First Cases of Natural Infections with Borrelia hispanica in Two Dogs and a Cat from Europe
Abstract
:1. Background
2. Case Reports
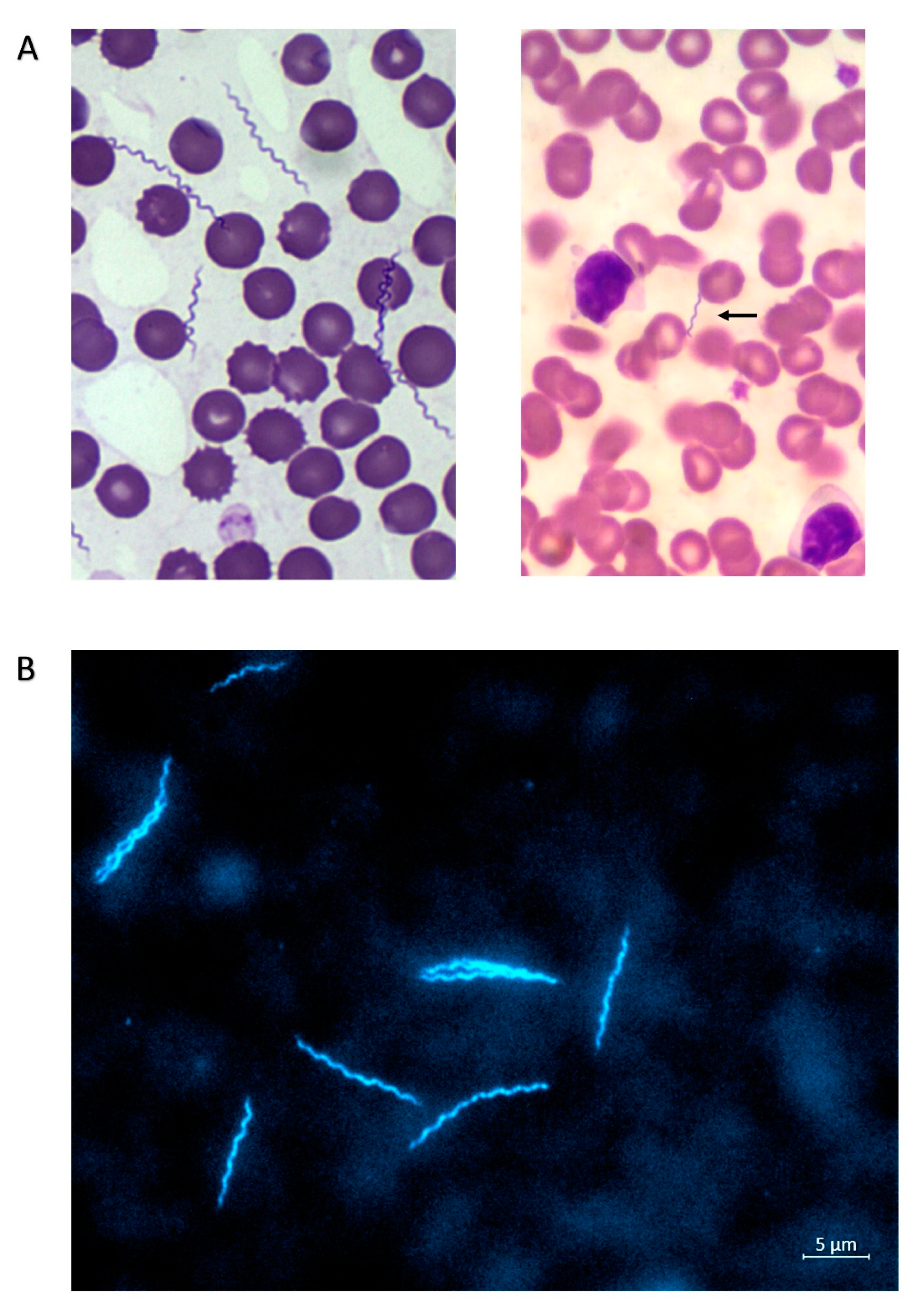
2.1. Canine Case 1 (Dog 1; Sample ID VM531519)
2.2. Canine Case 2 (Dog 2; Sample ID VM736940)
2.3. Feline Case (Cat; Sample ID 10827448)
3. Methods
3.1. Samples Included in Our Study
3.2. Serology IDEXX Ludwigsburg, Germany
3.3. PCRs IDEXX Ludwigsburg, Germany
3.4. In Vitro Culture from Cat Blood at NRZ Borrelia, Oberschleißheim, Germany
3.5. DNA Extraction and PCR (NRZ Borrelia, Oberschleißheim)
3.6. Molecular Analyses
3.7. Sequence Deposition
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gofton, A.; Margos, G.; Fingerle, V.; Hepner, S.; Loh, S.-M.; Ryan, U.M.; Irwin, P.; Oskam, C.L. Genome-wide analysis of Borrelia turcica and ‘Candidatus Borrelia tachyglossi’ shows relapsing fever-like genomes with unique genomic links to Lyme disease Borrelia. Infect. Genet. Evol. 2018, 66, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.-M.; Gillett, A.; Ryan, U.; Irwin, P.; Oskam, C.L. Molecular characterization of ‘Candidatus Borrelia tachyglossi’ (family Spirochaetaceae) in echidna ticks, Bothriocroton concolor. Int. J. Syst. Evol. Microbiol. 2017, 67, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantchev, N.; Pluta, S.; Huisinga, E.; Nather, S.; Scheufelen, M.; Vrhovec, M.G.; Schweinitz, A.; Hampel, H.; Straubinger, R. Tick-borne Diseases (Borreliosis, Anaplasmosis, Babesiosis) in German and Austrian Dogs: Status quo and Review of Distribution, Transmission, Clinical Findings, Diagnostics and Prophylaxis. Parasitol. Res. 2015, 114, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Vrhovec, M.G.; Pluta, S.; Straubinger, R. Seropositivity of Borrelia burgdorferi in a cohort of symptomatic cats from Europe based on a C6-peptide assay with discussion of implications in disease aetiology. Berl. und Munchener Tierarztliche Wochenschr. 2016, 129, 333–339. [Google Scholar]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell. Inf. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Palma, M.; De Carvalho, I.L.; Figueiredo, M.; Amaro, F.; Boinas, F.; Cutler, S.; Núncio, M.S. Borrelia hispanica in Ornithodoros erraticus, Portugal. Clin. Microbiol. Infect. 2012, 18, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Trape, J.-F.; Diatta, G.; Arnathau, C.; Bitam, I.; Sarih, M.; Belghyti, D.; Bouattour, A.; Elguero, E.; Vial, L.; Mané, Y.; et al. The Epidemiology and Geographic Distribution of Relapsing Fever Borreliosis in West and North Africa, with a Review of the Ornithodoros erraticus Complex (Acari: Ixodida). PLoS ONE 2013, 8, e78473. [Google Scholar] [CrossRef]
- Baneth, G.; Nachum-Biala, Y.; Halperin, T.; Hershko, Y.; Kleinerman, G.; Anug, Y.; Abdeen, Z.; Lavy, E.; Aroch, I.; Straubinger, R. Borrelia persica infection in dogs and cats: Clinical manifestations, clinicopathological findings and genetic characterization. Parasites Vectors 2016, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Piccione, J.; Levine, G.; Duff, C.; Kuhlman, G.; Scott, K.; Esteve-Gassent, M. Tick-Borne Relapsing Fever in Dogs. J. Vet. Intern. Med. 2016, 30, 1222–1228. [Google Scholar] [CrossRef]
- Dandrieux, J.R.; Sacchini, F.; Harms, G.; Globokar, M.; Balzer, H.-J.; Pantchev, N. Canine Leishmania infantum infection: An imported case in UK after staying in the Canary Islands. Parasitol. Res. 2017, 117, 331–334. [Google Scholar] [CrossRef]
- Dyachenko, V.; Pantchev, N.; Balzer, H.-J.; Meyersen, A.; Straubinger, R. First case of Anaplasma platys infection in a dog from Croatia. Parasites Vectors 2012, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preac-Mursic, V.; Wilske, B.; Schierz, G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1986, 263, 112–118. [Google Scholar] [CrossRef]
- Margos, G.; Stockmeier, S.; Hizo-Teufel, C.; Hepner, S.; Fish, D.; Dautel, H.; Sing, A.; Dzaferovic, E.; Rieger, M.; Jungnick, S.; et al. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks Tick-borne Dis. 2015, 6, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Radulović, Ž.; Milutinović, M.; Tomanović, S.; Mulenga, A. Detection of Borrelia-specific 16S rRNA sequence in total RNA extracted from Ixodes ricinus ticks. Arq. Bras. Med. Veterinária Zootec. 2010, 62, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Jungnick, S.; Margos, G.; Rieger, M.; Dzaferovic, E.; Bent, S.J.; Overzier, E.; Silaghi, C.; Walder, G.; Wex, F.; Koloczek, J.; et al. Borrelia burgdorferi sensu stricto and Borrelia afzelii: Population structure and differential pathogenicity. Int. J. Med Microbiol. 2015, 305, 673–681. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tamura, K.; Peterson, D.; Stecher, G.; Nei, M.; Kumar, S.; Peterson, N. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. In Some Mathematical Questions in Biology: DNA Sequence Analysis; Miura, R.M., Ed.; American Mathematical Society: Providence, RI, USA, 1986; Volume 17, pp. 57–86. [Google Scholar]
- Gettings, J.R.; Lopez, J.E.; Krishnavahjala, A.; Armstrong, B.A.; Thompson, A.T.; Yabsley, M.J. Antibodies to Borrelia turicatae in Experimentally Infected Dogs Cross-React with Borrelia burgdorferi Serologic Assays. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, C.; Krücken, J.; Beck, S.; Maaz, D.; Pachnicke, S.; Krieger, K.; Groß, M.; Kohn, B.; Von Samson-Himmelstjerna, G. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasites Vectors 2014, 7, 535. [Google Scholar] [CrossRef]
- Palomar, A.M.; Portillo, A.; Santibanez, P.; Santibanez, S.; Oteo, J.A. Borrelia miyamotoi: Should this pathogen be considered for the diagnosis of tick-borne infectious diseases in Spain? Enferm. Infecc. Microbiol. Clínica 2018, 36, 568–571. [Google Scholar] [CrossRef]
- Remesar, S.; Díaz, P.; Venzal, J.M.; Prieto, A.; Estrada-Peña, A.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; Morrondo, P. Longitudinal Study of Infection with Borrelia spp. in Questing Ticks from North-Western Spain. Vector Borne Zoonotic Dis. 2019, 19, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Díaz, P.; Arnal, J.L.; Remesar, S.; Pérez-Creo, A.; Venzal, J.M.; Vázquez-López, M.; Prieto, A.; Fernández, P.D.; López, C.M.; Panadero, R.; et al. Molecular identification of Borrelia spirochetes in questing Ixodes ricinus from northwestern Spain. Parasites Vectors 2017, 10, 615. [Google Scholar] [CrossRef] [Green Version]


| Sample/Date | Dog 1 | Dog 2 | Cat |
|---|---|---|---|
| Date of sampling | 30 October 2014 | 21 June 2018 | 21 July 2018 |
| Lab sample ID | VM531519/10015666 | VM73694/11024511 | 10827448 |
| Clinical signs | Lethargic | Lethargic | Cachexia |
| Pale mucosa, hematocrit 30.2% (reference range 38.3–56.5%) | High body temperature (39 °C) | Abdominal respiration | |
| Clinico-pathological abnormalities | Non-regenerative anemia; (erythrocytes 3.98 m/µL, hemoglobin 8.9 g/dL, hematocrit 29.4%, reticulocytes 58,108/µL) | Leukocytosis (20.3 g/L) | Regenerative anemia (low erythrocytes 2.45 m/µL, hemoglobin 3.3 g/dL, and hematocrit 12.9%; hematocrit reference range 25–45%) |
| Thrombocytopenia (23 tsd.) | Thrombocytopenia (92 tsd.) | High reticulocytes numbers (139,895/µL); mild monocytosis | |
| Blood taken | February 2014 March 2014 October 2014 | June 2018 | July 2018 |
| Blood smear | Borrelia positive (second presentation) | Borrelia positive (very few bacteria) Babesia spp. positive (low level) | Borrelia positive |
| Previous treatment | Doxycycline (2 courses; Feb 2014 and March 2014) | None | None |
| Locus | Primer Name | Primer Sequence |
|---|---|---|
| clpA | clpAF1258 | 5′-GATAAAGCTTTTGAYYTATTAGATGG-3 |
| clpA | clpAR2276 | 5′-TCATATTTDATRGTDTCGTC-3′ |
| clpX | clpXF104 | 5′-CTGTTGCYATTTGTTTTGAATGYTC-3′ |
| clpX | clpXR1277 | 5′-TAAAGTTCTTTTGCCCAAGG-3′ |
| pepX | pepF361 | 5′-AGAGAYTTAAGYTTAKCAGG-3′ |
| pepX | pepR1207 | 5′-CYATAGTTTCTCTTAAAGAYTGC-3 |
| pyrG | pyrF379 | 5′-TATTTAGGKAGAACTGTACAGC-3 |
| pyrG | pyrR1375 | 5′-CAAGTCGCATTGTWGCAC-3 |
| recG | recF898 | 5′-GCKTTTCTMTCTAGYATTCC-3 |
| recG | recR1779 | 5′-TTCRGTTAAAGGTTCCTTATAAAG-3 |
| rplB | rplF3 | 5′-GGAGAAAAATATGGGKATTAAGAC-3 |
| rplB | rplR769 | 5′-GRCCCCAAGGWGATAC-3 |
| uvrA | uvrF1170 | 5′-GAGGCGTTATCTTWCAAC-3 |
| uvrA | uvrR2181 | 5′-AGACTCTGGAAGCTTWGC-3 |
| Locus/Sample | Species and Isolate | Coverage | Similarity | GenBank Accession Number |
|---|---|---|---|---|
| clpX/dog1 (#allele 210)/cat (#allele 210) | Borrelia crocidurae str. Achema | 100% | 97% | CP003426.1 |
| Borrelia recurrentis A1 | 100% | 97% | CP000993.1 | |
| Borrelia duttonii Ly | 100% | 97% | CP000976.1 | |
| Borrelia crocidurae DOU | 100% | 96% | CP004267.1 | |
| Borrelia persica strain LMU-T01 | 99% | 91% | KP826804. | |
| pepX/cat (#allele 263) | Borrelia recurrentis A1 | 100% | 98% | CP000993.1 |
| Borrelia crocidurae DOU | 100% | 97% | CP004267.1 | |
| Borrelia crocidurae str. Achema | 100% | 97% | CP003426.1 | |
| Borrelia duttonii Ly | 99% | 97% | CP000976.1 | |
| Borrelia persica strain LMU-T01 | 100% | 90% | KP826805.1 | |
| pyrG/dog1 (#allele 274) | Borrelia crocidurae str. Achema | 100% | 98% | CP003426.1 |
| Borrelia recurrentis A1 | 100% | 97% | CP000993.1 | |
| Borrelia duttonii Ly | 100% | 97% | CP000976.1 | |
| Borrelia persica strain LMU-T01 | 100% | 89% | KP826806.1 | |
| pyrG/cat (#allele 273) | Borrelia crocidurae DOU | 100% | 98% | CP004267.1 |
| Borrelia crocidurae str. Achema | 100% | 98% | CP003426.1 | |
| Borrelia recurrentis A1 | 100% | 98% | CP000993.1 | |
| Borrelia duttonii Ly | 100% | 98% | CP000976.1 | |
| Borrelia persica strain LMU-T01 | 100% | 90% | KP826806.1 | |
| recG/cat (#allele 288) | Borrelia crocidurae str. Achema | 100% | 97% | CP003426.1 |
| Borrelia crocidurae DOU | 100% | 97% | CP004267.1 | |
| Borrelia duttonii Ly | 100% | 97% | CP000976.1 | |
| Borrelia recurrentis A1 | 100% | 96% | CP000993.1 | |
| Borrelia persica strain LMU-T01 | 99% | 89% | KP826807.1 | |
| rplB/dog1 (#allele 206) | Borrelia crocidurae str. Achema | 100% | 98% | CP003426.1 |
| Borrelia recurrentis A1 | 100% | 98% | CP000993.1 | |
| Borrelia duttonii Ly | 100% | 98% | CP000976.1 | |
| Borrelia crocidurae DOU | 100% | 98% | CP004267.1 | |
| Borrelia persica strain LMU-T01 | 98% | 92% | KP826807.1 | |
| 16S rRNA/dog2 (* MN175320)/cat (* MN173954) | Borrelia hispanica strain CR1 | 100% | 100% | GU350705.1 |
| Borrelia hispanica 16S rRNA gene | 100% | 100% | DQ057988.1 | |
| Borrelia crocidurae str. Achema | 100% | 99% | CP003426.1 | |
| Borrelia hispanica strain Sp3 | 100% | 99% | GU350706.1 | |
| Borrelia crocidurae strain 7-10TO58 | 100% | 99% | GQ358198.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margos, G.; Pantchev, N.; Globokar, M.; Lopez, J.; Rodon, J.; Hernandez, L.; Herold, H.; Salas, N.; Civit, A.; Fingerle, V. First Cases of Natural Infections with Borrelia hispanica in Two Dogs and a Cat from Europe. Microorganisms 2020, 8, 1251. https://doi.org/10.3390/microorganisms8081251
Margos G, Pantchev N, Globokar M, Lopez J, Rodon J, Hernandez L, Herold H, Salas N, Civit A, Fingerle V. First Cases of Natural Infections with Borrelia hispanica in Two Dogs and a Cat from Europe. Microorganisms. 2020; 8(8):1251. https://doi.org/10.3390/microorganisms8081251
Chicago/Turabian StyleMargos, Gabriele, Nikola Pantchev, Majda Globokar, Javier Lopez, Jaume Rodon, Leticia Hernandez, Heike Herold, Noelia Salas, Anna Civit, and Volker Fingerle. 2020. "First Cases of Natural Infections with Borrelia hispanica in Two Dogs and a Cat from Europe" Microorganisms 8, no. 8: 1251. https://doi.org/10.3390/microorganisms8081251





