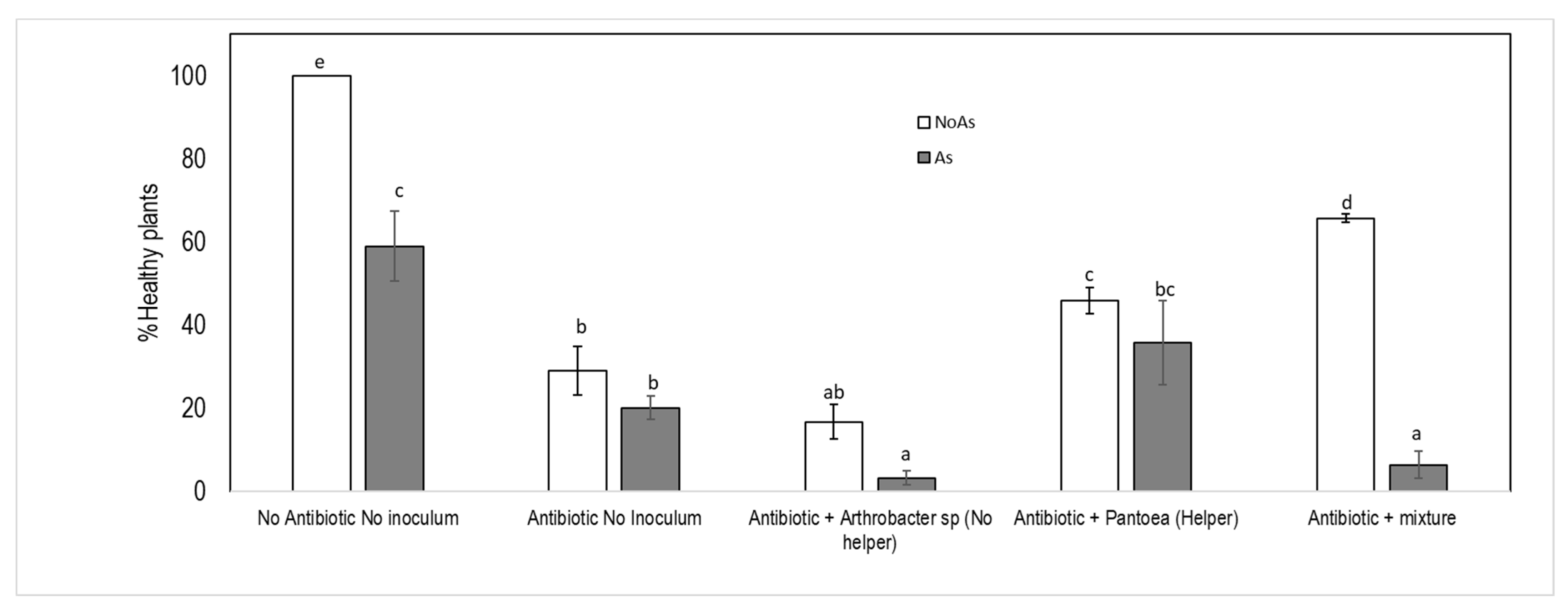
3.4. Seedling Growth Promotion Experiments with Single Bacterium or Bacterial Mixture (125 μM Arsenate)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mattusch, J.; Wennrich, R. Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci. Rep. 2017, 7, 40522. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef]
- Rafiq, M.; Shahid, M.; Abbas, G.; Shamshad, S.; Khalid, S.; Niazi, N.K.; Dumat, C. Comparative effect of calcium and EDTA on arsenic uptake and physiological attributes of Pisum sativum. Int. J. Phytoremediat. 2017, 19, 662–669. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.-Y.; Wang, L.-C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Talukdar, D. Arsenic-induced changes in growth and antioxidant metabolism of fenugreek. Russ. J. Plant Physiol. 2013, 60, 652–660. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller, A.L.W.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zhu, Y.-G.; Smith, F.A.; Smith, S.E. Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytol. 2004, 162, 481–488. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J.M. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2017, 25, 158–165. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.-H.; Cao, Y.; Zhu, Y.-G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Abedin, M.J.; Meharg, A.A. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 2002, 243, 57–66. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Babu, A.G.; Reddy, M.S. Dual inoculation of arbuscular mycorrhizal and phosphate solubilizing fungi contributes in sustainable maintenance of plant health in fly ash ponds. Water Air. Soil Pollut. 2011, 219, 3–10. [Google Scholar] [CrossRef]
- Tiwari, S.; Sarangi, B.K.; Thul, S.T. Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J. Environ. Manag. 2016, 180, 359–365. [Google Scholar] [CrossRef]
- Han, Y.H.; Fu, J.W.; Chen, Y.; Rathinasabapathi, B.; Ma, L.Q. Arsenic uptake, arsenite efflux and plant growth in hyperaccumulator Pteris vittata: Role of arsenic-resistant bacteria. Chemosphere 2016, 144, 1937–1942. [Google Scholar] [CrossRef]
- Salducci, M.-D.; Folzer, H.; Issartel, J.; Rabier, J.; Masotti, V.; Prudent, P.; Affre, L.; Hardion, L.; Tatoni, T.; Laffont-Schwob, I. How can a rare protected plant cope with the metal and metalloid soil pollution resulting from past industrial activities? Phytometabolites, antioxidant activities and root symbiosis involved in the metal tolerance of Astragalus tragacantha. Chemosphere 2019, 217, 887–896. [Google Scholar] [CrossRef]
- Cheng, C.; Nie, Z.W.; He, L.Y.; Sheng, X.F. Rice-derived facultative endophytic Serratia liquefaciens F2 decreases rice grain arsenic accumulation in arsenic-polluted soil. Environ. Pollut. 2020, 259, 113832. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of endophytic and rhizosphere bacteria to improve phytoremediation of arsenic-contaminated industrial soils by autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83, e03411-16. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Saha, C.; Naskar, N.; Mukherjee, A.; Mukherjee, A.; Lahiri, S.; Majumder, A.L.; Seal, A. An endophytic bacterial consortium modulates multiple strategies to improve arsenic phytoremediation efficacy in Solanum nigrum. Sci. Rep. 2018, 8, 6979. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Ohki, A.; Miyahara, K.; Takeshita, T.; Higashi, S. Growth characteristics and arsenic metabolism of two species of arsenic-tolerant bacteria. Appl. Organomet. Chem. 1990, 4, 245–250. [Google Scholar] [CrossRef]
- Bentley, R.; Chasteen, T.G. Microbial methylation of metalloids: Arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 2002, 66, 250–271. [Google Scholar] [CrossRef]
- Qin, J.; Rosen, B.P.; Zhang, Y.; Wang, G.J.; Franke, S.; Rensing, C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 2075–2080. [Google Scholar] [CrossRef]
- Páez-Espino, D.; Tamames, J.; de Lorenzo, V.; Cánovas, D. Microbial responses to environmental arsenic. Biometals 2009, 22, 117–130. [Google Scholar] [CrossRef]
- Wolfe-Simon, F.; Blum, J.S.; Kulp, T.R.; Gordon, G.W.; Hoeft, S.E.; Pett-Ridge, J.; Stolz, J.F.; Webb, S.M.; Weber, P.K.; Davies, P.C.W.; et al. A bacterium that can grow by using arsenic instead of phosphorus. Science 2011, 332, 1163–1166. [Google Scholar] [CrossRef]
- Dolphen, R.; Thiravetyan, P. Reducing arsenic in rice grains by leonardite and arsenic–resistant endophytic bacteria. Chemosphere 2019, 223, 448–454. [Google Scholar] [CrossRef]
- Retamal-Morales, G.; Mehnert, M.; Schwabe, R.; Tischler, D.; Zapata, C.; Chávez, R.; Schlömann, M.; Levicán, G. Detection of arsenic-binding siderophores in arsenic-tolerating Actinobacteria by a modified CAS assay. Ecotoxicol. Environ. Saf. 2018, 157, 176–181. [Google Scholar] [CrossRef]
- Vital, T.Z.; Román-Ponce, B.; Orduña, F.N.R.; de los Santos, P.E.; Vásquez-Murrieta, M.S.; Deng, Y.; Wang, E.T. An endophytic Kocuria palustris strain harboring multiple arsenate reductase genes. Arch. Microbiol. 2019, 201, 1285–1293. [Google Scholar] [CrossRef]
- Lampis, S.; Santi, C.; Ciurli, A.; Andreolli, M.; Vallini, G. Promotion of arsenic phytoextraction efficiency in the fern Pteris vittata by the inoculation of As-resistant bacteria: A soil bioremediation perspective. Front. Plant Sci. 2015, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; White, J.F. Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in brown top millet (Urochloa ramosa L.). J. Appl. Microbiol. 2018, 124, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, B.J. Effects of facilitation on community stability and dynamics: Synthesis and future directions. J. Ecol. 2009, 97, 1192–1201. [Google Scholar] [CrossRef]
- Parnell, J. Variation in Jasione montana L. (Campanulaceae) and related species in Europe and North Africa. J. Watsonia 1987, 16, 249–267. [Google Scholar]
- Pérez-Espona, S.; Sales, F.; Hedge, I.; Möller, M. Phylogeny and species relationships in Jasione (Campanulaceae) with emphasis on the ‘Montana-Complex’. Edinb. J. Bot. 2005, 62, 29–51. [Google Scholar] [CrossRef]
- García-Salgado, S.; García-Casillas, D.; Quijano-Nieto, M.A.; Bonilla-Simón, M.M. Arsenic and heavy metal uptake and accumulation in native plant species from soils polluted by mining activities. Water Air Soil Pollut. 2012, 223, 559–572. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Heavy metals in native mediterranean grassland species growing at abandoned mine sites: Ecotoxicological Assessment and Phytoremediation of Polluted Soils. In Soil Biology 44, Heavy Metal Contamination of Soils; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 159–178. [Google Scholar]
- Porter, E.K.; Peterson, P.J. Arsenic accumulation by plants on mine waste (United Kingdom). Sci. Total Environ. 1975, 4, 356–371. [Google Scholar] [CrossRef]
- Benson, L.M.; Porter, E.K.; Peterson, P.J. Arsenic accumulation, tolerance and genotypic variation in plants on arsenical mine wastes in S.W. England. J. Plant Nutr. 1981, 3, 655–666. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; McFall-Ngai, M.J. Metaorganisms as the new frontier. Zoology 2011, 114, 185–190. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Kurzbaum, E.; Kirzhner, F.; Armon, R. A hydroponic system for growing gnotobiotic vs. sterile plants to study phytoremediation processes. Int. J. Phytoremediat. 2014, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Herzog, V.; Fahimi, H.D. A new sensitive colorimetric assay for peroxidase using 3,3′-diaminobenzidine as hydrogen donor. Anal. Biochem. 1973, 55, 554–562. [Google Scholar] [CrossRef]
- White, J.F.; Torres, M.S.; Somu, M.P.; Johnson, H.; Irizarry, I.; Chen, Q.; Zhang, N.; Walsh, E.; Tadych, M.; Bergen, M. Hydrogen peroxide staining to visualize intracellular bacterial infections of seedling root cells. Microsc. Res. Technol. 2014, 77, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.C.; González, N.; Bautista, L.F.; Sanz, R.; Simarro, R.; Sánchez, I.; Sanz, J.L. Isolation and genetic identification of PAH degrading bacteria from a microbial consortium. Biodegradation 2009, 20, 789–800. [Google Scholar] [CrossRef] [PubMed]
- García-Salgado, S.; Quijano, M.A.; Bonilla, M.M. Arsenic speciation in edible alga samples by microwave-assisted extraction and high-performance liquid chromatography coupled to atomic fluorescence spectrometry. Anal. Chim. Acta 2012, 714, 38–46. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Kusaba, M.; Tsuge, T. Phylogeny of Alternaria fungi known to produce host-specific toxins on the basis of variation in internal transcribed spacers of ribosomal DNA. Curr. Genet. 1995, 28, 491–498. [Google Scholar] [CrossRef]
- Soares, M.A.; Li, H.-Y.; Bergen, M.; da Silva, J.M.; Kowalski, K.P.; White, J.F. Functional role of an endophytic Bacillus amyloliquefaciens in enhancing growth and disease protection of invasive English ivy (Hedera helix L.). Plant Soil 2016, 405, 107–123. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Hartley-Whitaker, J.; Ainsworth, G.; Meharg, A.A. Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001, 24, 713–722. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.-G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Harminder, P.S.; Daizy, R.B.; Ravinder, K.K.; Komal, A. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar]
- Gupta, M.; Sharma, P.; Sarin, N.B.; Sinha, A.L. Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 2009, 74, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, N.; Berova, M.; Zlatev, Z. Effect of arsenic on some physiological parameters in bean plants. Biol. Plantarum. 2005, 49, 293–296. [Google Scholar] [CrossRef]
- Farnese, F.S.; Oliveira, J.T.A.; Farnese, M.S.; Gusman, G.S.; Silveira, N.M.; Siman, L.I. Uptake arsenic by plants: Effects on mineral nutrition, growth and antioxidant capacity. Idesia 2014, 32, 99–106. [Google Scholar] [CrossRef]
- Bolhassani, A.; Khavari, S.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta 2014, 1845, 20–30. [Google Scholar] [CrossRef]
- Young, S.B.; Setlow, P. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 2003, 95, 54–67. [Google Scholar] [CrossRef]
- Verma, S.; Yadav, K.; Singh, N. Optimization of the protocols for surface sterilization, regeneration and acclimatization of Stevia rebaudiana Bertoni. Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 221–227. [Google Scholar]
- Robert, F.; Hevor, T.K. Abnormal organelles in cultured astrocytes are largely enhanced by streptomycin and intensively by gentamicin. Neuroscience 2007, 144, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef] [PubMed]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Klimova, S.Y.; Shestakov, A.I.; Botina, S.G.; Netrusov, A.I. Orchid-associated bacteria produce indole-3-acetic acid, promote seed germination, and increase their microbial yield in response to exogenous auxin. Arch. Microbiol. 2007, 188, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Cassán, F.; Perrig, D.; Sgroy, V.; Masciarelli, O.; Penna, C.; Luna, V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur. J. Soil Biol. 2009, 45, 28–35. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.I.; Kowalski, K.P.; Irizarry, I.; Micci, A.; Soares, M.A.; Bergen, M.S. Disease protection and allelopathic interactions of seed-transmitted endophytic pseudomonads of invasive reed grass (Phragmites australis). Plant Soil 2018, 422, 195–208. [Google Scholar] [CrossRef]
- Links, M.G.; Demeke, T.; Gräfenhan, T.; Hill, J.E.; Hemmingsen, S.M.; Dumonceaux, T.J. Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on Triticum and Brassica seeds. New Phytol. 2014, 202, 542–553. [Google Scholar] [CrossRef]
- White, J.F.; Crawford, H.; Torres, M.S.; Mattera, R.; Irizarry, I.; Bergen, M.A. A proposed mechanism for nitrogen acquisition by grass seedlings through oxidation of symbiotic bacteria. Symbiosis 2012, 57, 161–171. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araújo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Rahman, F.; Chen, Z.; Naidu, R. A comparative study of the extractability of arsenic species from silverbeet and amaranth vegetables. Environ. Geochem. Health 2009, 31, 103–113. [Google Scholar] [CrossRef]
- Escalante, G.; Campos, V.L.; Valenzuela, C.; Yañez, J.; Zaror, C.; Mondaca, M.A. Arsenic resistant bacteria isolated from arsenic contaminated river in the Atacama Desert (Chile). Bull. Environ. Contam. Toxicol. 2009, 83, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Du, J.; Zhuang, G.; Jing, C. Bacillus sp. SXB and Pantoea sp. IMH, aerobic As(V)-reducing bacteria isolated from arsenic-contaminated soil. J. Appl. Microbiol. 2013, 114, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Jing, C. Genome sequence of the aerobic arsenate-reducing bacterium Pantoea sp. strain IMH. Genome Announc. 2014, 2, e00267-14. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, W.; Zeng, X.-C.; Yang, Y.; Zhou, L.; Mu, Y.; Liu, Y. Draft genome sequence of Arthrobacter sp. strain B6 isolated from the high-arsenic sediments in Datong Basin, China. Stand. Genom. Sci. 2017, 12, 11. [Google Scholar] [CrossRef]
- Chopra, B.K.; Bhat, S.; Mikheenko, I.P.; Xu, Z.; Yang, Y.; Luo, X.; Chen, H.; van Zwieten, L.; Lilley, R.M.; Zhang, R. The characteristics of rhizosphere microbes associated with plants in arsenic-contaminated soils from cattle dip sites. Sci. Total Environ. 2007, 378, 331–342. [Google Scholar] [CrossRef]
- Prasad, K.S.; Ramanathan, A.L.; Paul, J.; Subramanian, V.; Prasad, R. Biosorption of arsenite (As+ 3) and arsenate (As+ 5) from aqueous solution by Arthrobacter sp. biomass. Environl. Technol. 2013, 34, 2701–2708. [Google Scholar] [CrossRef]
- Palaniappan, P.; Chauhan, P.S.; Saravanan, V.S.; Anandham, R.; Sa, T. Isolation and characterization of plant growth promoting endophytic bacterial isolates from root nodule of Lespedeza sp. Biol. Fertil. Soils 2010, 46, 807–816. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, N.; He, L.; Wang, L.; Liu, Z.; You, M.; Guan, F. Arthrobacter scleromae sp. nov. isolated from human clinical specimens. J. Clin. Microbiol. 2005, 43, 1451–1455. [Google Scholar] [CrossRef]
- González, N.; Simarro, R.; Molina, M.C.; Bautista, L.F.; Delgado, L.; Villa, J.A. Effects of surfactants on PAH biodegradation by a bacterial consortium and on the dynamics of the bacterial community during the process. Bioresour. Technol. 2011, 102, 9438–9446. [Google Scholar] [CrossRef]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant–microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]

| Molecular Approach * | P | IAA Production | % Fugal Inh. | AMIC | As(V) a | As(III) a | Total As b | As(V) Biot. c | |
|---|---|---|---|---|---|---|---|---|---|
| Strains | (μg mL−1 DO−1) | LB | mM | mgL−1 | mgL−1 | mgL−1 | % | ||
| MC-K1 | P.conspicuaT | + | 1.96 ± 0.07 | 40 | 450 | 0.125 ± 0.3 10−3 | 0.091 ± 0.001 | 0.63 ± 0.03 | 66 |
| MC-K2 | Kocuria sp. | − | 0.46 ± 0.10 | 53 | 450 | 0.561 ± 0.008 | n.d. | 0.88 ± 0.02 | 36 |
| MC-D1 | R. rhodochorusT | − | 0.76 ± 0.10 | 40 | 450 | 0.266 ± 0.005 | 0.022 ± 0.001 | 0.67 ± 0.03 | 57 |
| MC-D2 | K. roseaT | − | 1.05 ± 0.07 | 46.7 | 450 | 0.152 ± 0.001 | n.d. | 0.54 ± 0.04 | 72 |
| MC-D3A | Arthrobacter sp. | − | n.d. | 73 | 10 | n.a. | n.a. | n.a. | |
| Mixture bacteria | − | 4.46 ± 0.01 | 20 | 200 | n.a. | n.a. | n.a. |
| Parameter | Factor | df | DR | Pr (>/Chi/) |
|---|---|---|---|---|
| Germination | Treatment | 3 | 365.08 | <0.001 |
| Hypochloryte | 1 | 16.05 | <0.001 | |
| [As] | 1 | 258.75 | <0.001 | |
| Treatment:Hypochloryte | 3 | 84.21 | <0.001 | |
| Treatment:As | 3 | 21.61 | <0.001 | |
| Hypochloryte:As | 1 | 3.07 | ns | |
| Treatment:Hypochloryte:As | 3 | 6.77 | ns | |
| Hypocotiledons | Treatment | 3 | 1379.28 | <0.001 |
| Hypochloryte | 1 | 3.83 | ns | |
| [As] | 1 | 225.45 | <0.001 | |
| Treatment:Hypochloryte | 3 | 46.84 | <0.001 | |
| Treatment:As | 3 | 4.49 | ns | |
| Hypochloryte:As | 1 | 0.11 | ns | |
| Treatment:Hypochloryte:As | 3 | 11.01 | 0.011 |
| Parameter | Factor | df | DR | Pr(>/Chi/) |
|---|---|---|---|---|
| Germination | Treatment | 4 | 7.33 | 0.11 |
| [As] | 1 | 0.074 | 0.78 | |
| Treatment: [As] | 4 | 2.661 | 0.61 | |
| Healthy plants | Treatment | 4 | 170.315 | <0.001 |
| [As] | 1 | 46.072 | <0.001 | |
| Treatment: [As] | 4 | 27.251 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, M.d.C.; White, J.F.; García-Salgado, S.; Quijano, M.Á.; González-Benítez, N. A Gnotobiotic Model to Examine Plant and Microbiome Contributions to Survival under Arsenic Stress. Microorganisms 2021, 9, 45. https://doi.org/10.3390/microorganisms9010045
Molina MdC, White JF, García-Salgado S, Quijano MÁ, González-Benítez N. A Gnotobiotic Model to Examine Plant and Microbiome Contributions to Survival under Arsenic Stress. Microorganisms. 2021; 9(1):45. https://doi.org/10.3390/microorganisms9010045
Chicago/Turabian StyleMolina, María del Carmen, James F. White, Sara García-Salgado, M. Ángeles Quijano, and Natalia González-Benítez. 2021. "A Gnotobiotic Model to Examine Plant and Microbiome Contributions to Survival under Arsenic Stress" Microorganisms 9, no. 1: 45. https://doi.org/10.3390/microorganisms9010045
APA StyleMolina, M. d. C., White, J. F., García-Salgado, S., Quijano, M. Á., & González-Benítez, N. (2021). A Gnotobiotic Model to Examine Plant and Microbiome Contributions to Survival under Arsenic Stress. Microorganisms, 9(1), 45. https://doi.org/10.3390/microorganisms9010045









