Development and Inter-Laboratory Validation of Diagnostics Panel for Detection of Biothreat Bacteria Based on MOL-PCR Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and DNA Extraction
2.2. Internal Control
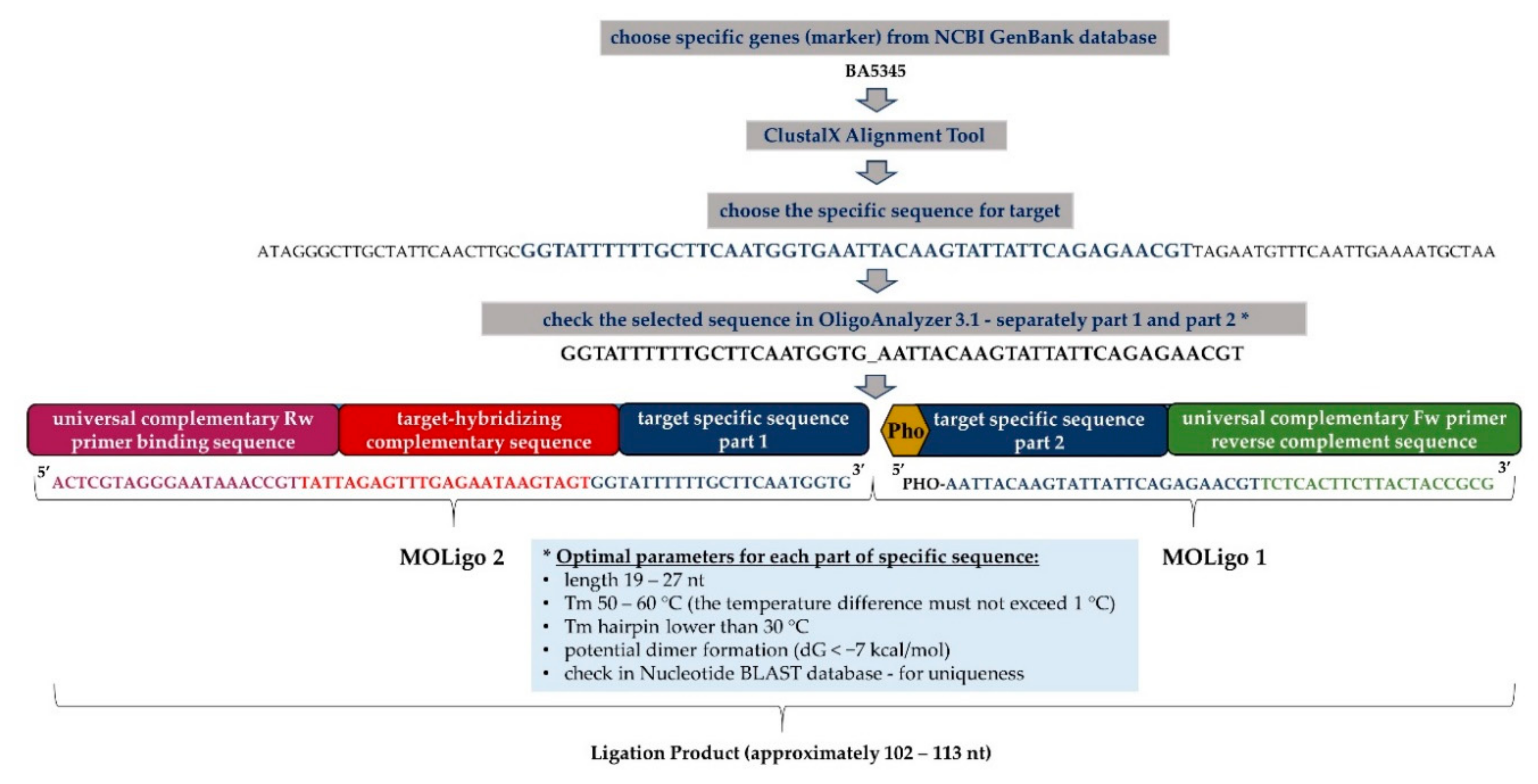
2.3. Design of MOLigo Probes (Short Specific Oligonucleotides)
2.4. MOL-PCR Assay
2.4.1. Coating the Magnetic Microspheres with Anti-xTAG Oligonucleotides
2.4.2. Multiplex Oligonucleotide Ligation
2.4.3. Singleplex PCR
2.4.4. Hybridization and MAGPIX Analysis
2.5. Specificity and Sensitivity of MOL-PCR Assay
2.6. Variability in Preparation of Ligation Mix and Stability during Long-Term Storage
2.7. Inter-Laboratory Validation of the Biothreat Detection Panel
2.8. Data Analysis and Interpretation
3. Results and Discussion
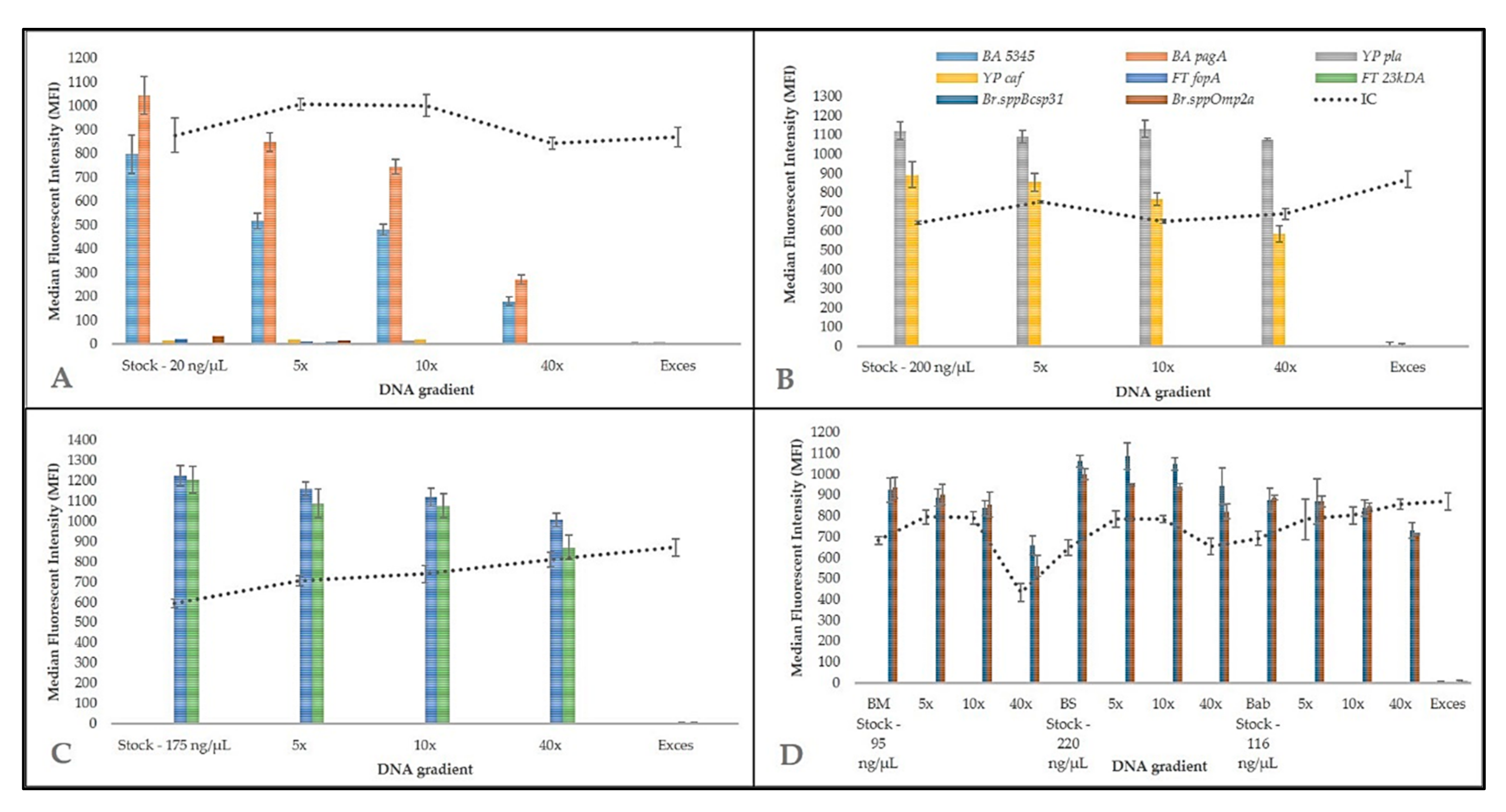
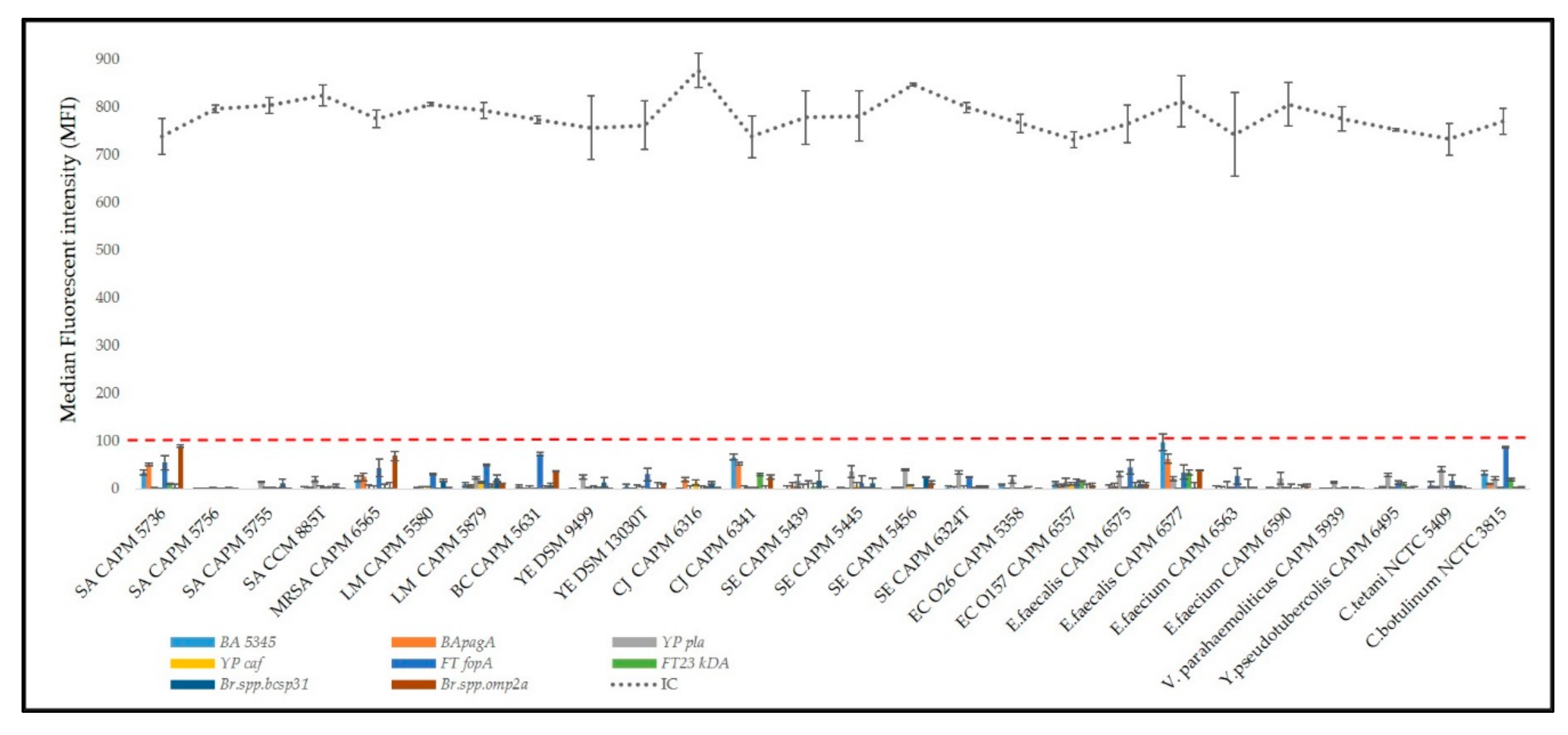
3.1. Biothreat Bacteria Panel Optimization
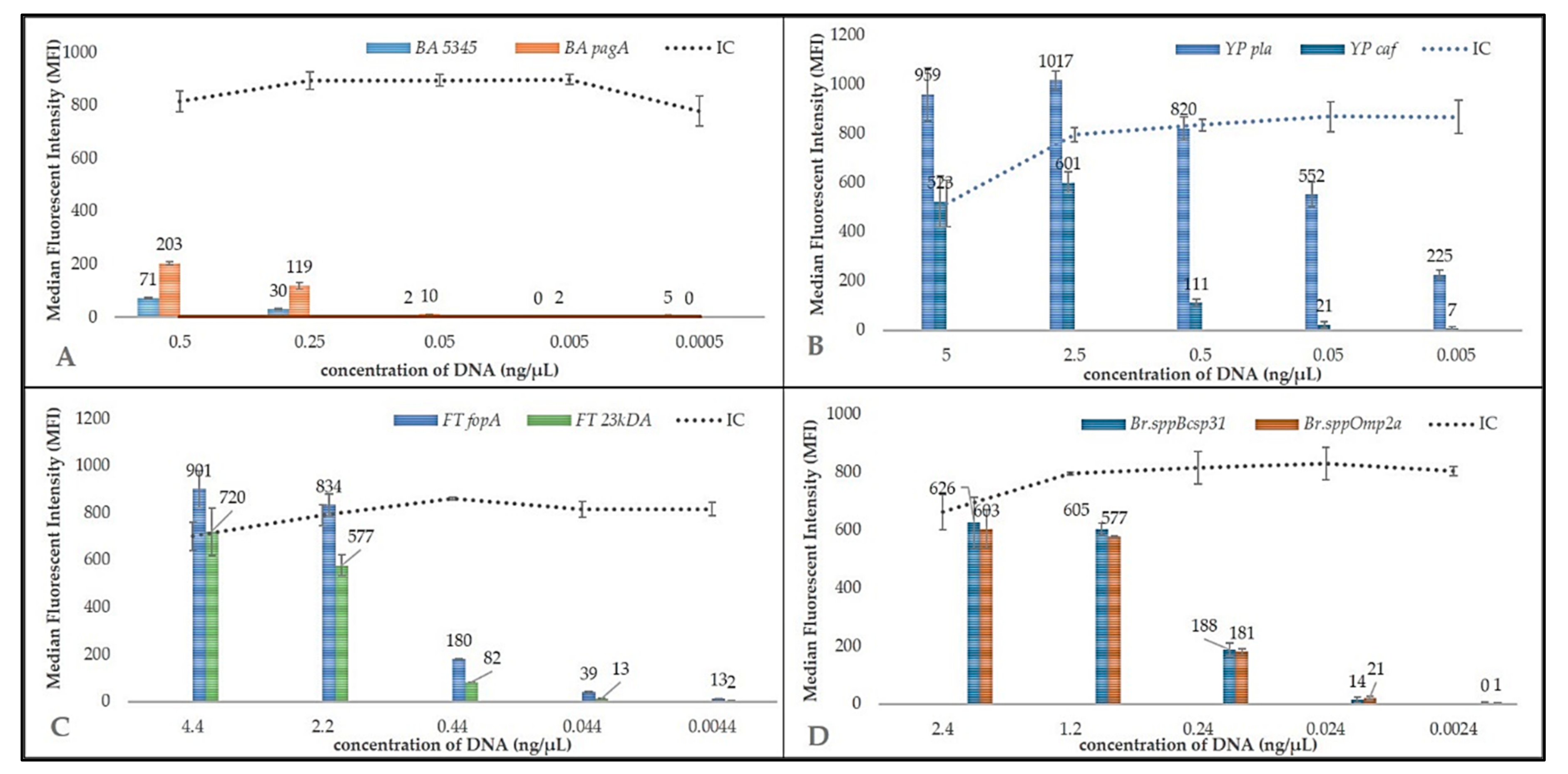
3.2. Sensitivity Test of the MOL-PCR Reaction
3.3. Long-Term Storage Effect of Ligation Mix
3.4. Inter-Laboratory Validation of Biothreat Detection Panel
3.5. On-Site Usability of the Panel
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arun Kumar, R.; Nishanth, T.; Ravi Teja, Y.; Sathish Kumar, D. Bio threat s bacterial warfare agents. J. Bioterr. Biodef. 2011, 2, 2. [Google Scholar]
- Christopher, L.G.W.; Cieslak, L.T.J.; Pavlin, J.A.; Eitzen, E.M. Biological warfare: A historical perspective. JAMA 1997, 278, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Wilkins, E. Electrochemical biosensors for detection of biological warfare agents. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2003, 15, 157–167. [Google Scholar] [CrossRef]
- Flora, S.; Pachauri, V. Handbook on Biological Warfare Preparedness; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Mölsä, M.; Hemmilä, H.; Katz, A.; Niemimaa, J.; Forbes, K.M.; Huitu, O.; Stuart, P.; Henttonen, H.; Nikkari, S. Monitoring biothreat agents (Francisella tularensis, Bacillus anthracis and Yersinia pestis) with a portable real-time PCR instrument. J. Microbiol. Methods 2015, 115, 89–93. [Google Scholar] [CrossRef]
- Peculi, A.; Campese, E.; Serrecchia, L.; Marino, L.; Boci, J.; Bijo, B. Genotyping of Bacillus anthracis strains circulating in Albania. J. Bioterror. Biodef. 2015, 6. [Google Scholar] [CrossRef]
- Carlson, C.J.; Kracalik, I.T.; Ross, N.; Alexander, K.A.; Hugh-Jones, M.E.; Fegan, M.; Elkin, B.T.; Epp, T.; Shury, T.K.; Zhang, W. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat. Microbiol. 2019, 4, 1337–1343. [Google Scholar] [CrossRef]
- Wilson, W.J.; Erler, A.M.; Nasarabadi, S.L.; Skowronski, E.W.; Imbro, P.M. A multiplexed PCR-coupled liquid bead array for the simultaneous detection of four biothreat agents. Mol. Cell. Probes 2005, 19, 137–144. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Davidow, A.L.; Miller, S.L.; Connell, N.; Ellner, J.J. Airborne infection with Bacillus anthracis—From mills to mail. Emerg. Infect. Dis. 2004, 10, 996. [Google Scholar] [CrossRef]
- World Health Organization. International Health Regulations (2005); World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Kool, J.L.; Weinstein, R.A. Risk of person-to-person transmission of pneumonic plague. Clin. Infect. Dis. 2005, 40, 1166–1172. [Google Scholar] [CrossRef]
- Gur, D.; Glinert, I.; Aftalion, M.; Vagima, Y.; Levy, Y.; Rotem, S.; Zauberman, A.; Tidhar, A.; Tal, A.; Maoz, S. Inhalational gentamicin treatment is effective against pneumonic plague in a mouse model. Front. Microbiol. 2018, 9, 741. [Google Scholar] [CrossRef]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis—Etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejek, M.A.; Hovde, C.J.; Minnich, S.A. Yersinia pestis Ail: Multiple roles of a single protein. Front. Cell. Infect. Microbiol. 2012, 2, 103. [Google Scholar] [CrossRef]
- Skládal, P.; Pohanka, M.; Kupská, E.; Šafář, B. Biosensors for Detection of Francisella tularensis and Diagnosis of Tularemia. In Biosensors; INTECH: Vienna, Austria, 2010; pp. 115–126. [Google Scholar]
- Gillard, J.J.; Laws, T.R.; Lythe, G.; Molina-Paris, C. Modeling early events in Francisella tularensis pathogenesis. Front. Cell. Infect. Microbiol. 2014, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.d.P.O.C.; Teles, J.A.A.; dos Santos, A.F.; Silva, S.O.F.; Cruz, M.V.R.A.; da Silva-Júnior, F.F. Prevalence of Brucella spp in humans. Rev. Latino-Am. Enferm. 2015, 23, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H.C.; Nöckler, K.; Göllner, C.; Bahn, P.; Vergnaud, G.; Tomaso, H.; Al Dahouk, S.; Kämpfer, P.; Cloeckaert, A.; Maquart, M. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evolut. Microbiol. 2010, 60, 801–808. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Wureli, H.; Xie, S.; Chen, C.; Wie, Q.; Cui, B.; Tu, C.; Wang, Y. Brucella melitensis and B. abortus in eggs, larvae and engorged females of Dermacentor marginatus. Ticks Tick-Borne Dis. 2018, 9, 1045–1048. [Google Scholar] [CrossRef]
- Abou Zaki, N.; Salloum, N.T.; Osman, M.; Rafei, R.; Hamze, M.; Tokajian, S. Typing and comparative genome analysis of Brucella melitensis isolated from Lebanon. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, H.J.; Gong, B.; Xiao, M.; Zhang, M.; Pang, Q.; Zhang, X.; Zhao, B.; Zhou, X. Screening and identification of a human domain antibody against Brucella abortus VirB5. Acta Trop. 2019, 197, 105026. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Wen, H.; Liu, H. Comparison of Two Suspension Arrays for Simultaneous Detection of Five Biothreat Bacterial in Powder Samples. J. Biomed. Biotechnol. 2012, 2012, 831052. [Google Scholar] [CrossRef]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef]
- Reslova, N.; Michna, V.; Kasny, M.; Mikel, P.; Kralik, P. xMAP technology: Applications in detection of pathogens. Front. Microbiol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Reslova, N.; Huvarova, V.; Hrdy, J.; Kasny, M.; Kralik, P. A novel perspective on MOL-PCR optimization and MAGPIX analysis of in-house multiplex foodborne pathogens detection assay. Sci. Rep. 2019, 9, 13. [Google Scholar]
- Deshpande, A.; Gans, J.; Graves, S.W.; Green, L.; Taylor, L.; Kim, H.B.; Kunde, Y.A.; Leonard, P.M.; Li, P.-E.; Mark, J.; et al. A rapid multiplex assay for nucleic acid-based diagnostics. J. Microbiol. Methods 2010, 80, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Stucki, D.; Mall, B.; Hostettler, S.; Huna, T.; Feldmann, J.; Yeboah-Manu, D.; Borrell, S.; Fenner, L.; Comas, I.; Coscolla, M. Two new rapid SNP-typing methods for classifying Mycobacterium tuberculosis complex into the main phylogenetic lineages. PLoS ONE 2012, 7, e41253. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, V.; Mattheus, W.; Roosens, N.H.; Marchal, K.; Bertrand, S.; De Keersmaecker, S.C. A multiplex oligonucleotide ligation-PCR as a complementary tool for subtyping of Salmonella Typhimurium. Appl. Microbiol. Biotechnol. 2015, 99, 8137–8149. [Google Scholar] [CrossRef]
- Ceyssens, P.-J.; Garcia-Graells, C.; Fux, F.; Botteldoorn, N.; Mattheus, W.; Wuyts, V.; De Keersmaecker, S.; Dierick, K.; Bertrand, S. Development of a Luminex xTAG® assay for cost-effective multiplex detection of β-lactamases in Gram-negative bacteria. J. Antimicrob. Chemother. 2016, 71, 2479–2483. [Google Scholar] [CrossRef][Green Version]
- Woods, T.A.; Mendez, H.M.; Ortega, S.; Shi, X.; Marx, D.; Bai, J.; Moxley, R.A.; Nagaraja, T.G.; Graves, S.W.; Deshpande, A. Development of 11-Plex MOL-PCR assay for the rapid screening of samples for Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2016, 6, 92. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wu, S.Q.; Jiang, L.; Xiao, R.H.; Li, T.; Mei, L.; Lv, J.Z.; Liu, J.J.; Lin, X.M.; Han, X.Q. Establishment and optimization of a liquid bead array for the simultaneous detection of ten insect-borne pathogens. Parasites Vectors 2018, 11, 442. [Google Scholar] [CrossRef]
- US Food & Drug Administration Office. Guidelines for the Validation of Analytical Methods for the Detection of Microbial Pathogens in Foods and Feeds; US Food & Drug Administration Office of Foods and Veterinary Medicine: Silver Spring, MD, USA, 2015. [Google Scholar]
- Slana, I.; Kaevska, M.; Kralik, P.; Horvathova, A.; Pavlik, I. Distribution of Mycobacterium avium subsp. avium and M. a. hominissuis in artificially infected pigs studied by culture and IS901 and IS1245 quantitative real time PCR. Vet. Microbiol. 2010, 144, 437–443. [Google Scholar] [CrossRef]
- Vasickova, P.; Slany, M.; Chalupa, P.; Holub, M.; Svoboda, R.; Pavlik, I. Detection and phylogenetic characterization of human hepatitis E virus strains, Czech Republic. Emerg. Infect. Dis. 2011, 17, 917. [Google Scholar] [CrossRef]
- Van Tongeren, S.P.; Roest, H.I.J.; Degener, J.E.; Harmsen, J.M. Bacillus anthracis-Like Bacteria and Other B. cereus Group Members in a Microbial Community Within the International Space Station: A Challenge for Rapid and Easy Molecular Detection of Virulent B. anthracis. PLoS ONE 2014, 9, e98871. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Uhl, J.R.; Hadfield, T.L.; David, J.C.; Meyer, R.F.; Smith, T.F.; Cockerill, F.R. Detection of Bacillus anthracis DNA by LightCycler PCR. J. Clin. Microbiol. 2002, 40, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Tomaso, H.; Reisinger, E.C.; Al Dahouk, S.; Frangoulidis, D.; Rakin, A.; Landt, O.; Neubauer, H. Rapid detection of Yersinia pestis with multiplex real-time PCR assays using fluorescent hybridisation probes. FEMS Immunol. Med. Microbiol. 2003, 38, 117–126. [Google Scholar] [CrossRef]
- Hatkoff, M.; Runco, L.M.; Pujol, C.; Jayatilaka, I.; Furie, M.B.; Bliska, J.B.; Thanassi, D.G. Roles of chaperone/usher pathways of Yersinia pestis in a murine model of plague and adhesion to host cells. Infect. Immun. 2012, 80, 3490–3500. [Google Scholar] [CrossRef] [PubMed]
- Versage, J.L.; Severin, D.D.M.; Chu, M.C.; Petersen, J.M. Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex specimens. J. Clin. Microbiol. 2003, 41, 5492–5499. [Google Scholar] [CrossRef]
- Fujita, O.; Tatsumi, M.; Tanabayashi, K.; Yamada, A. Development of a real-time PCR assay for detection and quantification of Francisella tularensis. Jpn. J. Infect. Dis. 2006, 59, 46–51. [Google Scholar]
- Probert, W.S.; Schrader, K.N.; Khuong, N.Y.; Bystrom, S.L.; Graves, M.H. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J. Clin. Microbiol. 2004, 42, 1290–1293. [Google Scholar] [CrossRef]
- Bounaadja, L.; Albert, D.; Chénais, B.; Hénault, S.; Zygmunt, M.S.; Poliak, S.; Garin-Bastuji, B. Real-time PCR for identification of Brucella spp.: A comparative study of IS711, bcsp31 and per target genes. Vet. Microbiol. 2009, 137, 156–164. [Google Scholar] [CrossRef]
- Thierry, S.; Hamidjaja, R.A.; Girault, G.; Löfström, C.; Ruuls, R.; Sylviane, D. A multiplex bead-based suspension array assay for interrogation of phylogenetically informative single nucleotide polymorphisms for Bacillus anthracis. J. Microbiol. Methods 2013, 95, 357–365. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Longo, C.M.; Berninger, M.S.; Hartley, J.L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 1990, 93, 125–128. [Google Scholar] [CrossRef]
- Wuyts, V.; Roosens, N.H.C.; Bertrand, S.; Marchal, K.; De Keersmaecker, S.C.J. Guidelines for Optimisation of a Multiplex Oligonucleotide Ligation-PCR for Characterisation of Microbial Pathogens in a Microsphere Suspension Array. BioMed Res. Int. 2015, 2015, 790170. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidelines for the Validation of Microbiological Methods for the FDA Foods Program, 3rd ed.; US Food and Drug Administration: Silver Spring, MA, USA, 2019. [Google Scholar]
- Yan, Y.; Luo, J.Y.; Chen, Y.; Wang, H.H.; Zhu, G.Y.; He, P.Y.; Guo, J.L.; Lei, Y.L.; Chen, Z.W. A multiplex liquid-chip assay based on Luminex xMAP technology for simultaneous detection of six common respiratory viruses. Oncotarget 2017, 8, 96913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, T.; Chidambara, V.A.; Andreasen, S.Z.; Golabi, M.; Huynh, V.N.; Linh, Q.T.; Bang, D.D.; Wolff, A. Point-of-care devices for pathogen detections: The three most important factors to realise towards commercialization. TrAC Trends Anal. Chem. 2020, 131, 116004. [Google Scholar] [CrossRef]

| Species | Accession No. |
|---|---|
| Bacillus anthracis | CAPM 5001 |
| Yersinia pestis | NCTC 5923 T |
| Francisella tularensis | CAPM 5600 |
| Brucella abortus | CAPM 5520 |
| Brucella suis | CAPM 6073 T |
| Brucella melitensis | CAPM 5659 T |
| Staphylococcus aureus | CCM 885 T |
| CAPM 5736 | |
| CAPM 5756 | |
| CAPM 5755 | |
| Methicillin-resistant Staphylococcus aureus | CAPM 6565 |
| Listeria monocytogenes | CAPM 5580 |
| CAPM 5879 | |
| Bacillus cereus | CAPM 5631 |
| Yersinia enterocolitica | DSM 9499 |
| Yersinia enterocolitica | DSM 13030 T |
| Campylobacter jejuni | CAPM 6316 |
| CAPM 6341 | |
| Salmonella enterica | CAPM 5439 |
| Salmonella enterica | CAPM 5445 |
| Salmonella enterica | CAPM 5456 |
| Salmonella enterica | CAPM 6324 T |
| Escherichia coli O26 | CAPM 5358 |
| Escherichia coli O157 | CAPM 6557 |
| Enterococcus faecalis | CAPM 6575 |
| CAPM 6577 | |
| Enterococcus faecium | CAPM 6563 |
| CAPM 6590 | |
| Vibrio parahaemoliticus | CAPM 5939 |
| Yersinia pseudotuberculosis | CAPM 6495 |
| Clostridium tetani | NCTC 5409 |
| Clostridium botulinum | NCTC 3815 |
| Pathogen | Marker | Reference | Sequence 5′-3′ | Size of LP | xTAG | Bead |
|---|---|---|---|---|---|---|
| Bacillus anthracis | BA5345 | [35] | PHO-AATTACAAGTATTATTCAGAGAACGTTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTtattagagtttgagaataagtagtGGTATTTTTTGCTTCAATGGTG | 112 | A033 | 033 |
| pagA | [36] | PHO-CTGTATCAGCGGTATTTAAACTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTtgagtaagtttgtatgtttaagtaATCTAATATCGGCATTTAATCTTG | 109 | A065 | 034 | |
| Yersinia pestis | pla | [37] | PHO-TTCTGTTGTTTTGCCTTGACATTCTCCTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTgtgttatagaagttaaatgttaagCATAATGACGGGGCGCTCA | 110 | A030 | 030 |
| caf1 | [38] | PHO-AGGAACCACTAGCACATCTTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTgtaagattagaagttaatgaagaaCTTACTCTTGGCGGCTATAAAAC | 106 | A051 | 051 | |
| Francisella tularensis | 23kDA | [39] | PHO-TGAGATGATAACAAGACAACAGTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTgtaagagtattgaaattagtaagaAACTAAAAAAAGGAGAATGATTATGAG | 113 | A066 | 020 |
| fopA | [40] | PHO-ACTATCTAGAAATGTTCAAGCAAGTGTTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTagtaagtgttagatagtattgaatGGGTGGTGGTCTTAAGTTTGA | 112 | A038 | 038 | |
| Brucella spp. | omp2a | [41] | PHO-CAGGCTACGAATCCAGAAATCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTatttgttatgataaatgtgtagtgCGCACTGAATCTCTGTTTTTC | 104 | A042 | 012 |
| bcsp31 | [42] | PHO-TATGCCATTCGCCGCCTGATCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTgtgattgaatagtagattgtttaaCATTCTTCACATCCAGGAAACCCGAC | 109 | A046 | 046 | |
| Internal control | aDNA | [34] | Pho-ATTAGCACAATGAATAATCATCGTCTCACTTCTTACTACCGCG ACTCGTAGGGAATAAACCGTattgtgaaagaaagagaagaaattTATACACACGCAATCACCAC | 107 | A014 | 036 |
| Forward primer | [43] | CGCGGTAGTAAGAAGTGAGA | ||||
| Reverse primer | BODIPY-TMRX-ACTCGTAGGGAATAAACCGT |
| Pathogen | Gene (Target) | LoD (ng/µL) |
|---|---|---|
| B. anthracis | 5345 | >0.5 |
| pagA | 0.25–0.05 | |
| Y. pestis | pla | <0.005 |
| caf | 0.5–0.05 | |
| F. tularensis | fopA | 0.44–0.044 |
| 23kDA | 2.2–0.44 | |
| B. melitensis | bcsp31 | 0.24–0.024 |
| omp2a | 0.24–0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelinkova, P.; Hrdy, J.; Markova, J.; Dresler, J.; Pajer, P.; Pavlis, O.; Branich, P.; Borilova, G.; Reichelova, M.; Babak, V.; et al. Development and Inter-Laboratory Validation of Diagnostics Panel for Detection of Biothreat Bacteria Based on MOL-PCR Assay. Microorganisms 2021, 9, 38. https://doi.org/10.3390/microorganisms9010038
Jelinkova P, Hrdy J, Markova J, Dresler J, Pajer P, Pavlis O, Branich P, Borilova G, Reichelova M, Babak V, et al. Development and Inter-Laboratory Validation of Diagnostics Panel for Detection of Biothreat Bacteria Based on MOL-PCR Assay. Microorganisms. 2021; 9(1):38. https://doi.org/10.3390/microorganisms9010038
Chicago/Turabian StyleJelinkova, Pavlina, Jakub Hrdy, Jirina Markova, Jiri Dresler, Petr Pajer, Oto Pavlis, Pavel Branich, Gabriela Borilova, Marketa Reichelova, Vladimir Babak, and et al. 2021. "Development and Inter-Laboratory Validation of Diagnostics Panel for Detection of Biothreat Bacteria Based on MOL-PCR Assay" Microorganisms 9, no. 1: 38. https://doi.org/10.3390/microorganisms9010038
APA StyleJelinkova, P., Hrdy, J., Markova, J., Dresler, J., Pajer, P., Pavlis, O., Branich, P., Borilova, G., Reichelova, M., Babak, V., Reslova, N., & Kralik, P. (2021). Development and Inter-Laboratory Validation of Diagnostics Panel for Detection of Biothreat Bacteria Based on MOL-PCR Assay. Microorganisms, 9(1), 38. https://doi.org/10.3390/microorganisms9010038





