New Clinical Applications of Electrolyzed Water: A Review
Abstract
1. Introduction
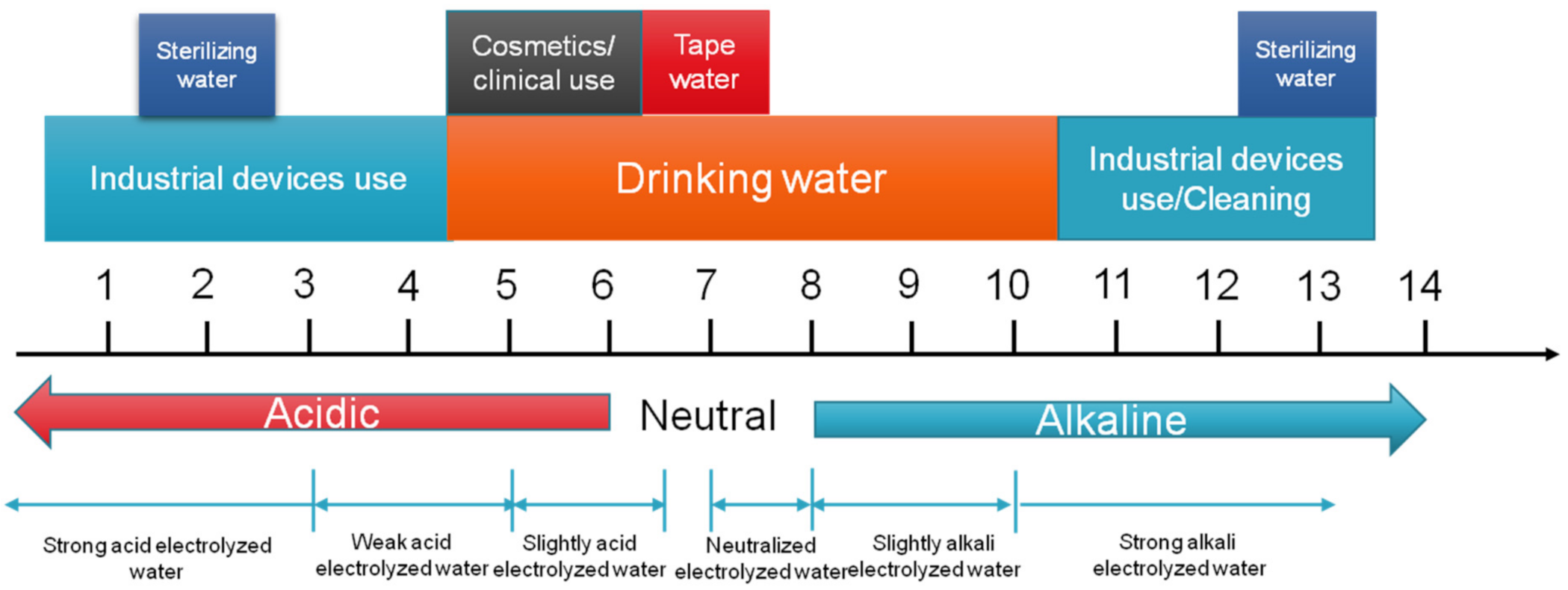
2. Principles and History of EW
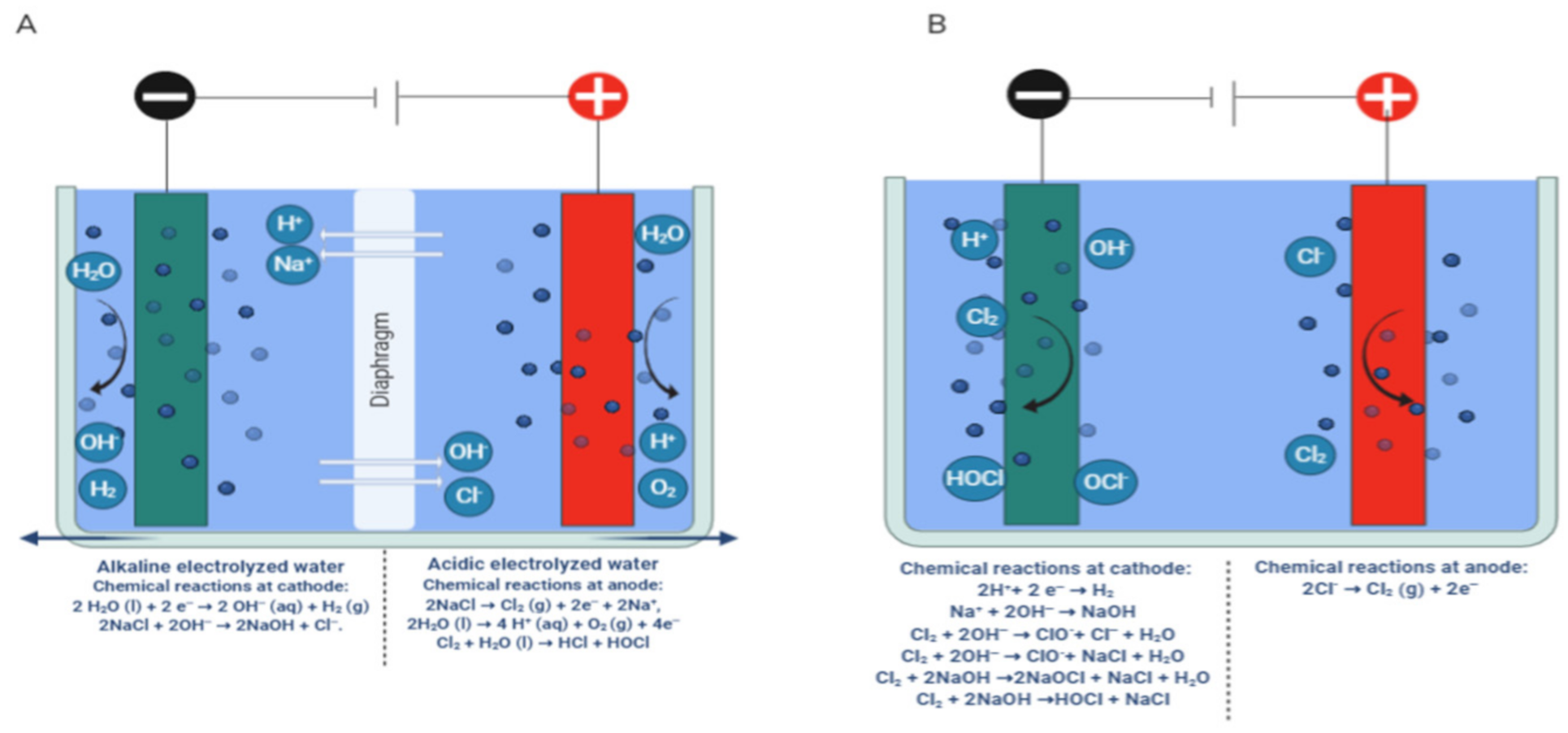
3. Systems for Generation of Electrolyzed Water
4. Factors Influencing Decontamination Efficacy of Electrolyzed Water
4.1. Direct Factors
4.2. Indirect Factors
5. The Advantages and Disadvantages of Electrolyzed Water
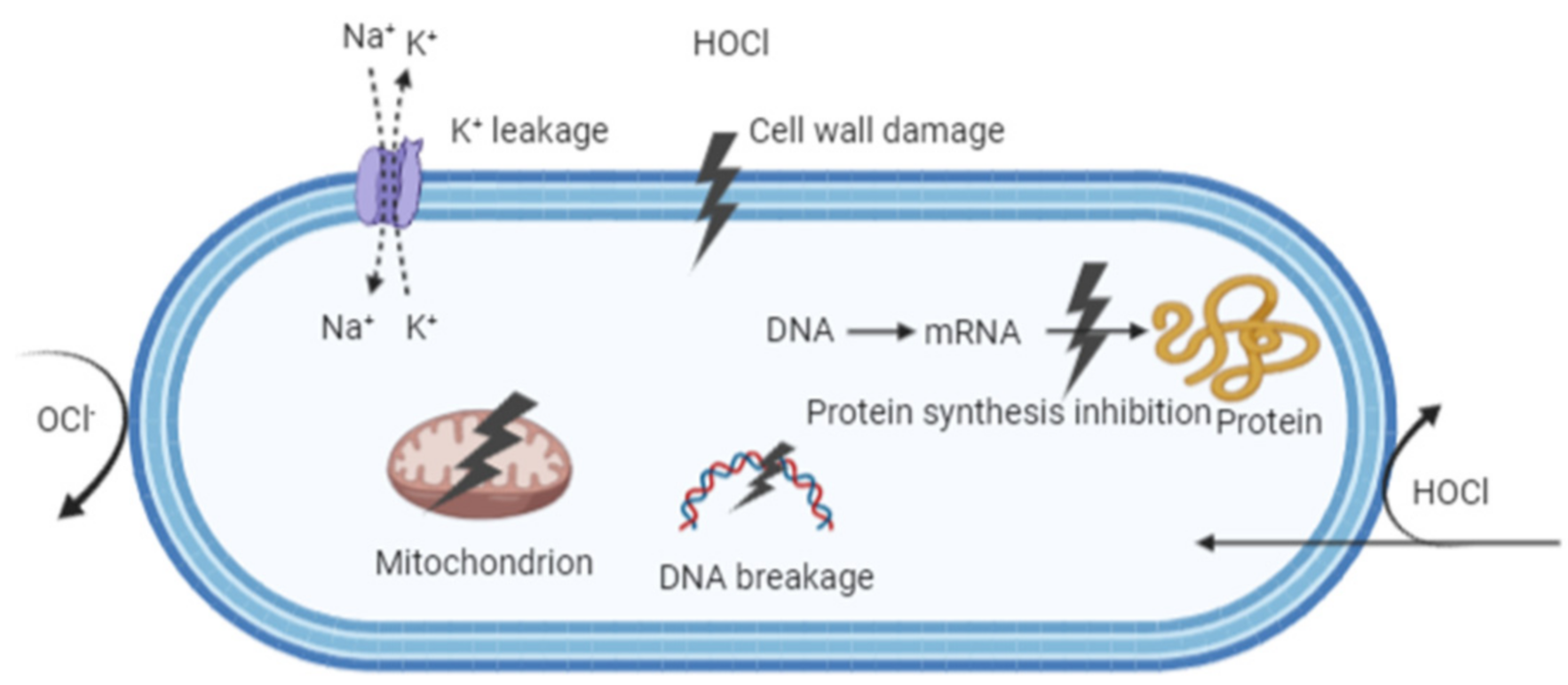
6. Disinfection Mechanisms of EW
7. Use of EW for Clinical Application
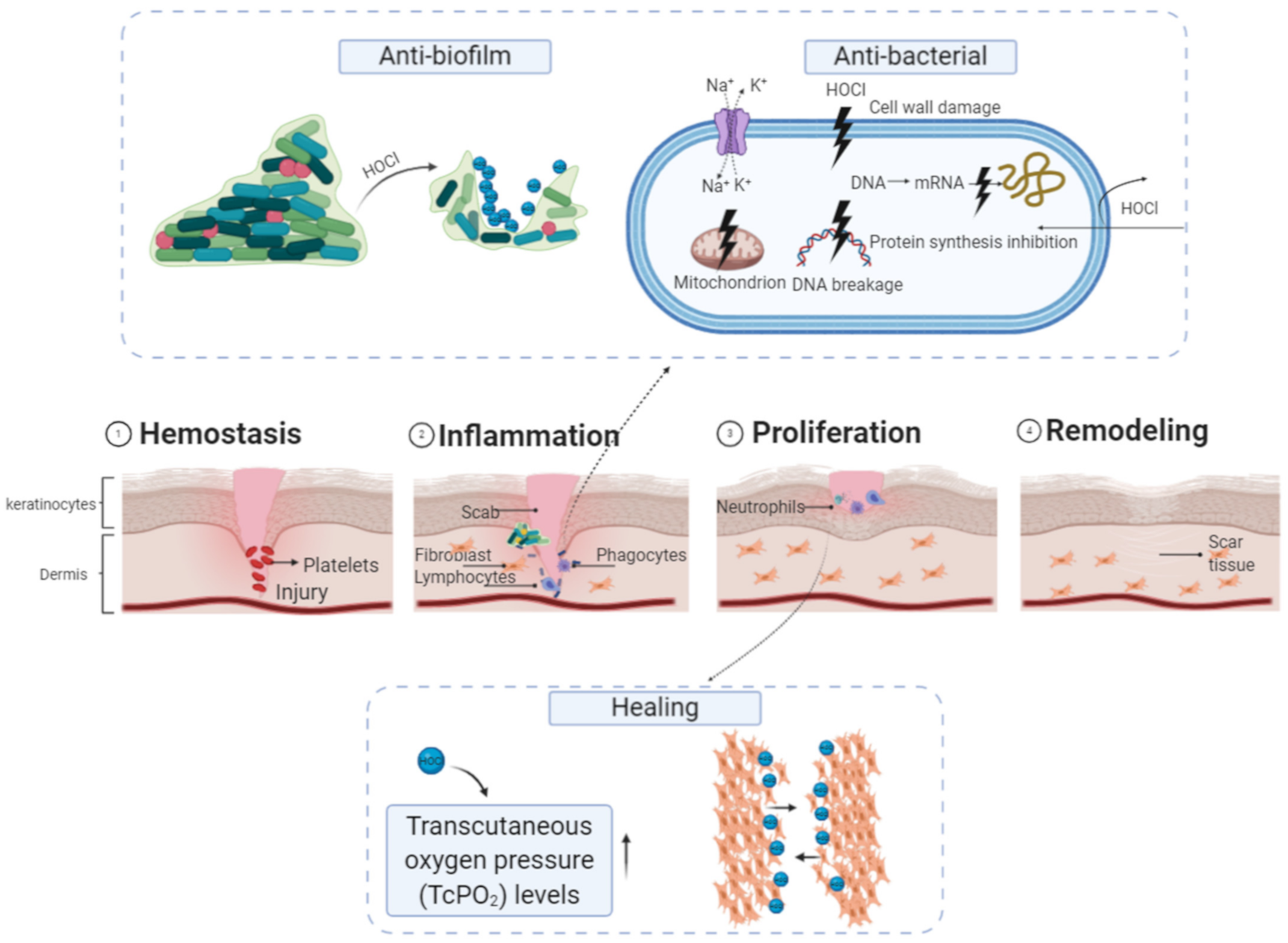
7.1. Wound Care
7.2. Hand Sanitizer
7.3. Oral Hygiene
7.4. Environmental Decontamination
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeshambel, A.; Endalamaw, A.; Belay, D.M.; Mekonen, D.K.; Birhan, B.M.; Bayih, W.A. Healthcare-associated infection and its determinants in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241073. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2019, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Savetamal, A. Infection in Elderly Burn Patients: What Do We Know? Surg. Infect. 2020, 21. [Google Scholar] [CrossRef]
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New insights on antimi-crobial efficacy of copper surfaces in the healthcare environment: A systematic review. Clin. Microbiol. Infec. 2018, 24, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Safaiefarahani, B. Indoor Damp Surfaces Harbor Molds with Clinical Significance. Curr. Med. Mycol. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Edmiston, C.E.; McBain, A.J.; Kiernan, M.; Leaper, D.J. A narrative review of microbial biofilm in postoperative surgical site infections: Clinical presentation and treatment. J. Wound Care 2016, 25, 693–702. [Google Scholar] [CrossRef]
- Iskandar, K.; Sartelli, M.; Tabbal, M.; Ansaloni, L.; Baiocchi, G.L.; Catena, F.; Coccolini, F.; Haque, M.; Labricciosa, F.M.; Moghabghab, A.; et al. Highlighting the gaps in quantifying the economic burden of surgical site infections associated with antimicrobial-resistant bacteria. World J. Emerg. Surg. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Stevens, D.L. Treatments for skin and soft-tissue and surgical site infections due to MDR Gram-positive bacteria. J. Infect. 2009, 59, S32–S39. [Google Scholar] [CrossRef]
- Gomila, A.; Carratalà, J.; Badia, J.M.; Camprubí, D.; Piriz, M.; Shaw, E.; Diaz-Brito, V.; Espejo, E.; Nicolas, C.; Brugués, M. Preoperative oral antibiotic prophylaxis reduces Pseudomonas aeruginosa surgical site infections after elective colorectal surgery: A multicenter prospective cohort study. BMC Infect. Dis. 2018, 18, 507. [Google Scholar] [CrossRef]
- Lim, S.L.; Ong, K.C.B.; Chan, Y.H.; Loke, W.C.; Ferguson, M.; Daniels, L. Malnutrition and its impact on cost of hos-pitalization, length of stay, readmission and 3-year mortality. Clin. Nutr. 2012, 31, 345–350. [Google Scholar] [CrossRef]
- Lingsma, H.F.; Bottle, A.; Middleton, S.; Kievit, J.; Steyerberg, E.W.; Marang-Van De Mheen, P.J. Evaluation of hospital outcomes: The relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv. Res. 2018, 18, 116. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Song, X.; Vossebein, L.; Zille, A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob. Resist. Infect. Control 2019, 8, 1–14. [Google Scholar] [CrossRef]
- Klemeš, J.J.; Fan, Y.V.; Jiang, P. The energy and environmental footprints of COVID-19 fighting measures-PPE, dis-infection, supply chains. Energy 2020, 211, 118701. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef]
- Stoica, M. Sustainable Sanitation in the Food Industry. In Sustainable Food Systems from Agriculture to Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–339. [Google Scholar]
- Sharma, A.; Das, P.; Buschmann, M.; Gilbert, J.A. The Future of Microbiome-Based Therapeutics in Clinical Applications. Clin. Pharmacol. Ther. 2020, 107, 123–128. [Google Scholar] [CrossRef]
- Goh, C.F.; Ming, L.C.; Wong, L.C. Dermatologic reactions to disinfectant use during the COVID-19 pandemic. Clin. Dermatol. 2020. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D.-H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Arya, R.; Bryant, M.; Degala, H.L.; Mahapatra, A.K.; Kannan, G. Effectiveness of a low-cost household electrolyzed water generator in reducing the populations of Escherichia coli K12 on inoculated beef, chevon, and pork surfaces. J. Food Process. Preserv. 2018, 42, e13636. [Google Scholar] [CrossRef]
- Veasey, S.; Muriana, P.M. Evaluation of electrolytically-generated hypochlorous acid (‘electrolyzed water’) for sanitation of meat and meat-contact surfaces. Foods 2016, 5, 42. [Google Scholar] [CrossRef]
- Graça, A.; Santo, D.; Quintas, C.; Nunes, C. Growth of Escherichia coli, Salmonella enterica and Listeria spp., and their inactivation using ultraviolet energy and electrolyzed water, on ‘Rocha’ fresh-cut pears. Food Control 2017, 77, 41–49. [Google Scholar] [CrossRef]
- Xuan, X.; Ling, J. Generation of Electrolyzed Water. In Electrolyzed Water in Food: Fundamentals and Applications; Springer Science and Business Media LLC: Cham, Switzerland, 2019; pp. 1–16. [Google Scholar]
- Takeda, Y.; Uchiumi, H.; Matsuda, S.; Ogawa, H. Acidic electrolyzed water potently inactivates SARS-CoV-2 de-pending on the amount of free available chlorine contacting with the virus. Biochem. Bioph. Res. Commun. 2020, 530, 1–3. [Google Scholar] [CrossRef]
- Izumi, H.; Inoue, A. Viability of sublethally injured coliform bacteria on fresh-cut cabbage stored in high CO2 atmospheres following rinsing with electrolyzed water. Int. J. Food Microbiol. 2018, 266, 207–212. [Google Scholar] [CrossRef]
- Hyun-Ji, K.; Tango, C.N.; Ramachandran, C.; Deog-Hwan, O. Sanitization Efficacy of Slightly Acidic Electrolyzed Water against pure cultures of Escherichia coli, Salmonella enterica, Typhimurium, Staphylococcus aureus and Bacillus cereus spores, in Comparison with Different Water Hardness. Sci. Rep. 2019, 9, 1–14. [Google Scholar]
- Lemos, J.G.; Stefanello, A.; Bernardi, A.O.; Garcia, M.V.; Magrini, L.N.; Cichoski, A.J.; Wagner, R.; Copetti, M.V. Antifungal efficacy of sanitizers and electrolyzed waters against toxigenic Aspergillus. Food Res. Int. 2020, 137, 109451. [Google Scholar] [CrossRef]
- Salisbury, A.-M.; Percival, S.L. The Efficacy of an Electrolysed Water Formulation on Biofilms. In Neurotransmitter Interactions and Cognitive Function; Springer Science and Business Media LLC: Cham, Switzerland, 2018; pp. 1–8. [Google Scholar]
- Eftekharizadeh, F.; Dehnavieh, R.; Hekmat, S.N.; Mehrolhassani, M.H. Health technology assessment on super oxidized water for treatment of chronic wounds. Med. J. Islamic Repub. Iran 2016, 30, 384. [Google Scholar]
- Samara, F.; Badran, R.; Dalibalta, S. Are Disinfectants for the Prevention and Control of COVID-19 Safe? Health Secur. 2020, 18, 496–498. [Google Scholar] [CrossRef]
- Supardi, E.; Yusuf, S.; Massi, M.N.; Haeruddin, H. Evaluation of different type of electrolyzed water against bacterial colonization of diabetic foot ulcers: Study in vitro. Med. Clínica Práctica 2020, 3, 100090. [Google Scholar] [CrossRef]
- Chittoria, R.K.; Yootla, M.; Sampatrao, L.; Raman, S.V. The role of super oxidized solution in the management of diabetic foot ulcer: Our experience. Nepal Med. Coll. J. 2007, 9, 125–128. [Google Scholar]
- Bongiovanni, C.M. Effects of Hypochlorous Acid Solutions on Venous Leg Ulcers (VLU): Experience With 1249 VLUs in 897 Patients. J. Am. Coll. Clin. Wound Spéc. 2016, 6, 32–37. [Google Scholar] [CrossRef]
- Thekdi, P.I.; Bathla, V.; Koradi, P.; Jhala, D.; Patel, D. A study on newer dressing materials versus conventional dressing materials in ulcer healing. Int. Surg. J. 2016, 3, 108–112. [Google Scholar] [CrossRef]
- Hopkins, J. Electrolyzed water treatment for feminine hygiene. Google Patents US20060275502A1, 7 December 2006. [Google Scholar]
- Morris, C.D.; Stone, J.K. Method for remediating mold and mildew using acidic electrolyzed water. U.S. Patent 7445800, 4 November 2008. [Google Scholar]
- Jo, H.-Y.; Tango, C.N.; Oh, D.-H. Influence of different organic materials on chlorine concentration and sanitization of slightly acidic electrolyzed water. LWT 2018, 92, 187–194. [Google Scholar] [CrossRef]
- Xuan, X.; Wang, M.; Ahn, J.; Ma, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Storage Stability of Slightly Acidic Electrolyzed Water and Circulating Electrolyzed Water and Their Property Changes after Application. J. Food Sci. 2016, 81, E610–E617. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.-H. Hurdle technology: A novel approach for enhanced food quality and safety-A review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Almås, G.H. Acetic acid and hypochlorous acid compositions for treatment of skin trauma. U.S. Patent Application 15/852603, 2018. [Google Scholar]
- Almås, G.H. Compositions and methods for treating biofilms without inducing antimicrobial resistance. U.S. Patent Application 16/672393, 2020. [Google Scholar]
- Oh, D.-H.; Khan, I.; Tango, C.N. Hurdle Enhancement of Electrolyzed Water with Other Techniques. In Electrolyzed Water in Food: Fundamentals and Applications; Springer Science and Business Media LLC: Cham, Switzerland, 2019; pp. 231–260. [Google Scholar]
- Zhiznin, S.; Timokhov, V.; Gusev, A. Economic aspects of nuclear and hydrogen energy in the world and Russia. Int. J. Hydrog. Energy 2020, 45, 31353–31366. [Google Scholar] [CrossRef]
- Forghani, F. Application of Electrolyzed Water in Agriculture. In Electrolyzed Water in Food: Fundamentals and Applications; Springer Science and Business Media LLC: Cham, Switzerland, 2019; pp. 223–230. [Google Scholar]
- Zheng, W.; Li, Z.; Shah, S.B.; Li, B. Removal of ammonia and airborne culturable bacteria by proof-of-concept wind-break wall with slightly acidic electrolyzed water spray for a layer breeding house. Appl. Eng. Agric. 2016, 32, 393–399. [Google Scholar]
- Graça, A.; Santo, D.; Pires-Cabral, P.; Quintas, C. The effect of UV-C and electrolyzed water on yeasts on fresh-cut apple at 4 °C. J. Food Eng. 2020, 282, 110034. [Google Scholar] [CrossRef]
- Hakim, H.; Alam, S.; Sangsriratanakul, N.; Nakajima, K.; Kitazawa, M.; Ota, M.; Toyofuku, C.; Yamada, M.; Thammakarn, C.; Shoham, D.; et al. Inactivation of bacteria on surfaces by sprayed slightly acidic hypochlorous acid water: In vitro experiments. J. Vet. Med. Sci. 2016, 78, 1123–1128. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Applications of Electrolyzed Water in Agriculture & Food Industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar] [CrossRef]
- Shiroodi, S.G.; Ovissipour, M. Electrolyzed Water Application in Fresh Produce Sanitation. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–89. [Google Scholar]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Environmental Assessment for Food Contact Notification FCN 1811; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2017. Available online: https://www.fda.gov/food/environmental-decisions/environmental-decision-memo-food-contact-notification-no-1811 (accessed on 1 October 2017).
- Rahman, S.; Ding, T.; Oh, D.-H. Effectiveness of low concentration electrolyzed water to inactivate foodborne pathogens under different environmental conditions. Int. J. Food Microbiol. 2010, 139, 147–153. [Google Scholar] [CrossRef]
- Ding, T.; Oh, D.-H.; Liu, D. Electrolyzed Water in Food: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Naito, Y.; Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas. Res. 2018, 8, 6–11. [Google Scholar] [CrossRef]
- Kitaori, N.; Uno, M.; Nishiki, Y.; Furuta, T. Method of sterilization and electrolytic water ejecting apparatus. U.S. Patent 7,887, 679, 15 February 2011. [Google Scholar]
- Kim, E.S. Electrolysis apparatus capable of producing disinfectant or cleaning agent, and electrolysis method therefor. U.S. Pat. Appl. 16/569, 153, 2020. [Google Scholar]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. LWT 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Zheng, W.; Xie, C.; Liang, J.; Yu, Q.-D.; Bai, D.; Huang, J. Effects of weak acidic electrolytic water ice and modified packaging on shrimp quality of Litopenaeus vannamei. Sci. Technol. Food Ind. 2018, 34. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-REEF201704010.htm (accessed on 1 November 2020).
- Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Pa-ez-Esquiliano, D.; Andrade-Esquivel, E.; Cano-Buendia, J.A. The effect of neutral electrolyzed water as a disinfectant of eggshells artificially contaminated with Listeria monocytogenes. Food Sci. Nutr. 2019, 7, 2252–2260. [Google Scholar] [CrossRef]
- Naka, A.; Yakubo, M.; Nakamura, K.; Kurahashi, M. Effectiveness of slightly acidic electrolyzed water on bacteria reduction: In vitro and spray evaluation. PeerJ 2020, 8, e8593. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Zhao, Z.; Liu, W.; Chen, Y.; Yang, G.; Xia, X.; Cao, Y. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout. Food Control 2019, 104, 83–90. [Google Scholar] [CrossRef]
- Mansur, A.R.; Oh, D.-H. Modeling the Growth of Epiphytic Bacteria on Kale Treated by Thermosonication Combined with Slightly Acidic Electrolyzed Water and Stored under Dynamic Temperature Conditions. J. Food Sci. 2016, 81, M2021–M2030. [Google Scholar] [CrossRef]
- Bansal, V.; Prasad, P.; Mehta, D.; Siddiqui, M.W. Ultrasound Techniques in Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–177. [Google Scholar]
- Zhang, J.; Yang, H.; Chan, J.Z.Y. Development of Portable Flow-Through Electrochemical Sanitizing Unit to Generate Near Neutral Electrolyzed Water. J. Food Sci. 2018, 83, 780–790. [Google Scholar] [CrossRef]
- Xuan, X.T.; Fan, Y.F.; Ling, J.G.; Hu, Y.; Liu, D.H.; Chen, S.G.; Ye, X.Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Da Cruz Nizer, W.S.; Inkovskiy, V.; Overhage, J. Surviving reactive chlorine stress: Responses of gram-negative bacteria to hypochlorous acid. Microorganisms 2020, 8, 1220. [Google Scholar] [CrossRef]
- Ding, T.; Xuan, X.T.; Li, J.; Chen, S.; Liu, D.H.; Ye, X.; Shi, J.; Xue, S.J. Disinfection efficacy and mechanism of slightly acidic electrolyzed water on Staphylococcus aureus in pure culture. Food Control 2016, 60, 505–510. [Google Scholar] [CrossRef]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parametersin vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Simic, N.; Ahlberg, E. Exploring the mechanism of hypochlorous acid decomposition in aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 19342–19348. [Google Scholar] [CrossRef]
- Hung, Y.C.; Waters, B.W.; Yemmireddy, V.K.; Huang, C.H. pH effect on the formation of THM and HAA disinfection byproducts and potential control strategies for food processing. J. Integr. Agric. 2017, 16, 2914–2923. [Google Scholar] [CrossRef]
- Sam, C.H.; Lu, H.K. The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J. Dent. Sci. 2009, 4, 45–54. [Google Scholar] [CrossRef]
- Medina-Gudiño, J.; Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Andrade-Esquivel, E.; Cano-Buendia, J.A. Analysis of Neutral Electrolyzed Water anti-bacterial activity on contaminated eggshells with Salmonella enterica or Escherichia coli. Int. J. Food Microbiol. 2020, 320, 108538. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Hsu, S.-Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Hsu, S.-Y. Effects of water flow rate, salt concentration and water temperature on efficiency of an electrolyzed oxidizing water generator. J. Food Eng. 2003, 60, 469–473. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food in-dustry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F.; Tarlak, F.; García-Gimeno, R.M. Quantifying and modelling the inactivation of Listeria monocytogenes by electrolyzed water on food contact surfaces. J. Food Eng. 2021, 290, 110287. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Lee, S.W.H.; Robinson, G.M.; Greenman, J.; Reynolds, D.M. Electrochemically activated solutions: Evidence for antimicrobial efficacy and applications in healthcare environments. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 641–653. [Google Scholar] [CrossRef]
- Al-Qadiri, H.M.; Smith, S.; Sielaff, A.C.; Govindan, B.N.; Ziyaina, M.; Al-Alami, N.; Rasco, B. Bactericidal activity of neutral electrolyzed water against Bacillus cereus and Clostridium perfringens in cell suspensions and artificially inoculated onto the surface of selected fresh produce and polypropylene cutting boards. Food Control 2019, 96, 212–218. [Google Scholar] [CrossRef]
- Dragana, O.; Richard, K.A.; Holdsworth, S.R. Neutrophil-mediated regulation of innate and adaptive immunity: The role of myeloperoxidase. J. Immunol. Res. 2016, 6, 1–11. [Google Scholar]
- Hricova, D.; Stephan, R.; Zweifel, C. Electrolyzed water and its application in the food industry. J. Food Protect. 2008, 71, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Dewi, F.R.; Stanley, R.; Powell, S.M.; Burke, C.M. Application of electrolysed oxidising water as a sanitiser to extend the shelf-life of seafood products: A review. J. Food Sci. Technol. 2017, 54, 1321–1332. [Google Scholar] [CrossRef]
- Park, H.; Puligundla, P.; Mok, C. Microbial Decontamination of Mung Bean Sprouts Using Electrolyzed Water and Its Effects on The Physicochemical and Sensory Properties of The Sprouts. Chiang Mai J. Sci. 2020, 47, 28–38. [Google Scholar]
- Gómez-López, V.M.; Gil, M.I.; Allende, A. A novel electrochemical device as a disinfection system to maintain water quality during washing of ready to eat fresh produce. Food Control 2017, 71, 242–247. [Google Scholar] [CrossRef]
- Block, Z.; Eyles, A.; Corkrey, R.; Stanley, R.; Ross, T.; Kocharunchitt, C. Effect of Storage Conditions on Shelf Stability of Undiluted Neutral Electrolyzed Water. J. Food Protect. 2020, 83, 1838–1843. [Google Scholar] [CrossRef]
- Feliziani, E.; Lichter, A.; Smilanick, J.L.; Ippolito, A. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol. Technol. 2016, 122, 53–69. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, K.; Bai, L.; Minakata, D.; Seo, Y.; Göktaş, R.K.; Dionysiou, D.D.; Tang, C.-J.; Wei, Z.; Spinney, R. Inactivation of pathogenic microorganisms by sulfate radical: Present and future. Chem. Eng. J. 2019, 371, 222–232. [Google Scholar] [CrossRef]
- Akbulut, M.B.; Eldeniz, A.U. In vitro antimicrobial activity of different electrochemically-activated solutions on enterococcus faecalis. Eur. Oral Res. 2019, 53, 44. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Block, M.S.; Rowan, B.G. Hypochlorous acid–a review. J. Oral Maxil. Surg. 2020, 78, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeng, X.; Zhao, Y.; Ye, G.; Gui, W.; Ni, Y. Disinfection effect and its mechanism of electrolyzed oxidizing water on spores of Bacillus subtilis var. niger. Food Sci. Biotechnol. 2011, 20, 889–895. [Google Scholar] [CrossRef]
- Zhao, L. Electrolysed Water Combined With Levulinic Acid and Ultrasound for Sanitisation and Its Antimicrobial Mechanism. Master’s Thesis, National University of Singapore, Singapore, 2017. [Google Scholar]
- Nybo, T.; Dieterich, S.; Gamon, L.F.; Chuang, C.Y.; Hammer, A.; Hoefler, G.; Malle, E.; Rogowska-Wrzesinska, A.; Davies, M.J. Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol. 2019, 20, 496–513. [Google Scholar] [CrossRef]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576. [Google Scholar] [CrossRef]
- National Criterion of China. Hygienic Requirement for Disinfectants with Chlorine GB/T 36758-2018; National Criterion of China. Available online: http://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=CFCB58954F3CA1C01908B0407FF97D8E (accessed on 1 April 2019).
- National Criterion of China. Genaral Requirements for Disfectant of Mucous Membrane GB/T 27954-2020; National Criterion of China. Available online: http://std.samr.gov.cn/gb/search/gbDetailed?id=A327FCEA3049B9B7E05397BE0A0A8A97 (accessed on 1 November 2020).
- Fukuyama, T.; Martel, B.C.; Linder, K.E.; Ehling, S.; Ganchingco, J.R.; Bäumer, W. Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin. Exp. Allergy 2017, 48, 78–88. [Google Scholar] [CrossRef]
- Alimi, H. Method of using oxidative reductive potential water solution in dental applications. U.S. Patent 9498548, 22 November 2016. [Google Scholar]
- Arai, N.; Hayashi, N. Washing machine, electrolyte for generating electrolyzed water, and electrolyzed water for rinse. U.S. Patent Application 15/511754, 2017. [Google Scholar]
- Garcia, J.P.; Michel, B.A.P.; Moctezuma, M.V.; Enciso, I.D. Neutral Electrolyzed Water and Uses Thereof. U.S. Patent Application 15/791927, 2018. [Google Scholar]
- Ministry of Health, Labour and Welfare. Advanced Acid Electrolyzed Water Generator; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2005. [Google Scholar]
- Ministry of Health, Labour and Welfare. Guidelines for Standardization of Cleaning and Disinfection of Digestive Endoscopy; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2018. [Google Scholar]
- Ministry of Health, Labour and Welfare. The Contents of the Consideration (Draft) Which Should be Provided with Regard to the Material Specified as a Specified Agricultural Chemical (Specified Control Material); Ministry of Health, Labour and Welfare: Tokyo, Japan, 2003. [Google Scholar]
- European commission. Expert Group for Technical Advice on Organic Production; European Commission: Brussels, Belgium, 2016; Available online: http://www.envirolyte.com/Expert-Group.pdf (accessed on 1 November 2020).
- Herruzo, R.; Herruzo, I. Antimicrobial efficacy of a very stable hypochlorous acid formula compared with other anti-septics used in treating wounds: In-vitro study on micro-organisms with or without biofilm. J. Hosp. Infect. 2020, 105, 289–294. [Google Scholar] [CrossRef]
- Stroman, D.W.; Mintun, K.; Epstein, A.B.; Brimer, C.M.; Patel, C.R.; Branch, J.D.; Najafi-Tagol, K. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin. Ophthalmol. 2017, 11, 707. [Google Scholar] [CrossRef]
- Hiebert, J.M.; Robson, M.C. The Immediate and Delayed Post-Debridement Effects on Tissue Bacterial Wound Counts of Hypochlorous Acid Versus Saline Irrigation in Chronic Wounds. Eplasty 2016, 16, e32. [Google Scholar] [PubMed]
- Sasai-Takedatsu, M.; Kojima, T.; Yamamoto, A.; Hattori, K.; Yoshijima, S.; Taniuchi, S.; Namura, S.; Akamatsu, H.; Horio, T.; Kobayashi, Y. Reduction of Staphylococcus aureus in atopic skin lesions with acid electrolytic water-a new therapeutic strategy for atopic dermatitis. Allergy 1997, 52, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- You, H.S.; Fadriquela, A.; Sajo, M.E.J.; Bajgai, J.; Ara, J.; Kim, C.S.; Kim, S.-K.; Oh, J.R.; Shim, K.Y.; Lim, H.K. Wound healing effect of slightly acidic electrolyzed water on cutaneous wounds in hairless mice via immune-redox modulation. Biol. Pharm. Bull. 2017, 40, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Sato, Y.; Ishihara, M.; Takayama, T.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H.; Kiyosawa, T. Healing of Pseudomonas aeruginosa-infected wounds in diabetic db/db mice by weakly acidic hypochlorous acid cleansing and silver nanoparticle/chitin-nanofiber sheet covering. Wound Med. 2020, 28, 100183. [Google Scholar] [CrossRef]
- Chen, K.K.; Wu, J.H.; Wei, S.I.; Du, J.K. Influence of the acidity of electrolyzed water on the microhardness of inner layer dentin. J. Dent. Sci. 2019, 14, 419–425. [Google Scholar] [CrossRef]
- Kiamco, M.M.; Zmuda, H.M.; Mohamed, A.; Call, D.R.; Raval, Y.S.; Patel, R.; Beyenal, H. Hypochlorous-Acid-Generating Electrochemical Scaffold for Treatment of Wound Biofilms. Sci. Rep. 2019, 9, 2683. [Google Scholar] [CrossRef]
- Johani, K.; Malone, M.; Jensen, S.O.; Dickson, H.G.; Gosbell, I.B.; Hu, H.; Yang, Q.; Schultz, G.; Vickery, K. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: From in vitro to in vivo. J. Antimicrob. Chemother. 2018, 73, 494–502. [Google Scholar] [CrossRef]
- Reis, R.; Sipahi, H.; Dinc, O.; Kavaz, T.; Charehsaz, M.; Dimoglo, A.; Aydın, A. Toxicity, mutagenicity and stability assessment of simply produced electrolyzed water as a wound healing agent in vitro. Hum. Exp. Toxicol. 2020. [Google Scholar] [CrossRef]
- Vahabi, S.; Shokri, M.; Lazar, M. Effects of Electrolyzed Water on the Growth of Oral Pathologic Bacteria Species and its Cytotoxic Effects on Fibroblast and Epithelial Cells at Different pH Values. Iran. J. Basic Med. Sci. 2020, 45, 277–285. [Google Scholar]
- Ishiyama, K.; Nakamura, K.; Kanno, T.; Niwano, Y. Bactericidal Action of Photodynamic Antimicrobial Chemotherapy (PACT) with Photosensitizers Used as Plaque-Disclosing Agents against Experimental Biofilm. Biocontrol. Sci. 2016, 21, 187–191. [Google Scholar] [CrossRef][Green Version]
- Kubota, A.; Goda, T.; Tsuru, T.; Yonekura, T.; Yagi, M.; Kawahara, H.; Yoneda, A.; Tazuke, Y.; Tani, G.; Ishii, T. Efficacy and safety of strong acid electrolyzed water for peritoneal lavage to prevent surgical site infection in patients with perforated appendicitis. Surg. Today 2015, 45, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, C.C.; Ding, S.J. Effectiveness of hypochlorous acid to reduce the biofilms on titanium alloy sur-faces in vitro. Int. J. Mol. Sci. 2016, 17, 1161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, B.K. Antibacterial effect of electrolyzed water on oral bacteria. J. Microbiol. 2006, 44, 417–422. [Google Scholar] [PubMed]
- Xin, P.J.; Huang, N.; Sun, H.H.; Wang, J.Q.; Su, J. Research for continuous disinfection on the dental unit water lines. Chin. J. Disinfect. 2017, 34, 422–425. [Google Scholar]
- Galvin, S.; Boyle, M.; Russell, R.; Coleman, D.; Creamer, E.; O’Gara, J.P.; Fitzgerald-Hughes, D.; Humphreys, H. Evaluation of vaporized hydrogen peroxide, Citrox and pH neutral Ecasol for decontamination of an enclosed area: A pilot study. J. Hosp. Infect. 2012, 80, 67–70. [Google Scholar] [CrossRef]
- Kim, S.B. Development of a mouthwash alternative using a low-level hypochlorous acid solution with macroporous platinum electrodes and its application to oral health. Int. J. Clin. Exp. Med. 2016, 9, 21304–21311. [Google Scholar]
- Park, G.W.; Boston, D.M.; Kase, J.A.; Sampson, M.N.; Sobsey, M.D. Evaluation of Liquid- and Fog-Based Application of Sterilox Hypochlorous Acid Solution for Surface Inactivation of Human Norovirus. Appl. Environ. Microbiol. 2007, 73, 4463–4468. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Joachim, D. Wound cleansing: Benefits of hypochlorous acid. J. Wound Care 2020, 29, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nakhi, M.E.; Eltayeb, H.I. First Middle East Experience with Novel Foam Dressing Together with Negative Pres-sure Wound Therapy and Instillation. Cureus 2018, 10, e3415. [Google Scholar] [PubMed]
- Tirado-Sánchez, A.; Ponce-Olivera, R.M. Efficacy and tolerance of superoxidized solution in the treatment of mild to moderate inflammatory acne. A double-blinded, placebo- controlled, parallel-group, randomized, clinical trial. J. Dermatol. Treat. 2009, 20, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H.; Andriessen, A.; Bhatia, A.C.; Bitter, P., Jr.; Chilukuri, S.; Cohen, J.L.; Robb, C.W. Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures. J. Cosmet. Dermatol. 2020, 19, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.L.J.; Yi, T.P.; Bose, R.J.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, H.; Reis, R.; Dinc, O.; Kavaz, T.; Dimoglo, A.; Aydın, A. In vitro biocompatibility study approaches to evaluate the safety profile of electrolyzed water for skin and eye. Hum. Exp. Toxicol. 2019, 38, 1314–1326. [Google Scholar] [CrossRef]
- Ding, T.; Ge, Z.; Shi, J.; Xu, Y.-T.; Jones, C.L.; Liu, D.-H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT Food Sci. Technol. 2015, 60, 1195–1199. [Google Scholar] [CrossRef]
- Almås, G.H.; Bignami, A. Hypochlorous acid preparation with organic acids. U.S. Patent 10029917, 24 July 2018. [Google Scholar]
- Dodoo, C.C.; Stapleton, P.; Basit, A.W.; Gaisford, S. The potential of Streptococcus salivarius oral films in the management of dental caries: An inkjet printing approach. Int. J. Pharmaceut. 2020, 591, 119962. [Google Scholar] [CrossRef]
- Lin, Y.T.J.; Chou, C.C.; Hsu, C.Y.S. Effects of Lactobacillus casei Shirota intake on caries risk in children. J. Dent. Sci. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Pormohammad, A.; Eslami, H.; Shokouhi, B.; Fakhrzadeh, V.; Kafil, H.S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. 2017, 113, 303–311. [Google Scholar] [CrossRef]
- Ab Malik, N.; Razak, F.A.; Lam, O.L.T.; Jin, L.; Li, L.S.; McGrath, C. Oral Health Interventions Using Chlorhexidine-Effects on the Prevalence of Oral Opportunistic Pathogens in Stroke Survivors: A Randomized Clinical Trial. J. Evid. Based Dent. Pract. 2018, 18, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.L.; Yao, J.C.; Hsieh, S.C.; Teng, N.C.; Chu, Y.T.; Yu, W.X.; Chen, C.H.; Chang, L.Y.; Huang, C.S.; Lee, T.H.; et al. The In Vivo Toxicity and Antimicrobial Properties for Electrolyzed Oxidizing (EO) Water-Based Mouthwashes. Materials 2020, 13, 4299. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fedele, G.; Doğramacı, E.J.; Steier, L.; De Figueiredo, J.A.P. Some factors influencing the stability of Sterilox®, a superoxidised water. Br. Dent. J. 2011, 210, E23. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Mohanty, S.K.; Pradhan, P.K.; Patri, G.; Sinha, S.P.; Agrawal, P. Anti bacterial effectiveness of electro-chemically activated (ECA) water as a root canal irrigant-An in-vitro comparative study. J. Clin. Diagn. Res. 2016, 10, ZC138. [Google Scholar] [CrossRef]
- Nakano, M.; Takao, A.; Maeda, N.; Hosoya, N. Efficacy of Slightly Acidic Electrolyzed Water against Contamination of Water Line of Dental Units. Nippon Eiseigaku Zasshi (Jpn. J. Hyg.) 2020, 75, 19021. [Google Scholar] [CrossRef]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Ni, L.; Zheng, W.; Zhang, Q.; Cao, W.; Li, B. Application of slightly acidic electrolyzed water for decontamination of stainless steel surfaces in animal transport vehicles. Prev. Vet. Med. 2016, 133, 42–51. [Google Scholar] [CrossRef]
- Stewart, M.; Bogusz, A.; Hunter, J.; Devanny, I.; Yip, B.; Reid, D.; Robertson, C.; Dancer, S.J. Evaluating Use of Neutral Electrolyzed Water for Cleaning Near-Patient Surfaces. Infect. Control Hosp. Epidemiol. 2014, 35, 1505–1510. [Google Scholar] [CrossRef]
- Meakin, N.; Bowman, C.; Lewis, M.; Dancer, S. Comparison of cleaning efficacy between in-use disinfectant and electrolysed water in an English residential care home. J. Hosp. Infect. 2012, 80, 122–127. [Google Scholar] [CrossRef]
- Tango, C.N.; Hussain, M.S.; Oh, D.-H. Application of Electrolyzed Water on Environment Sterilization. In Electrolyzed Water in Food: Fundamentals and Applications; Springer Science and Business Media LLC: Cham, Switzerland, 2019; pp. 177–204. [Google Scholar]
- Hao, X.; Shen, Z.; Wang, J.; Zhang, Q.; Li, B.; Wang, C.; Cao, W. In vitro inactivation of porcine reproductive and respiratory syndrome virus and pseudorabies virus by slightly acidic electrolyzed water. Vet. J. 2013, 197, 297–301. [Google Scholar] [CrossRef]
- Bui, V.N.; Nguyen, K.V.; Pham, N.T.; Bui, A.N.; Imai, K. Potential of electrolyzed water for disinfection of foot-and-mouth disease virus. J. Vet. Med. Sci. 2017, 79, 726–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakim, H.; Thammakarn, C.; Suguro, A.; Ishida, Y.; Nakajima, K.; Kitazawa, M.; Takehara, K. Aerosol Disinfection Capacity of Slightly Acidic Hypochlorous Acid Water Towards Newcastle Disease Virus in the Air: An In Vivo Experiment. Avian. Dis. 2015, 59, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19′s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Esua, O.J.; Cheng, J.H.; Sun, D.W. Functionalization of water as a nonthermal approach for ensuring safety and quality of meat and seafood products. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–19. [Google Scholar]

| Japan [101,102,103] | The United States [51] | EU [104] | China [95,96] | |
|---|---|---|---|---|
| Administration | Ministry of Health, Labor, and Welfare | Administration of US Food and Drug | European Commission Directorate-General for Agriculture and Rural Development | Standardization administration |
| Application | Strong acid electrolyzed water (pH < 2.7): 20–60 ppm: hand washing in operation, cleaning and disinfection of endoscope and food additives. | Poultry Processing Facilities | Buildings and installations Aquaculture (only in the absence of animals) | Indoor air environment General object surface Medical equipment Surface of secondary water supply equipment and facilities |
| Slightly acid electrolyzed water (2.7–5.0): 10–60 ppm: food additives and designation of specified pesticides (specific control materials) | Meat Processing | In general agriculture and in organic farming Plant and animal production Food processing | Vegetables and fruits | |
| Slightly acid electrolyzed water (ph:5.0–6.0): 10–80 ppm: food additives | Fruit and Vegetable Processing Facilities | Fabric | ||
| Fish and Seafood Processing | Utensils | |||
| Processed and Preformed Meat and Poultry | Hands | |||
| Shell Egg Wash Organic Production and Handling | Skin and mucous membrane | |||
| ACC concentration | Strong acid electrolyzed water (ph < 2.7): 20–60 ppm Slightly acid electrolyzed water (2.7–5.0): 10–60 ppm Slightly acid electrolyzed water (pH:5.0–6.0): 10–80 ppm | <60 ppm Organic production and Handling(≤4 ppm) | Electrolyzed water usually contains 20–60 ppm (hypochlorite and hypochlorous acid, in a pH-dependent equilibrium). | Requirement of different application of toxicity |
| Requirement | Electrolyzed water must be decomposed or removed before completion of the final food | The treatment will be followed by either a 10 min drain step or a potable water rinse to remove | Non toxicity |
| Type of EW | Diaphragm Electrolyzer | Electrolyte | pH | ORP (mV) | ACC |
|---|---|---|---|---|---|
| Acidic electrolyzed water/electrolyzed oxidizing water | Two-cell chambers /anode Three-cell chambers/anode | NaCl water (<0.2%) | 2–2.7 | >1100 | 20–60 |
| Weak acid electrolyzed water | Two-cell chambers Three-cell chambers | NaCl water (<0.2%) | 2.7–5.0 | - | 10–60 |
| Slightly acid electrolyzed water | Single-cell chamber (without diaphragm) | HCl water (2–6%)/ The mixture water of NaCl and HCl | 5–6.5 | 850 | 10–80 |
| Neutralized electrolyzed water | Single-cell unit (without diaphragm) | NaCl or HCl | 7–8 | 750–900 | 30–200 |
| Alkaline electrolyzed water | Two-cell chambers /cathode | NaCl water | 10–13 | −800–900 | 80–100 |
| Application | Target | EW Type (Product) | Exposure Time | Observations (log CFU) | ACC | pH | ORP (Mv) | Reference |
|---|---|---|---|---|---|---|---|---|
| Wound | These comprised three Gram-positive bacteria (Enterococcus faecium; S. epidermidis and S. aureus); three Gram-negative bacteria (Morganella morganii; Enterobacter cloacae and P. aeruginosa) and two yeasts (Candida albicans and Torulopsis glabrata). | EW Clortech® | 5 | 4.57 log CFU/cm2 | 500 | - | - | [105] |
| Eye | S. epidermidis colony-forming units | EW Avenova® | 20 | >99.5% | 100 | 4 | - | [106] |
| Wound | X Pseudomonas Staphylococcus aureus | Slightly acid electrolyzed water (SAEW) Vashe Wound Solution | - | 3.78 log/g 4.44 log/g | - | 5.5 | - | [107] |
| Atopic dermatitis on skin | Staphylococcus aureus | Acidic electrolyzed water (AEW) | 3 min after spraying (P < 0.05) and after 1 week of skin treatment | 3.80 log/cm2 | - | ≤2.7 | 1000≥ | [108] |
| Wound healing | Hairless mice (wound size) | Slightly acid electrolyzed water (SAEW) | Hairless mice three times a day for seven days | Wound size reduced to 22.4% | 25 | 5.5–6.5 | 800 | [109] |
| Wound healing | Pseudomonas aeruginosa-infected wounds | Weakly acidic hypochlorous acid | Cleansing effects of HOCl and covering with CNFS/Ag NP composites daily for 3 days | Wound size reduced to 23% | 200 | 6.5 | - | [110] |
| Inner layer dentin | The time dependent microhardness values at 25 μm depth | AEW | 15 min | 75% decrease | 49 | 2.4 | - | [111] |
| Wound biofilms | S. aureus biofilms A. baumannii biofilms P. aeruginosa biofilms | EW | 180 120 60 | 100% 100% 100% | 892 524 367 | 6.0 | - | [112] |
| Wound biofilm | Staphylococcus aureus biofilm in vitro Pseudomonas aeruginosa biofilm in vitro Pseudomonas aeruginosa biofilm in an ex vivo porcine skin explant model | Microcyn® | 15 | 4.3 log10 CFU/mL reduction 7 log10 CFU/mL reduction 0.77 log10 CFU /mL reduction | - | - | - | [113] |
| Atopic dermatitis | NC/Nga mouse model of Atopic dermatitis | EW | Twice a day | less skin lesions prevent scratching bouts nontoxicity | 500 | 6.0 | - | [97] |
| Wound healing | Cytotoxicity in L929 mice fibroblast cells Wound healing activity | Strong acid electrolyzed water (StAEW) | Scratch assay | 88.84% wound healing ratio No mutagenic activity | 32.87 | 2.4 | 1140.67 | [114] |
| Oral Pathologic Bacteria Species | A. actinomycetemcomitans S. salivarius L. casei S. aureus | AEW | 0.5 | 100% 99.92% 99.99% 98.04% | - | 3 | - | [115] |
| Dental plaque (biofilm) | Streptococcus mutans biofilm | SIEW | 3 log reduction CFU/cm2 | 5 | 11.4–11.7 | −868 | [116] | |
| Ascetic fluid | Surgical site infection including Escherichia coli, Bacteroides fragilis, γ-hemolytic Streptococcus) | StAEW | - | No one infection in 24 patients | 40 | 2.5–2.7 | 1000–11000 | [117] |
| Titanium alloy surfaces | E. coli P. gingivalis E. faecalis S. sanguinis | EW | 1.5 | 100% 100% 100% 100% | 180 | 5.5 | - | [118] |
| Toothbrushes | A.actinomycetemcomitans F. nucleatum P. intermedia P. gingivalis | EW | 0.5 | 11.0–12.4% | 30 | 8.4 | - | [119] |
| Oral comprehensive treatment table | Pseudomonas aeruginosa and Legionella pneumophila | SAEW | Flush the oral comprehensive treatment table | 4.30 log/mL | 10 | 5.5–6.5 | 982 | [120] |
| Floor, table, mattress, sheet, blanket, curtain | Escherichia coli Staphylococcus aureus Enterococcus faecalis Pseudomonas aeruginosa Aspergillus fumigatus Acinetobacter baumannii Clostridium difficile | Ecasol™ | 1.5 h | ≥7 log/cm2 | 1000 | Ph neutral | - | [121] |
| Oral bacteria strains | Porphyromonas gingivalis Prevotella intermedia Prevotella nigrescens Fusobacterium nucleatum Streptococcus mutans Streptococcus sobrinus Streptococcus gordonii Streptococcus oralis Streptococcus salivarius | SAEW | 1 | ≥99.999% ≥99.999% ≥99.9999% ≥99.9999% ≥99.9999% ≥99.999% ≥99.99% ≥99.99999% ≥99.9999% | 3–5 | 5–7 | - | [122] |
| Porous | Noroviruses | EW | 10 | 3 log/cm2 | 200 | 5.5–6.2 | - | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, P.; Daliri, E.B.-M.; Oh, D.-H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. https://doi.org/10.3390/microorganisms9010136
Yan P, Daliri EB-M, Oh D-H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms. 2021; 9(1):136. https://doi.org/10.3390/microorganisms9010136
Chicago/Turabian StyleYan, Pianpian, Eric Banan-Mwine Daliri, and Deog-Hwan Oh. 2021. "New Clinical Applications of Electrolyzed Water: A Review" Microorganisms 9, no. 1: 136. https://doi.org/10.3390/microorganisms9010136
APA StyleYan, P., Daliri, E. B.-M., & Oh, D.-H. (2021). New Clinical Applications of Electrolyzed Water: A Review. Microorganisms, 9(1), 136. https://doi.org/10.3390/microorganisms9010136







