Genomic Insight of VIM-harboring IncA Plasmid from a Clinical ST69 Escherichia coli Strain in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Presentation, Antimicrobial Susceptibility Test and Molecular Investigations
2.2. Conjugation Assay
2.3. Whole-Genome Sequencing (WGS)
2.4. Whole-Genome-Sequencing-Data Analysis
2.5. Nucleotide Accession Numbers
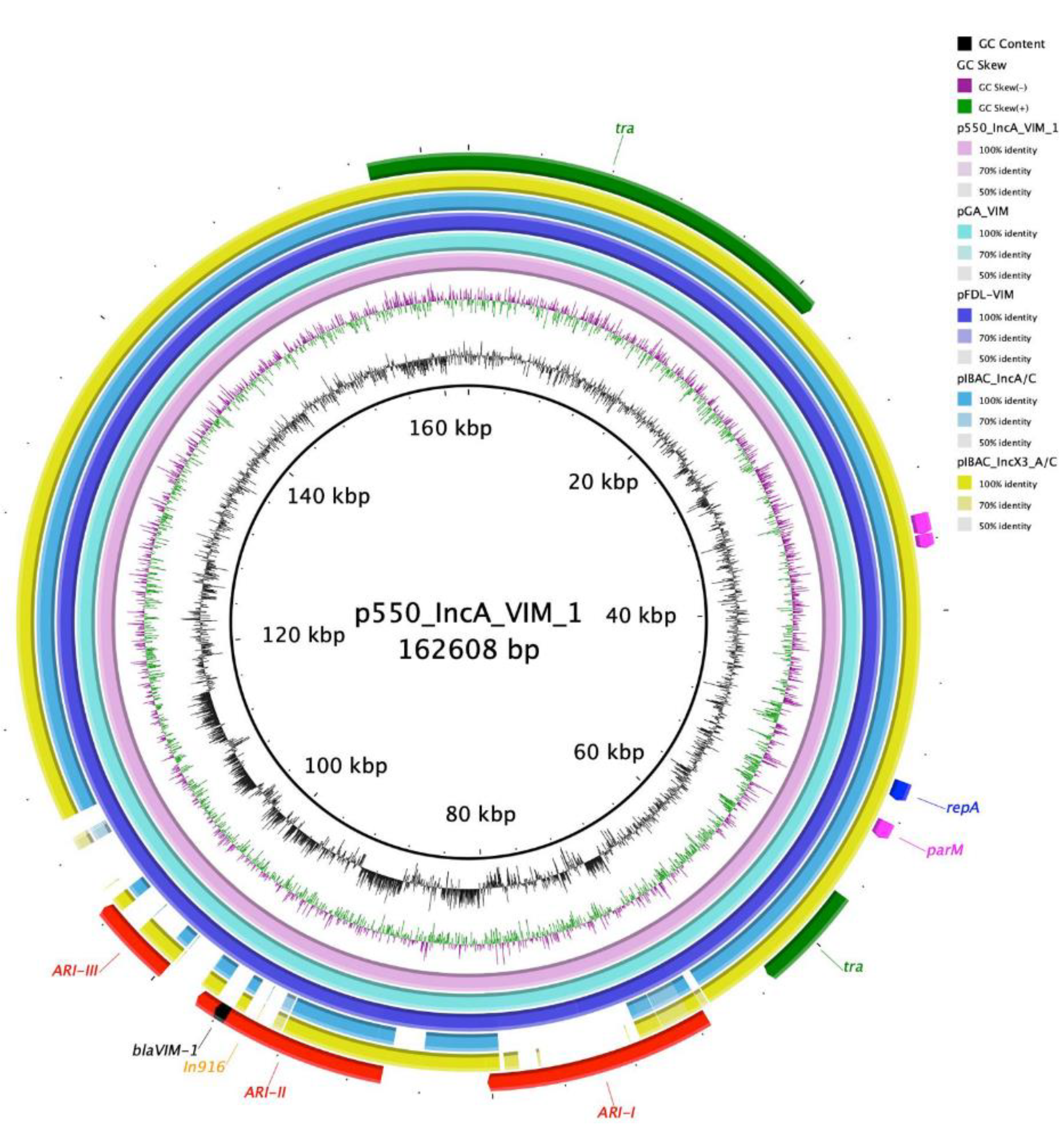
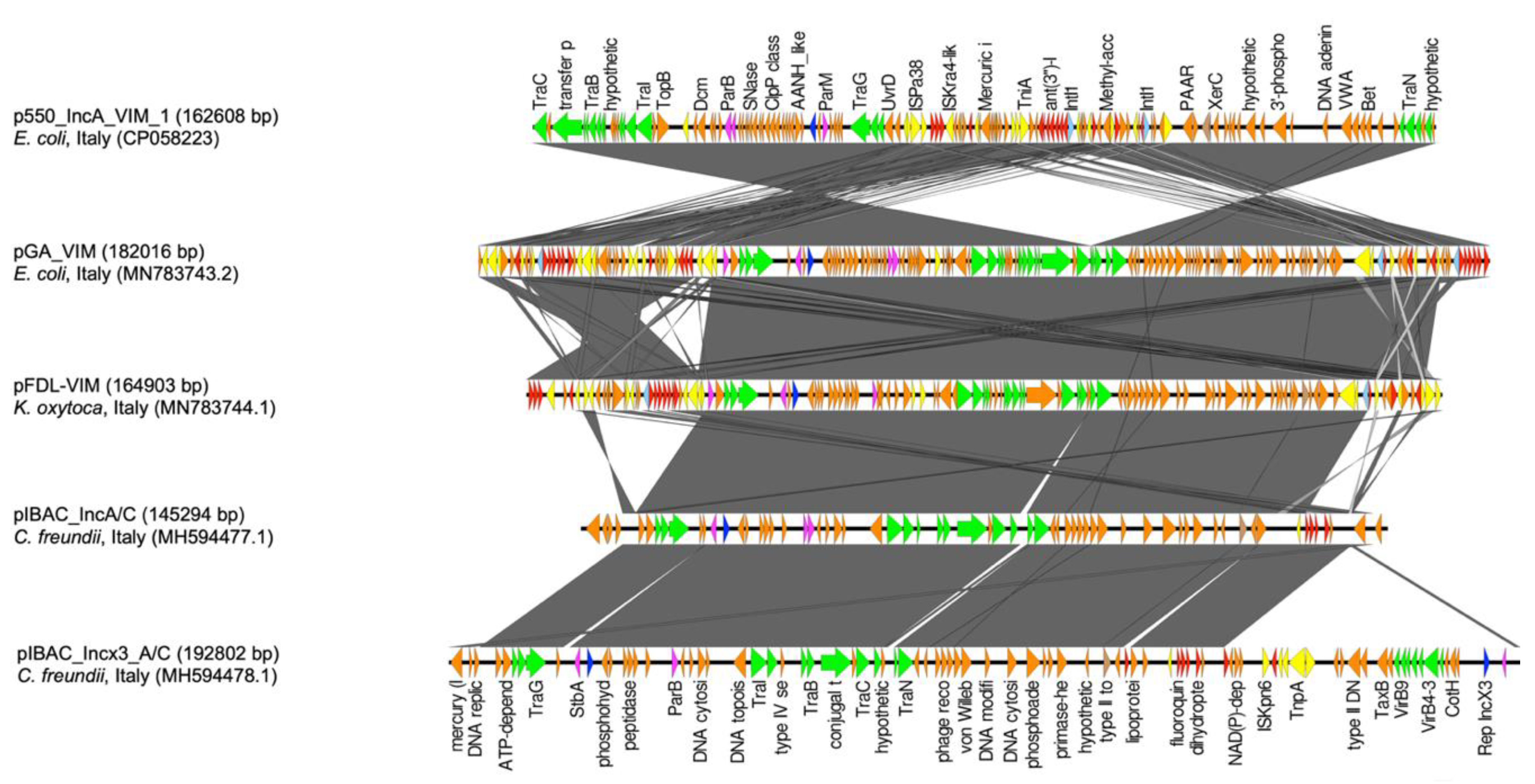
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lauretti, L.; Riccio, M.L.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-Metallo-β-Lactamases: Where Do We Stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef]
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and Characteristics of Metallo-β-Lactamase-Producing Pseudomonas aeruginosa. Infect. Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.J.; Peirano, G.; Pitout, J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobact. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Peirano, G.; Devinney, R.; Bradford, P.A.; Motyl, M.R.; Adams, M.D.; Chen, L.; Kreiswirth, B.; Johann, D.; Pitout, D. Genomic epidemiology of global VIM-producing Enterobact. J. Antimicrob. Chemother. 2017, 72, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Aschbacher, R.; March, A.; Larcher, C.; Livermore, D.M.; Woodford, N. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the USA. J. Antimicrob. Chemother. 2010, 65, 2070–2075. [Google Scholar] [CrossRef] [Green Version]
- Miriagou, V.; Papagiannitsis, C.C.; Kotsakis, S.D.; Loli, A.; Tzelepi, E.; Legakis, N.J.; Tzouvelekis, L.S. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-beta-lactamase gene in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2010, 54, 4497–4502. [Google Scholar] [CrossRef] [Green Version]
- Roschanski, N.; Guenther, S.; Vu, T.T.T.; Fischer, J.; Semmler, T.; Huehn, S.; Alter, T.; Roesler, U. VIM-1 carbapenemase-producing Escherichia coli isolated from retail seafood, Germany 2016. Euro. Surveill. 2017, 22, 17-00032. [Google Scholar] [CrossRef] [Green Version]
- Drieux, L.; Decré, D.; Frangeul, L.; Arlet, G.; Jarlier, V.; Sougakoff, W. Complete nucleotide sequence of the large conjugative pTC2 multireplicon plasmid encoding the VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 2013, 68, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Arcari, G.; Di Lella, F.M.; Bibbolino, G.; Mengoni, F.; Beccaccioli, M.; Antonelli, G.; Faino, L.; Carattoli, A. A Multispecies Cluster of VIM-1 Carbapenemase-Producing Enterobacterales Linked by a Novel, Highly Conjugative, and Broad-Host-Range IncA Plasmid Forebodes the Reemergence of VIM-1. Antimicrob. Agents Chemother. 2020, 64, e02435-19. [Google Scholar] [CrossRef]
- Bitar, I.; Caltagirone, M.; Villa, L.; Mattioni, M.V.; Nucleo, E.; Sarti, M.; Migliavacca, R.; Carattoli, A. Interplay among IncA and blaKPC-Carrying Plasmids in Citrobacter freundii. Antimicrob. Agents Chemother. 2019, 63, e02609-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaibani, P.; Ambretti, S.; Scaltriti, E.; Cordovana, M.; Berlingeri, A.; Pongolini, S.; Landini, M.P.; Re, M.C. A novel IncA plasmid carrying blaVIM-1 in a Kluyvera cryocrescens strain. J. Antimicrob. Chemother. 2018, 73, 3206–3208. [Google Scholar] [CrossRef] [PubMed]
- Rotova, V.; Papagiannitsis, C.C.; Skalova, A.; Chudejova, K.; Hrabak, J. Comparison of imipenem and meropenem antibiotics for the MALDI-TOF MS detection of carbapenemase activity. J. Microbiol. Methods. 2017, 137, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lim, Y.S.; Yong, D.; Yum, J.H.; Chong, Y. Evaluation of the hodge test and the imipenem-EDTA double-disk synergy test for differentiating Metallo-β-Lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2003, 41, 4623–4629. [Google Scholar] [CrossRef] [Green Version]
- Doi, Y.; Potoski, B.A.; Adams-Haduch, J.M.; Sidjabat, H.E.; Pasculle, A.W.; Paterson, D.L. Simple disk-based method for detection of Klebsiella pneumoniae Carbapenemase-type -lactamase by use of a boronic acid compound. J. Clin. Microbiol. 2008, 46, 4083–4086. [Google Scholar] [CrossRef] [Green Version]
- Glupczynski, Y.; Huang, T.D.; Bouchahrouf, W.; Castro, R.R.D.; Bauraing, C.; Geérard, M.; Verbruggen, A.; Deplano, A.; Denis, O.; Bogaerts, P. Rapid emergence and spread of OXA-48-producing carbapenem-resistant Enterobacteriaceae isolates in Belgian hospitals. Int. J. Antimicrob. Agents 2012, 39, 168–172. [Google Scholar] [CrossRef]
- Shirani, K.; Ataei, B.; Roshandel, F. Antibiotic resistance pattern and evaluation of metallo-beta lactamase genes (VIM and IMP) in Pseudomonas aeruginosa strains producing MBL enzyme, isolated from patients with secondary immunodeficiency. Adv. Biomed. Res. 2016, 5, 124. [Google Scholar]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Voldby Larsen, M. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby, L.M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli using whole genome sequencing (WCS) data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roer, L.; Tchesnokova, V.; Allesøe, R.; Muradova, M.; Chattopadhyay, S.; Ahrenfeldt, J.; Thomsen, M.C.F.; Lund, O.; Hansen, F.; Hammerum, A.M.; et al. Development of a Web Tool for Escherichia coli Subtyping Based on fimH Alleles. J. Clin. Microbiol. 2017, 55, 2538–2543. [Google Scholar] [CrossRef] [Green Version]
- Roer, L.; Johannesen, T.B.; Hansen, F.; Stegger, M.; Tchesnokova, V.; Sokurenko, E.; Garibay, N.; Allesøe, R.; Thomsen, M.C.F.; Lund, O.; et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH Alleles. J. Clin. Microbiol. 2018, 56, e00063-18. [Google Scholar] [CrossRef] [Green Version]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Kjaergaard, K.; Schembri, M.A.; Hasman, H.; Klemm, P. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas Fluoresc. J. Bacteriol. 2000, 182, 4789–4796. [Google Scholar] [CrossRef] [Green Version]
- Manges, A.R.; Johnson, J.R.; Foxman, B.; O’Bryan, T.T.; Fullerton, K.E.; Riley, L.W. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 2001, 345, 1007–1013. [Google Scholar] [CrossRef]
- Johnson, J.R.; Magistro, G.; Clabots, C.; Stephen, P.; Amee, M.; Paul, T.; Sören, S. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 (“clonal group A”) cystitis isolate in murine models of urinary tract infection and sepsis. Microb. Pathog. 2018, 120, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghoribi, M.F.; Gibreel, T.M.; Dodgson, A.R.; Beatson, S.A.; Upton, M. Galleria mellonella infection model demonstrates high lethality of ST69 and ST127 uropathogenic E. coli. PLoS ONE 2014, 9, e101547. [Google Scholar] [CrossRef] [Green Version]
- Giacobbe, D.R.; Del Bono, V.; Coppo, E.; Marchese, A.; Viscoli, C. Emergence of a KPC-3-Producing Escherichia coli ST69 as a Cause of Bloodstream Infections in Italy. Microb. Drug Resist. 2015, 21, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.I.; Ghany, M.; Sharaf, H.; Al-Agamy, M.H.; Shibl, A.; Hill-Cawthorne, G.A.; Hong, P.Y. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS ONE 2018, 13, e0201613. [Google Scholar] [CrossRef]
- Hammad, A.M.; Hoffmann, M.; Gonzalez-Escalona, N.; Nasser, H.A.; Yao, K.; Koenig, S.; Allué-Guardia, A.; Eppinger, M. Genomic features of colistin resistant Escherichia coli ST69 strain harboring mcr-1 on IncHI2 plasmid from raw milk cheese in Egypt. Infect. Genet. Evol. 2019, 73, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Fibke, C.D.; Croxen, M.A.; Geum, H.M.; Glass, M.; Wong, E.; Avery, B.P.; Daignault, D.; Mulvey, M.R.; Reid-Smith, R.J.; Parmley, E.J.; et al. Genomic Epidemiology of Major Extraintestinal Pathogenic Escherichia coli Lineages Causing Urinary Tract Infections in Young Women Across Canada. Open Forum. Infect. Dis. 2019, 6, ofz431. [Google Scholar] [CrossRef]
- Boll, E.J.; Overballe-Petersen, S.; Hasman, H.; Roer, L.; Ng, K.; Scheutz, F.; Hammerum, A.M.; Dungu, A.; Hansen, F.; Johannesen, T.B.; et al. Emergence of Enteroaggregative Escherichia coli within the ST131 Lineage as a Cause of Extraintestinal Infections. mBio 2020, 11, e00353-20. [Google Scholar] [CrossRef]
- Loconsole, D.; Accogli, M.; De Robertis, A.L.; Capozzo, L.; Bianco, A.; Morea, A.; Mallamaci, R.; Quarto, M.; Parisi, A.; Chironna, M. Emerging high-risk ST101 and ST307 carbapenem-resistant Klebsiella pneumoniae clones from bloodstream infections in Southern Italy. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 24. [Google Scholar] [CrossRef]
- Flores, C.; Bianco, K.; de Filippis, I.; Clementino, M.M.; Romão, C.M.C. Genetic Relatedness of NDM-Producing Klebsiella pneumoniae Co-occurring VIM, KPC, and OXA-48 Enzymes from Surveillance Cultures from an Intensive Care Unit. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef]
- Caltagirone, M.; Bitar, I.; Piazza, A.; Spalla, M.; Nucleo, E.; Navarra, A.; Migliavacca, R. Detection of an IncA/C plasmid encoding VIM-4 and CMY-4 β-lactamases in Klebsiella oxytoca and Citrobacter koseri from an inpatient in a cardiac rehabilitation unit. New Microbiol. 2015, 38, 387–392. [Google Scholar] [PubMed]
- Johnson, T.J.; Lang, K.S. IncA/C plasmids: An emerging threat to human and animal health? Mob. Genet. Elem. 2012, 2, 55–58. [Google Scholar] [CrossRef] [PubMed]
| AMP | AMS | ATM | CTX | FEP | CAZ | CIP | CO | FOS | ETP | MP | IMP | CN | TOB | TGC | TMX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coliA15 | 2 S | 2 S | ≤0.125 S | ≤0.068 S | ≤0.125 S | ≤0.25 S | ≤0.068 S | ≤0.5 S | ≤0.125 S | ≤0.032 S | ≤0.125 S | ≤0.125 S | ≤0.25 S | ≤0.125 S | ≤0.125 S | ≤0.068 S |
| E. coli550 | >128 R | >128 R | 16 R | >8 R | 16 R | >16 R | 0.25 S | 0.25 S | 8 S | 1 R | 1 S | 2 S | 1 S | 4 R | 0.25 S | >4 R |
| A15* E. coli550 | 128 R | 128 R | 16 R | >8 R | 8 R | >16 R | 0.25 S | 0.25 S | 8 S | 0.38 S | 1 S | 2 S | 0.5 S | 2 S | 0.12 5S | >4 R |
| Position | Antibiotic Resistance | Adhesion | Autotransporter | Invasion | Iron Uptake | Secretion | Toxin |
|---|---|---|---|---|---|---|---|
| Chromosome | mdf(A) | Pilus, EaeH, Type I fimbriae | ag43, cah, air, vat | ibeB, ibeC | chu, sit | Type III Secretion System | clyA |
| p550_IncA_VIM_1 | aac(6′)-lb4, ant(3″)-Ia, aph(3″)-lb, aph(3′)-XV, aph(6)-ld, blaSHV-12, blaVIM-1, mph(A), catB2, qnrS1, sul1, sul2, dfrA14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioni Marchetti, V.; Bitar, I.; Piazza, A.; Mercato, A.; Fogato, E.; Hrabak, J.; Migliavacca, R. Genomic Insight of VIM-harboring IncA Plasmid from a Clinical ST69 Escherichia coli Strain in Italy. Microorganisms 2020, 8, 1232. https://doi.org/10.3390/microorganisms8081232
Mattioni Marchetti V, Bitar I, Piazza A, Mercato A, Fogato E, Hrabak J, Migliavacca R. Genomic Insight of VIM-harboring IncA Plasmid from a Clinical ST69 Escherichia coli Strain in Italy. Microorganisms. 2020; 8(8):1232. https://doi.org/10.3390/microorganisms8081232
Chicago/Turabian StyleMattioni Marchetti, Vittoria, Ibrahim Bitar, Aurora Piazza, Alessandra Mercato, Elena Fogato, Jaroslav Hrabak, and Roberta Migliavacca. 2020. "Genomic Insight of VIM-harboring IncA Plasmid from a Clinical ST69 Escherichia coli Strain in Italy" Microorganisms 8, no. 8: 1232. https://doi.org/10.3390/microorganisms8081232
APA StyleMattioni Marchetti, V., Bitar, I., Piazza, A., Mercato, A., Fogato, E., Hrabak, J., & Migliavacca, R. (2020). Genomic Insight of VIM-harboring IncA Plasmid from a Clinical ST69 Escherichia coli Strain in Italy. Microorganisms, 8(8), 1232. https://doi.org/10.3390/microorganisms8081232





