Escherichia coli HS and Enterotoxigenic Escherichia coli Hinder Stress Granule Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Bacteria Strains
2.2. Bacterial Infection and Stress Treatment
2.3. Immunofluorescence and Imaging Analyses
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
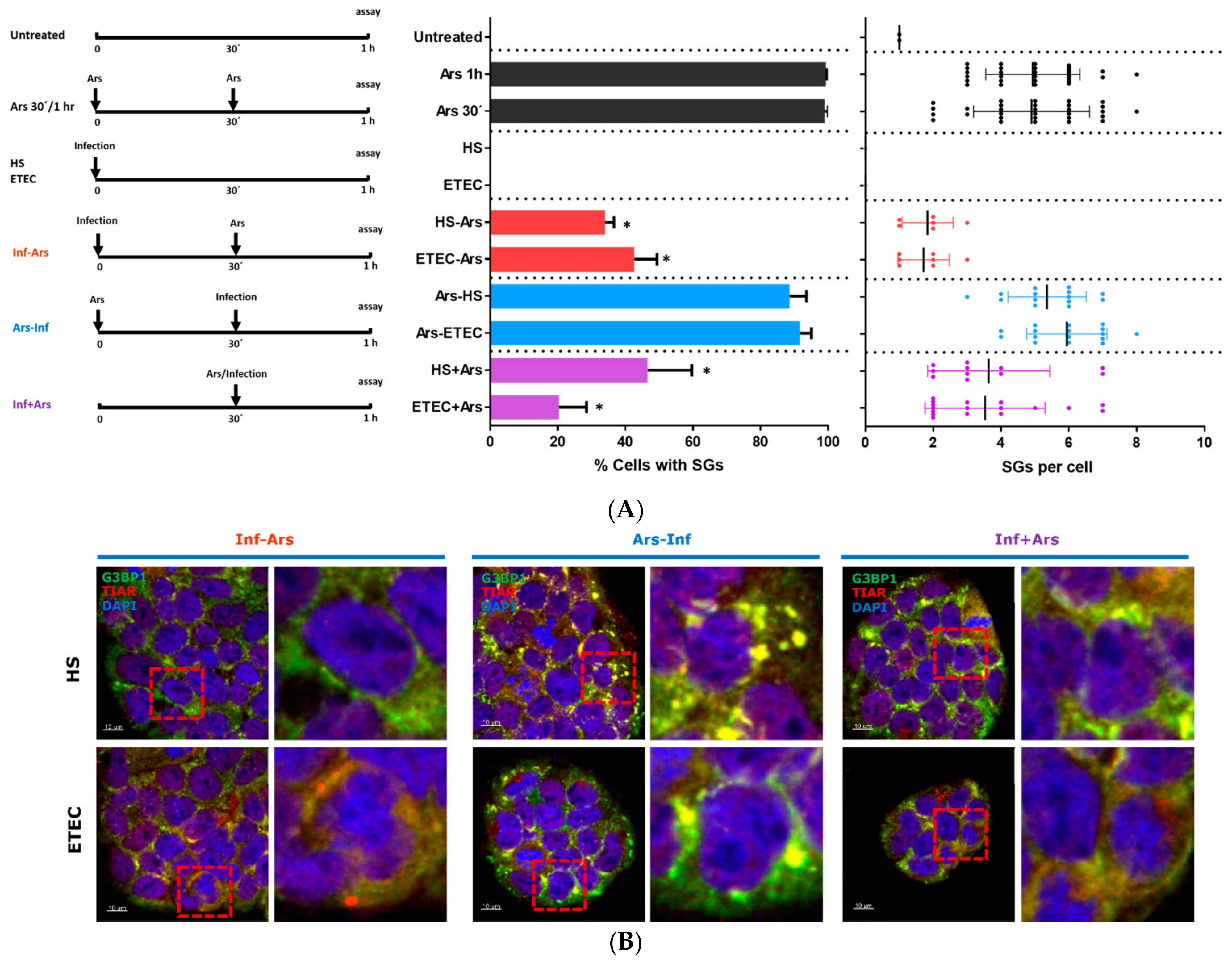
3.1. Non-Pathogenic E. coli HS Strain and ETEC 1766a Pathogenic Strain Do Not Induce Stress Granule Assembly
3.2. E. coli HS and ETEC 1766a Strains Prevent Arsenite-Induced Stress Granule Assembly in Caco-2 Cells
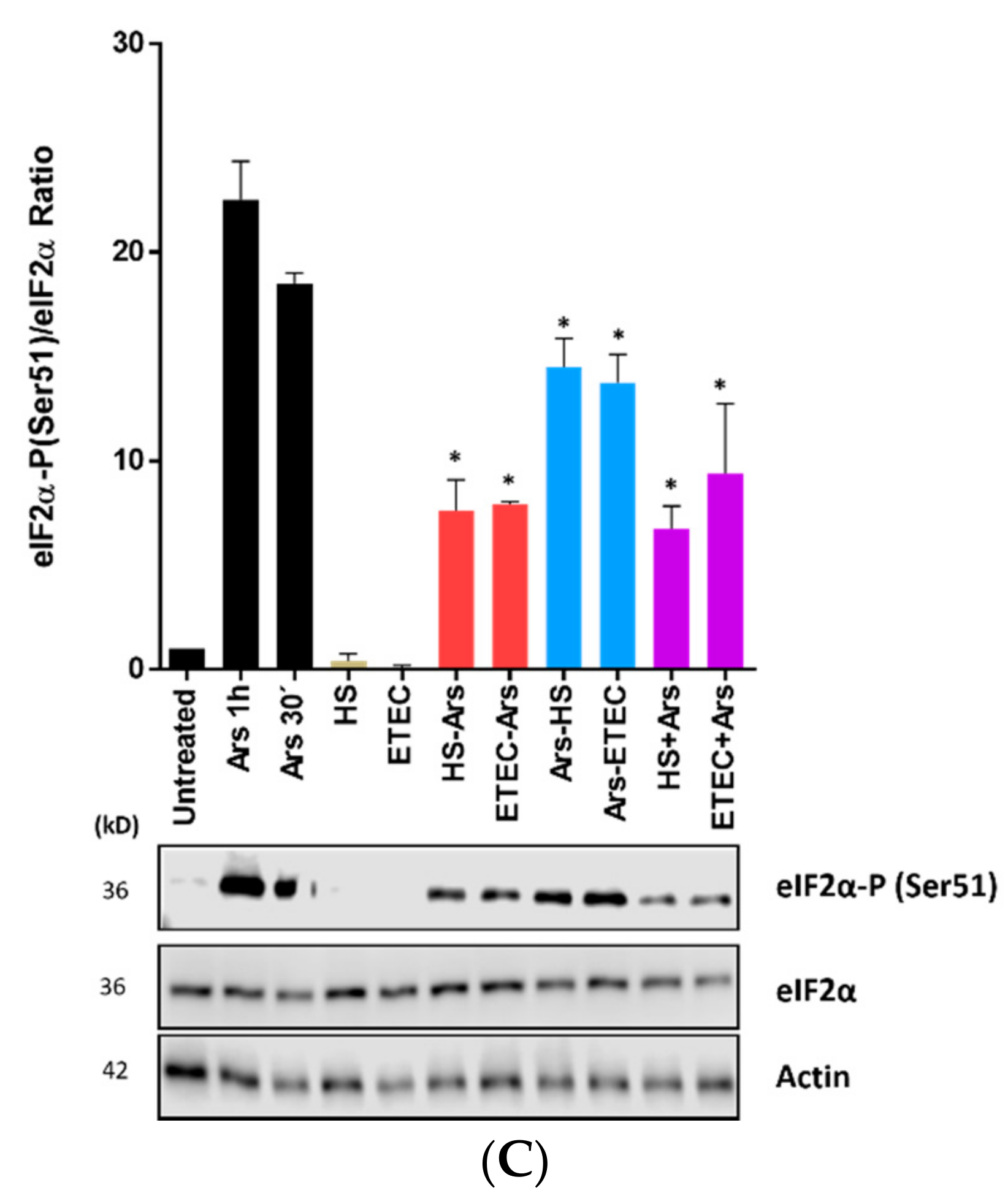
3.3. E. coli HS and ETEC 1766a Do Not Induce eIF2α Phosphorylation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, P.; Kedersha, N. Stressful initiations. J. Cell Sci. 2002, 115 Pt 16, 3227–3234. [Google Scholar] [PubMed]
- Anderson, P.; Kedersha, N. RNA granules. J. Cell Biol. 2006, 172, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Gaete-Argel, A.; Marquez, C.L.; Barriga, G.P.; Soto-Rifo, R.; Valiente-Echeverria, F. Strategies for Success. Viral Infections and Membraneless Organelles. Front. Cell. Infect. Microbiol. 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.R. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014, 11, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Campbell-Valois, F.X.; Dufour, A.; Sansonetti, P.J.; Schnupf, P. Shigella flexneri modulates stress granule composition and inhibits stress granule aggregation. Cell. Microbiol. 2016, 18, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Sansonetti, P.J.; Schnupf, P. Immunofluorescence Analysis of Stress Granule Formation after Bacterial Challenge of Mammalian Cells. J. Vis. Exp. Jove 2017, 125, e55536. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuki, H.; Yahiro, K.; Ogura, K.; Ichimura, K.; Iyoda, S.; Ohnishi, M.; Nagasawa, S.; Seto, K.; Moss, J.; Noda, M. Subtilase cytotoxin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli induces stress granule formation. Cell. Microbiol. 2016, 18, 1024–1040. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Rosovitz, M.J.; Myers, G.S.; Mongodin, E.F.; Fricke, W.F.; Gajer, P.; Crabtree, J.; Sebaihia, M.; Thomson, N.R.; Chaudhuri, R.; et al. The pangenome structure of Escherichia coli: Comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 2008, 190, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Del Canto, F.; Botkin, D.J.; Valenzuela, P.; Popov, V.; Ruiz-Perez, F.; Nataro, J.P.; Levine, M.M.; Stine, O.C.; Pop, M.; Torres, A.G.; et al. Identification of Coli Surface Antigen 23, a novel adhesin of enterotoxigenic Escherichia coli. Infect. Immun. 2012, 80, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.; Pardo, M.; Montero, D.; Onate, A.; Farfan, M.J.; Ruiz-Perez, F.; Del Canto, F.; Vidal, R. TleA, a Tsh-like autotransporter identified in a human enterotoxigenic Escherichia coli strain. Infect. Immun. 2015, 83, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Formal, S.B.; Hornick, R.B.; Snyder, M.J.; Libonati, J.P.; Sheahan, D.G.; LaBrec, E.H.; Kalas, J.P. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 1971, 285, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Ferreccio, C.; Prado, V.; Cayazzo, M.; Abrego, P.; Martinez, J.; Maggi, L.; Baldini, M.M.; Martin, W.; Maneval, D.; et al. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am. J. Epidemiol. 1993, 138, 849–869. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Aubel, D.; Chauviere, G.; Rich, C.; Bourges, M.; Servin, A.; Joly, B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect. Immun. 1990, 58, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Duran, N.; Prades-Perez, Y.; Vera-Otarola, J.; Soto-Rifo, R.; Valiente-Echeverria, F. Who Regulates Whom? An Overview of RNA Granules and Viral Infections. Viruses 2016, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Graca, R.S.F.; Carneiro, L.A.M. Integrated Stress Responses to Bacterial Pathogenesis Patterns. Front. Immunol. 2018, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Mishchuk, D.O.; Shah, J.; Weimer, B.C.; Slupsky, C.M. Cross-talk between E. coli strains and a human colorectal adenocarcinoma-derived cell line. Sci. Rep. 2013, 3, 3416. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Li, C.; Li, P.; Fu, E.; Xie, Y.; Jin, F. Heat-Labile Enterotoxin-Induced PERK-CHOP Pathway Activation Causes Intestinal Epithelial Cell Apoptosis. Front. Cell. Infect. Microbiol. 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- McEwen, E.; Kedersha, N.; Song, B.; Scheuner, D.; Gilks, N.; Han, A.; Chen, J.J.; Anderson, P.; Kaufman, R.J. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005, 280, 16925–16933. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nour, M.; Carneiro, L.A.M.; Downey, J.; Tsalikis, J.; Outlioua, A.; Prescott, D.; Da Costa, L.S.; Hovingh, E.S.; Farahvash, A.; Gaudet, R.G.; et al. The heme-regulated inhibitor is a cytosolic sensor of protein misfolding that controls innate immune signaling. Science 2019, 365, eaaw4144. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, F.; Marín-Rojas, J.; Soto-Rifo, R.; Torres, A.; Del Canto, F.; Valiente-Echeverría, F. Escherichia coli HS and Enterotoxigenic Escherichia coli Hinder Stress Granule Assembly. Microorganisms 2021, 9, 17. https://doi.org/10.3390/microorganisms9010017
Velásquez F, Marín-Rojas J, Soto-Rifo R, Torres A, Del Canto F, Valiente-Echeverría F. Escherichia coli HS and Enterotoxigenic Escherichia coli Hinder Stress Granule Assembly. Microorganisms. 2021; 9(1):17. https://doi.org/10.3390/microorganisms9010017
Chicago/Turabian StyleVelásquez, Felipe, Josefina Marín-Rojas, Ricardo Soto-Rifo, Alexia Torres, Felipe Del Canto, and Fernando Valiente-Echeverría. 2021. "Escherichia coli HS and Enterotoxigenic Escherichia coli Hinder Stress Granule Assembly" Microorganisms 9, no. 1: 17. https://doi.org/10.3390/microorganisms9010017
APA StyleVelásquez, F., Marín-Rojas, J., Soto-Rifo, R., Torres, A., Del Canto, F., & Valiente-Echeverría, F. (2021). Escherichia coli HS and Enterotoxigenic Escherichia coli Hinder Stress Granule Assembly. Microorganisms, 9(1), 17. https://doi.org/10.3390/microorganisms9010017




