Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases
Abstract
1. Introduction
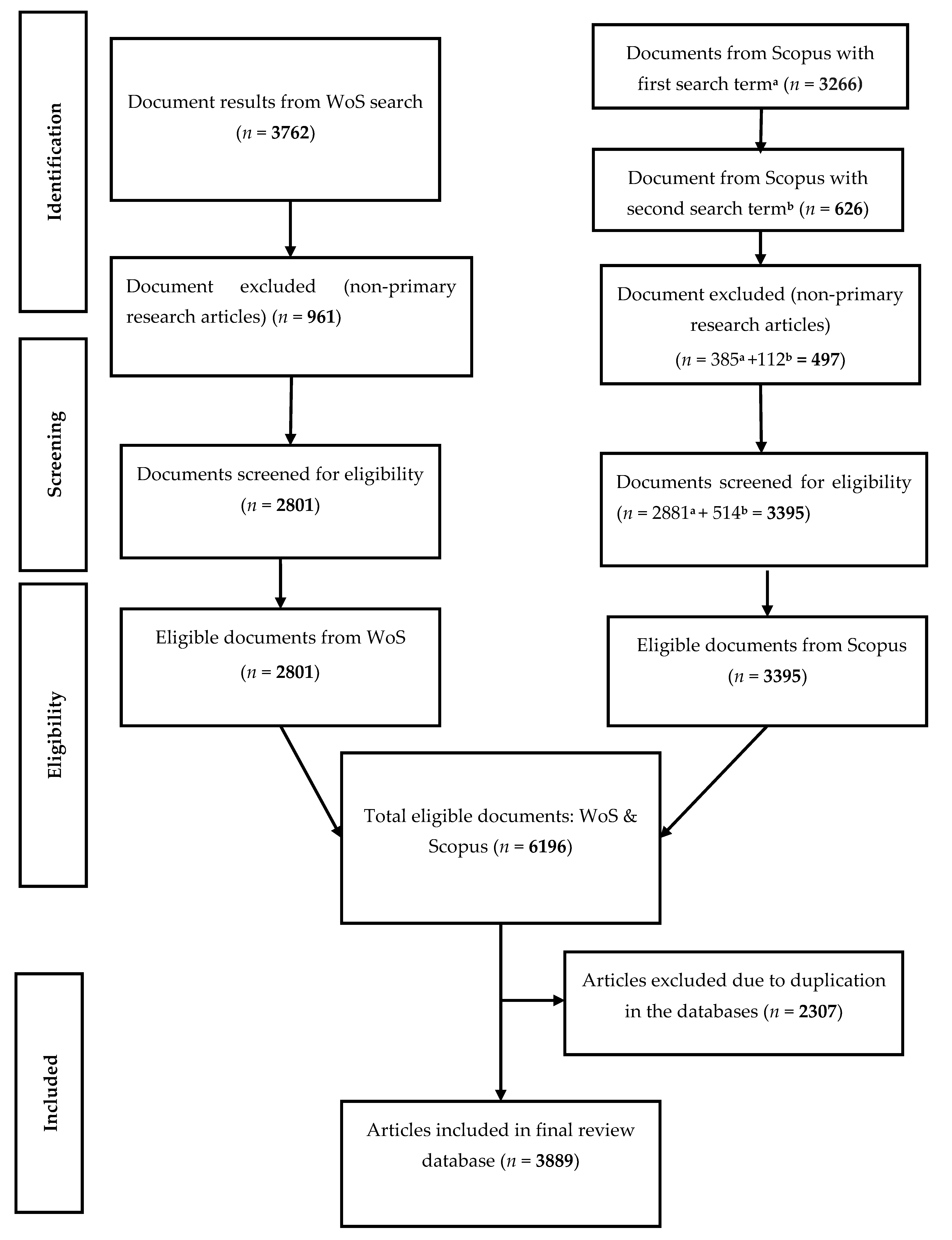
2. Materials and Methods
2.1. Mycobacterium avium Subsp. Paratuberculosis (MAP) Data Sources
2.2. Pre-Analytic and Bibliometric Assessment of the Data
2.3. Determination of Growth of MAP Research
2.4. Determination of Scientific-Networking in MAP Research
2.5. Software
3. Results and Discussion
3.1. MAP Bibliometrics
3.1.1. Characteristics of MAP Research Documents
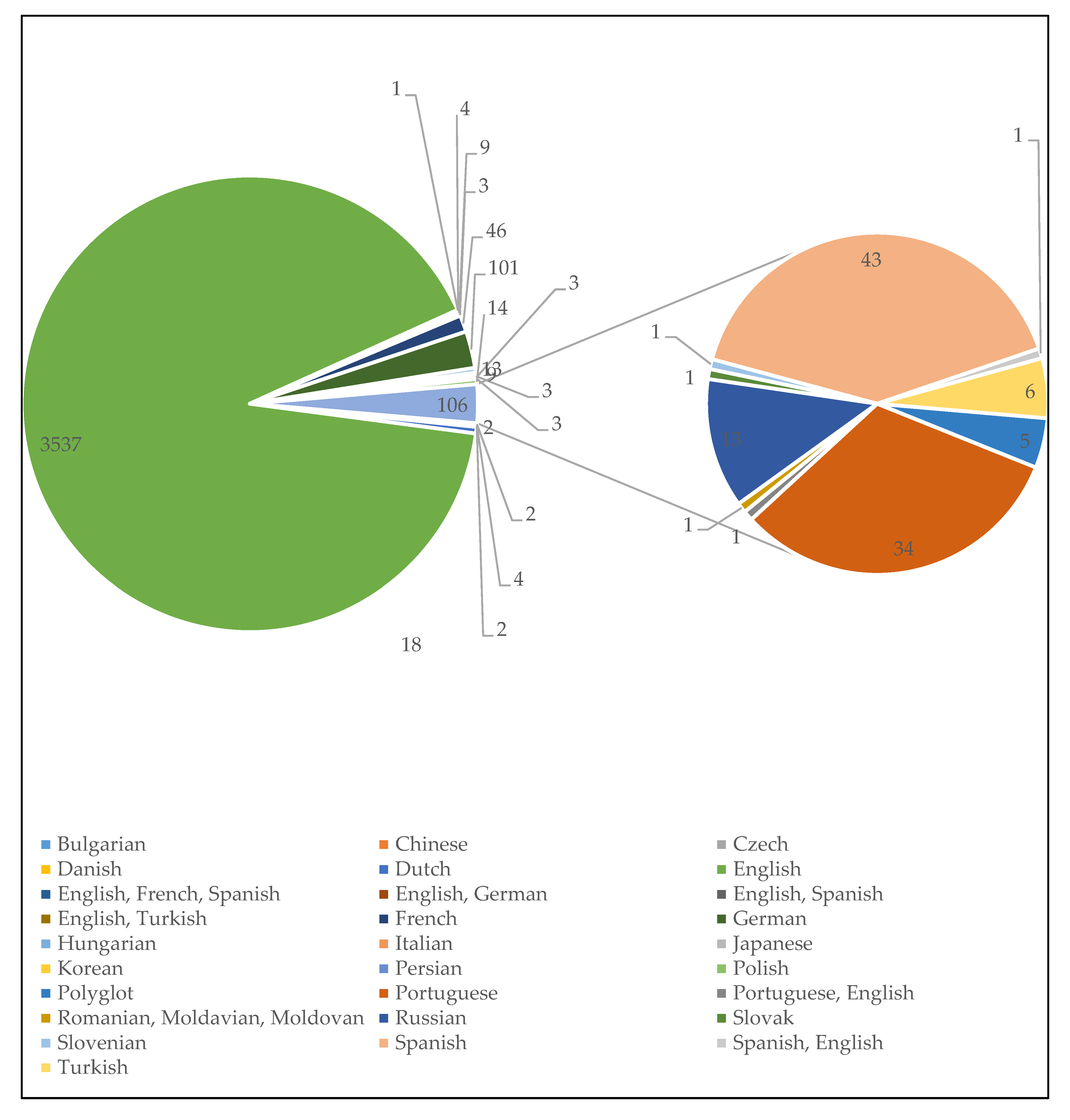
3.1.2. Language Diversity in MAP Research Communications
3.1.3. Annual Productivity of MAP Research Landscape
3.1.4. Authors’ Productivity
3.1.5. Most Cited/Articles with Highest Contributions in MAP Research
3.1.6. Country’s Productivity in MAP Research
3.1.7. Journal’s Productivity in MAP Research
3.2. Growth of MAP Research from 2017–2019
3.2.1. Growth of MAP Research Related to Discipline Classification
3.2.2. Growth of MAP Research per Country
3.2.3. Growth of MAP Research Based on Institution Participations
3.3. Trend and Growth of MAP Related to Some Hot Research Topics
3.3.1. Growth of MAP Research in Wildlife-Related Discipline
3.3.2. Growth of MAP Research Related to Environmental Milieu
3.3.3. Growth of MAP Research Related to Virulence and Disease Resistance
3.3.4. Growth of MAP Related to Anaerobic Processes
3.3.5. Growth of MAP Research Related to Waste Milk Feeding in Animals
3.3.6. MAP and Other Research Topics
3.4. Growth and Trend of MAP Research Related to Autoimmune Diseases
3.4.1. Growth of MAP Research Related to Type 1 Diabetes Mellitus (T1DM)
3.4.2. Growth of MAP Research Related to Multiple Sclerosis Diseases
3.4.3. Growth of MAP Research Related to Sarcoidosis
3.4.4. MAP Importance in Thyroid Disorders (TDs)
3.4.5. Growth of MAP Research Related to Psoriasis
3.4.6. Growth of MAP Research Related to Irritable Bowel Syndrome (Crohn’s Disease and Ulcerative Colitis)
3.4.7. Growth of MAP Research Related to Parkinson’s Disease
3.4.8. Role of MAP Arthritis and Osteoporosis (Blau Syndrome; Granulomatoud, Rheumatoid Arthritis)
3.4.9. Contributions of Osteopontin in MAP
3.4.10. MAP and Neuromyelitis Optica Spectrum Disorder
3.5. Resource-, Intellectual- and Knowledge-Sharing in MAP Research
3.5.1. Country Collaboration Network (CCN) in MAP Research
3.5.2. Author Collaboration Network (ACN) in MAP Research
3.5.3. Institution Collaboration Network (ICN) in MAP Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Whittington, R.J.; Begg, D.J.; de Silva, K.; Plain, K.M.; Purdie, A.C. Comparative immunological and microbiological aspects of Para tuberculosis as a model mycobacterial infection. Vet. Immunol. Immunopathol. 2012, 148, 29–47. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, L.C.; Jia, B.Y.; Chen, J.R.; Dong, Y.; Jiang, X.Y.; Ma, H.X. Analysis of the microRNA Expression Profile of Bovine Monocyte-derived Macrophages Infected with Mycobacterium avium subsp. Paratuberculosis Reveals that miR-150 Suppresses Cell Apoptosis by Targeting PDCD4. Int. J. Mol. Sci. 2019, 20, 2708. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; El-Deeb, W.; Abdel-Moein, K.; El-Sayed, A.; Fayed, A.; Housawi, F.; AlNaeem, A.; Zschöck, M. Detection of Mycobacterium avium subsp. paratuberculosis in an Egyptian mixed breeding farm and comparative molecular characterisation of isolates from cattle, camels and cats—A case report. Bulg. J. Vet. Med. 2019, 22, 41–49. [Google Scholar] [CrossRef]
- Kim, J.M.; Ku, B.K.; Lee, H.N.; Hwang, I.Y.; Jang, Y.B.; Kim, J.; Hyun, B.H.; Jung, S.C. Mycobacterium avium paratuberculosis in wild boars in Korea. J. Wildl. Dis. 2013, 49, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Thibault, V.C.; Smith, D.G.; McLuckie, J.; Heron, I.; Sevilla, I.A.; Biet, F.; Harris, S.R.; Maskell, D.J.; Bentley, S.D.; et al. Phylogenomic exploration of the relationships between strains of Mycobacterium avium subspecies paratuberculosis. BMC Genom. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Cho, E.H.; Huh, H.J.; Song, D.J.; Moon, S.M.; Lee, S.H.; Shin, S.Y.; Kim, C.K.; Ki, C.S.; Koh, W.J.; Lee, N.Y. Differences in drug susceptibility pattern between Mycobacterium avium and Mycobacterium intracellulare isolated in respiratory specimens. J. Infect. Chemother. 2018, 24, 315–318. [Google Scholar] [CrossRef]
- Davis, W.C.; Kuenstner, J.T.; Singh, S.V. Resolution of Crohn’s (Johne’s) disease with antibiotics: What are the next steps? Expert Rev. Gastroenterol. Hepatol. 2017, 11, 393–396. [Google Scholar] [CrossRef][Green Version]
- Feller, M.; Huwiler, K.; Stephan, R.; Altpeter, E.; Shang, A.; Furrer, H.; Pfyffer, G.E.; Jemmi, T.; Baumgartner, A.; Egger, M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 607–613. [Google Scholar] [CrossRef]
- Sechi, L.A.; Dow, C.T. Mycobacterium avium ssp. paratuberculosis zoonosis—The Hundred Year War—beyond Crohn’s disease. Front. Immunol. 2015, 6, 96. [Google Scholar] [CrossRef]
- Singh, S.V.; Kumar, N.; Sohal, J.S.; Singh, A.V.; Singh, P.K.; Agrawal, N.D.; Gupta, S.; Chaubey, K.K.; Deb, R.; Dhama, R.; et al. First mass screening of the human population to estimate the bio-load of Mycobacterium avium sub-species paratuberculosis in North India. JPHE 2014, 6, 20–29. [Google Scholar]
- Singh, S.V.; Kuenstner, J.T.; Davis, W.C.; Agarwal, P.; Kumar, N.; Singh, D.; Gupta, S.; Chaubey, K.K.; Kumar, A.; Misri, J.; et al. Concurrent resolution of chronic diarrhea likely due to Crohn’s disease and infection with Mycobacterium avium paratuberculosis. Front. Med. 2016, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Beard, P.M.; Daniels, M.J.; Henderson, D.; Pirie, A.; Rudge, K.; Buxton, D.; Rhind, S.; Greig, A.; Hutchings, M.R.; McKendrick, I.; et al. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 2001, 39, 1517–1521. [Google Scholar] [CrossRef]
- Chiodini, R.J.; Van Kruiningen, H.J.; Merkal, R.S. Ruminant paratuberculosis (Johne’s disease): The current status and future prospects. Cornell Vet. 1984, 74, 218–262. [Google Scholar] [PubMed]
- Bates, A.; O’Brien, R.; Liggett, S.; Griffin, F. Control of Mycobacterium avium subs paratuberculosis infection on a New Zealand pastoral dairy farm. BMC Vet. Res. 2019, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Koets, A.; Ravesloot, L.; Ruuls, R.; Dinkla, A.; Eisenberg, S.; Lievaart-Peterson, K. Effects of age and environment on adaptive immune responses to Mycobacterium avium subsp. paratuberculosis (MAP) vaccination in dairy goats in relation to paratuberculosis control strategies. Vet. Sci. 2019, 6, 62. [Google Scholar] [CrossRef]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef]
- Mazzone, P.; Corneli, S.; Di Paolo, A.; Maresca, C.; Felici, A.; Biagetti, M.; Ciullo, M.; Sebastiani, C.; Pezzotti, G.; Leo, S.; et al. Survival of Mycobacterium avium subsp. paratuberculosis in the intermediate and final digestion products of biogas plants. J. Appl. Microbiol. 2018, 125, 36–44. [Google Scholar] [CrossRef]
- Salgado, M.; Alfaro, M.; Salazar, F.; Badilla, X.; Troncoso, E.; Zambrano, A.; González, M.; Mitchell, R.M.; Collins, M.T. Application of cattle slurry containing Mycobacterium avium subsp. paratuberculosis (MAP) to grassland soil and its effect on the relationship between MAP and free-living amoeba. Vet. Microbiol. 2015, 175, 26–34. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Robino, E.; Cochard, T.; Branger, M.; Delafont, V.; Aucher, W.; Wambeke, W.; Bannantine, J.P.; Biet, F.; Héchard, Y. Environmental Mycobacterium avium subsp. paratuberculosis hosted by free-living amoebae. Front. Cell Infect. Microbiol. 2018, 8, 28. [Google Scholar] [CrossRef]
- Mishina, D.; Katsel, P.; Brown, S.T.; Gilberts, E.C.; Greenstein, R.J. On the etiology of Crohn disease. Proc. Natl. Acad. Sci. USA 1996, 93, 9816–9820. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ekundayo, T.C.; Okoh, A.I. A global bibliometric analysis of Plesiomonas-related research (1990–2017). PLoS ONE 2018, 13, e0207655. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rosero, J.; Ramirez-Gonzalez, G.; Viveros-Delgado, J. Software survey: ScientoPy, a scientometric tool for topics trend analysis in scientific publications. Scientometrics 2019, 121, 1165–1188. [Google Scholar] [CrossRef]
- Jaccard, P. Distribution of alpine flora in the basin of Dranses and in some neighboring regions. Bull. Soc. Vaud. Sci. Nat. 1901, 37, 241–272. [Google Scholar]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Bannantine, J.P.; Baechler, E.; Zhang, Q.; Li, L.; Kapur, V. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J. Clin. Microbiol. 2002, 40, 1303–1310. [Google Scholar] [CrossRef]
- Stabel, J.R. Johne’s disease: A hidden threat. J. Dairy Sci. 1998, 81, 283–288. [Google Scholar] [CrossRef]
- Collins, M.T.; Wells, S.J.; Petrini, K.R.; Collins, J.E.; Schultz, R.D.; Whitlock, R.H. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 2005, 12, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, I.X.; Singh, S.V.; Garrido, J.M.; Aduriz, G.; Rodriguez, S.; Geijo, M.V.; Whittington, R.J.; Saunders, V.; Whitlock, R.H.; Juste, R.A. Molecular typing of Mycobacterium avium subspecies paratuberculosis strains from different hosts and regions. Rev. Sci. Tech. 2005, 24, 1061. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Green, E.P.; Tizard, M.L.V.; Moss, M.T.; Thompson, J.; Winterbourne, D.J.; McFadden, J.J.; Hermon-Taylor, J. Sequence and characteristics or IS 900, an insertion element identified in a human Crohn’s disease isolate or Mycobacterium paratuberculosis. Nucleic Acids Res. 1989, 17, 9063–9073. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.D.; Moss, M.T.; Tizard, M.L.; Hermon-Taylor, J. Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut 1992, 33, 890–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Bannantine, J.P.; Zhang, Q.; Amonsin, A.; May, B.J.; Alt, D.; Banerji, N.; Kanjilal, S.; Kapur, V. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. USA 2005, 102, 12344–12349. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 1992, 30, 166–171. [Google Scholar] [CrossRef]
- Vary, P.H.; Andersen, P.R.; Green, E.; Hermon-Taylor, J.; McFadden, J.J. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne’s disease. J. Clin. Microbiol. 1990, 28, 933–937. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis isolated from fetuses of infected cows not manifesting signs of the disease. Am. J. Vet. Res. 1992, 53, 477–480. [Google Scholar]
- Fischer, O.A.; Matlova, L.; Dvorska, L.; Svastova, P.; Pavlik, I. Nymphs of the Oriental cockroach (Blatta orientalis) as passive vectors of causal agents of avian tuberculosis and paratuberculosis. Med. Vet. Entomol. 2003, 17, 145–150. [Google Scholar] [CrossRef]
- Fischer, O.A.; Matlova, L.; Dvorska, L.; Svastova, P.; Bartl, J.; Weston, R.T.; Pavlik, I. Blowflies Calliphora vicina and Lucilia sericata as passive vectors of Mycobacterium avium subsp. avium, M. a. paratuberculosis and M. a. hominissuis. Med. Vet. Entomol. 2004, 18, 116–122. [Google Scholar] [CrossRef]
- Fischer, O.A.; Matlova, L.; Dvorska, L.; Svastova, P.; Bartos, M.; Weston, R.T.; Kopecna, M.; Trcka, I.; Pavlik, I. Potential risk of Mycobacterium avium subspecies paratuberculosis spread by syrphid flies in infected cattle farms. Med. Vet. Entomol. 2005, 19, 360–366. [Google Scholar] [CrossRef]
- Okafor, C.; Grooms, D.; Alocilja, E.; Bolin, S. Fabrication of a novel conductometric biosensor for detecting Mycobacterium avium subsp paratuberculosis antibodies. Sensors 2008, 8, 6015–6025. [Google Scholar] [CrossRef] [PubMed]
- Okafor, C.; Grooms, D.; Alocilja, E.; Bolin, S. Comparison between a Conductometric Biosensor and ELISA in the Evaluation of Johne’s Disease. Sensors 2014, 14, 19128–19137. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.; Wang, Y.L.; Kelton, D.; Neethirajan, S. Isothermal DNA amplification with functionalized graphene and nanoparticle assisted electroanalysis for rapid detection of Johne’s disease. Sens. Actuators B Chem. 2018, 261, 31–37. [Google Scholar] [CrossRef]
- Aboagye, G.; Rowe, M.T. Occurrence of Mycobacterium avium subsp. paratuberculosis in raw water and water treatment operations for the production of potable water. Water Res. 2011, 45, 3271–3278. [Google Scholar] [CrossRef] [PubMed]
- Chern, E.C.; King, D.; Haugland, R.; Pfaller, S. Evaluation of quantitative polymerase chain reaction assays targeting Mycobacterium avium, M. intracellulare, and M. avium subspecies paratuberculosis in drinking water biofilms. J. Water Health 2015, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.S. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis… and colorectal cancer? Infect. Agents Cancer 2018, 13, 1. [Google Scholar] [CrossRef]
- Fechner, K.; Schäfer, J.; Münster, P.; Ternes, K.; Döring, S.; Völkel, I.; Kaup, F.J.; Czerny, C.P. Detection of Mycobacterium avium subspecies paratuberculosis in rock hyraxes (Procavia capensis) imported from South Africa. J. Zoo Wildl. Med. 2017, 48, 1086–1094. [Google Scholar] [CrossRef]
- Lobão-Tello, E.R.; Herbach, E.P.; Navarrete-Talloni, M.J. Paratuberculosis: New histopathological findings in red deer (Cervus elaphus) and fallow deer (Dama dama) in Chile. Pesq. Vet. Brasil 2017, 37, 749–753. [Google Scholar] [CrossRef]
- Bonovska, M.; Savova, T.; Petrova, R.; Valcheva, V.; Najdenski, H. Cases of Paratuberculosis in Deer in Bulgaria. Comptes Rend. Acad. Bulg. Sci. 2019, 72, 422–428. [Google Scholar]
- Rhim, H.R.; Cho, Y.I.; Jang, H.J.; Na, K.J.; Han, J.I. High Prevalence of Mycobacterium avium subsp. paratuberculosis in Wild Ducks in the Middle Area of South Korea. J. Vet. Clin. 2018, 35, 7–9. [Google Scholar] [CrossRef]
- Wolf, R.; Orsel, K.; De Buck, J.; Kanevets, U.; Barkema, H.W. Evaluation of sampling socks for detection of Mycobacterium avium ssp. paratuberculosis on dairy farms. J. Dairy Sci. 2016, 99, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.; Rhodes, G.; Henrys, P.; Sedda, L.; Weightman, A.J.; Pickup, R.W. Presence of Mycobacterium avium subspecies paratuberculosis monitored over varying temporal and spatial scales in river catchments: Persistent routes for human exposure. Microorganisms 2019, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Chamchoy, T.; Williams, D.R.; Adaska, J.M.; Anderson, R.J.; Aly, S.S. Environmental sampling to assess the bioburden of Mycobacterium avium subspecies paratuberculosis in drylot pens on California dairies. PeerJ 2019, 7, e8081. [Google Scholar] [CrossRef] [PubMed]
- Correa-Valencia, N.M.; Ramírez, N.F.; Arango-Sabogal, J.C.; Fecteau, G.; Fernández-Silva, J.A. Prevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds in Northern Antioquia (Colombia) and associated risk factors using environmental sampling. Prev. Vet. Med. 2019, 170, 104739. [Google Scholar] [CrossRef]
- Donat, K.; Eisenberg, S.W.F.; Einax, E.; Reinhold, G.; Zoche-Golob, V. Reduction of viable Mycobacterium avium ssp. paratuberculosis in slurry subjected to anaerobic digestion in biogas plants. J. Dairy Sci. 2019, 102, 6485–6494. [Google Scholar] [CrossRef]
- Corbett, C.S.; Naqvi, S.A.; Bauman, C.A.; De Buck, J.; Orsel, K.; Uehlinger, F.; Kelton, D.F.; Barkema, H.W. Prevalence of Mycobacterium avium ssp. paratuberculosis infections in Canadian dairy herds. J. Dairy Sci. 2018, 101, 11218–11228. [Google Scholar] [CrossRef]
- Corbett, C.S.; Naqvi, S.A.; De Buck, J.; Kanevets, U.; Kastelic, J.P.; Barkema, H.W. Environmental sample characteristics and herd size associated with decreased herd-level prevalence of Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2018, 101, 8092–8099. [Google Scholar] [CrossRef]
- Hahn, N.; Failing, K.; Eisenberg, T.; Schlez, K.; Zschöck, P.M.; Donat, K.; Einax, E.; Köhler, H. Evaluation of different diagnostic methods for the detection of Mycobacterium avium subsp. paratuberculosis in boot swabs and liquid manure samples. BMC Vet. Res. 2017, 13, 259. [Google Scholar] [CrossRef]
- Kochler, J.; Gschaider, S.; Spergser, J.; Tichy, A.; Mader, C.; Vill, M.; Ortners, P.; Kossler, J.; Khol, J.L. Reproducibility of negative boot swab samples for paratuberculosis in cattle herds in Tyrol (Austria). Berl. Münch Tierärztl. Wochensch. 2017, 130, 29–33. [Google Scholar]
- Korou, L.M.; Liandris, E.; Gazouli, M.; Ikonomopoulos, J. Investigation of the association of the SLC11A1 gene with resistance/sensitivity of goats (Capra hircus) to paratuberculosis. Vet. Microbiol. 2010, 144, 353–358. [Google Scholar] [CrossRef]
- Marfell, B.J.; O’Brien, R.; Griffin, J.F.T. Global gene expression profiling of monocyte-derived macrophages from red deer (Cervus elaphus) genotypically resistant or susceptible to Mycobacterium avium subspecies paratuberculosis infection. Dev. Comp. Immunol. 2013, 40, 210–217. [Google Scholar] [CrossRef]
- Toman, M.; Faldyna, M.; Pavlik, I. Immunological characteristics of cattle with Mycobacterium avium subsp. paratuberculosis infection. Vet. Med. 2003, 48, 147–154. [Google Scholar] [CrossRef]
- Singh, S.V.; Dhama, K.; Chaubey, K.K.; Kumar, N.; Singh, P.K.; Sohal, J.S.; Gupta, S.; Singh, A.V.; Verma, A.K.; Tiwari, R.; et al. Impact of host genetics on susceptibility and resistance to Mycobacterium avium subspecies Paratuberculosis infection in domestic ruminants. Pak. J. Biol. Sci. 2013, 16, 251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Homuth, M.; Valentin-Weigand, P.; Rohde, M.; Gerlach, G.F. Identification and characterization of a novel extracellular ferric reductase from Mycobacterium paratuberculosis. Infect. Immune 1998, 66, 710–716. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Singh, R.V.; Chauhan, A.; Kumar, A.; Bharati, J.; Singh, S.V. Association of Bovine CLEC7A gene polymorphism with host susceptibility to paratuberculosis disease in Indian cattle. Res. Vet. Sci. 2019, 123, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Singh, R.V.; Chauhan, A.; Kumar, A.; Sulabh, S.; Bharati, J.; Singh, S.V. Genetic association of polymorphisms in bovine TLR2 and TLR4 genes with Mycobacterium avium subspecies paratuberculosis infection in Indian cattle population. Vet. Res. Commun. 2019, 43, 105–114. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.P.; Purfield, D.C.; Ring, S.C.; Carthy, T.R.; Graham, D.A.; Berry, D.P. Candidate genes associated with the heritable humoral response to Mycobacterium avium ssp. paratuberculosis in dairy cows have factors in common with gastrointestinal diseases in humans. J. Dairy Sci. 2019, 102, 4249–4263. [Google Scholar] [CrossRef]
- Alonso-Hearn, M.; Magombedze, G.; Abendaño, N.; Landin, M.; Juste, R.A. Deciphering the virulence of Mycobacterium avium subsp. paratuberculosis isolates in animal macrophages using mathematical models. J. Theoret. Biol. 2019, 468, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Erre, G.L.; Bach, H.; Slavin, Y.N.; Manchia, P.A.; Passiu, G.; Sechi, L.A. PtpA and PknG Proteins Secreted by Mycobacterium avium subsp. paratuberculosis are recognized by sera from patients with rheumatoid arthritis: A aase–control study. J. Inflamm. Res. 2019, 12, 301. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bo, M.; Caggiu, E.; Sechi, G.; Arru, G.; Bach, H.; Sechi, L.A. High levels of antibodies against PtpA and PknG secreted by Mycobacterium avium ssp. paratuberculosis are present in neuromyelitis optica spectrum disorder and multiple sclerosis patients. J. Neuroimmunol. 2018, 323, 49–52. [Google Scholar] [CrossRef]
- Leao, C.; Botelho, A.; Martins, E.; Aguiar, C.; Rebelo, I.; Nunes, T.; Bexiga, R. Presence of Mycobacterium avium subs. paratuberculosis DNA in milk used to feed calves in Portugal. J. Dairy Sci. 2017, 84, 124–127. [Google Scholar]
- Fechner, K.; Dreymann, N.; Schimkowiak, S.; Czerny, C.P.; Teitzel, J. Efficacy of dairy on-farm high-temperature, short-time pasteurization of milk on the viability of Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 11280–11290. [Google Scholar] [CrossRef] [PubMed]
- Advancing Human MAP Research. Available online: https://humanpara.org/educational-pack-for-patients/ (accessed on 22 February 2020).
- Cossu, A.; Rosu, V.; Paccagnini, D.; Cossu, D.; Pacifico, A.; Sechi, L.A. MAP3738c and MptD are specific tags of Mycobacterium avium Subsp. paratuberculosis infection in type I diabetes mellitus. Clin. Immunol. 2011, 141, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Masala, S.; Frau, J.; Cocco, E.; Marrosu, M.G.; Sechi, L.A. Anti-Mycobacterium avium subsp. paratuberculosis heat shock protein 70 antibodies in the sera of Sardinian patients with multiple sclerosis. J. Neurol. Sci. 2013, 335, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Masala, S.; Cocco, E.; Paccagnini, D.; Tranquilli, S.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Association of Mycobacterium avium subsp. paratuberculosis and SLC11A1 polymorphisms in Sardinian multiple sclerosis patients. J. Infect. Dev. Countries 2013, 7, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Abdel-Aty, A.; Abu-Suwa, H.; Naser, S.A. Oxidative stress due to Mycobacterium avium subspecies paratuberculosis (MAP) infection upregulates selenium-dependent GPx activity. Gut Pathog. 2016, 8, 12. [Google Scholar] [CrossRef]
- Masala, S.; Cossu, D.; Pacifico, A.; Molicotti, P.; Sechi, L.A. Sardinian Type 1 diabetes patients, Transthyretin and Mycobacterium avium subspecies paratuberculosis infection. Gut Pathog. 2012, 4, 24. [Google Scholar] [CrossRef]
- Bo, M.; Arru, G.; Niegowska, M.; Erre, G.L.; Manchia, P.A.; Sechi, L.A. Association between lipoprotein levels and humoral reactivity to Mycobacterium avium subsp. paratuberculosis in multiple sclerosis, type 1 diabetes mellitus and rheumatoid arthritis. Microorganisms 2019, 7, 423. [Google Scholar] [CrossRef]
- Mameli, G.; Cossu, D.; Cocco, E.; Masala, S.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein–Barr virus and Mycobacterium avium subsp. paratuberculosis peptides are cross recognized by anti-myelin basic protein antibodies in multiple sclerosis patients. J. Neuroimmunol. 2014, 270, 51–55. [Google Scholar] [CrossRef]
- Cossu, D.; Masala, S.; Cocco, E.; Paccagnini, D.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Are Mycobacterium avium subsp. paratuberculosis and Epstein–Barr virus triggers of multiple sclerosis in Sardinia? Mult. Scler. J. 2012, 18, 1181–1184. [Google Scholar] [CrossRef]
- Frau, J.; Cossu, D.; Coghe, G.; Lorefice, L.; Fenu, G.; Porcu, G.; Sardu, C.; Murru, M.R.; Tranquilli, S.; Marrosu, M.G.; et al. Role of interferon-beta in Mycobacterium avium subspecies paratuberculosis antibody response in Sardinian MS patients. J. Neurol. Sci. 2015, 349, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Cossu, D.; Caggiu, E.; Arru, G.; Niegowska, M.; Cocco, E.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Soluble BAFF level is not correlated to Mycobacterium avium subspecies paratuberculosis antibodies and increases after interferon-β therapy in multiple sclerosis patients. J. Mol. Neurosci. 2016, 60, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Frau, J.; Cossu, D.; Coghe, G.; Lorefice, L.; Fenu, G.; Melis, M.; Paccagnini, D.; Sardu, C.; Murru, M.R.; Tranquilli, S.; et al. Mycobacterium avium subsp. paratuberculosis and multiple sclerosis in Sardinian patients: Epidemiology and clinical features. Mult. Scler. J. 2013, 19, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Lisby, G.; Milman, N.; Jacobsen, G.K. Search for Mycobacterium paratuberculosis DNA in tissue from patients with sarcoidosis by enzymatic gene amplification. APMIS 1993, 101, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Celler, B.G. Case study: Cardiac sarcoidosis resolved with Mycobacterium avium paratuberculosis antibiotics (MAP). Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 171–177. [Google Scholar]
- Gupta, S.; Singh, S.V.; Gururaj, K.; Chaubey, K.K.; Singh, M.; Lahiri, B.; Agarwal, P.; Kumar, A.; Misri, J.; Hemati, Z.; et al. Comparison of IS900 PCR with ‘Taqman probe PCR’and ‘SYBR Green Real time PCR’assays in patients suffering with thyroid disorder and sero-positive for Mycobacterium avium subspecies paratuberculosis. IJBT 2017, 16, 228–234. Available online: http://nopr.niscair.res.in/handle/123456789/42918 (accessed on 22 February 2020).
- Cossu, D.; Yokoyama, K.; Sechi, L.A.; Otsubo, S.; Tomizawa, Y.; Momotani, E.; Hattori, N. Humoral response against host-mimetic homologous epitopes of Mycobacterium avium subsp. paratubrculosis in Japanes multiple sclerosis patients. Sci. Rep. 2016, 6, 29227. [Google Scholar] [CrossRef]
- Del Prete, R.; Quaranta, M.; Lippolis, A.; Giannuzzi, V.; Mosca, A.; Jirillo, E.; Miragliotta, G. Detection of Mycobacterium paratuberculosis in stool samples of patients with inflammatory bowel disease by IS900-based PCR and colorimetric detection of amplified DNA. J. Microbiol. Methds 1998, 33, 105–114. [Google Scholar] [CrossRef]
- Elguezabal, N.; Chamorro, S.; Molina, E.; Garrido, J.M.; Izeta, A.; Rodrigo, L.; Juste, R.A. Lactase persistence, NOD2 status and Mycobacterium avium subsp. paratuberculosis infection associations to Inflammatory Bowel Disease. Gut Pathog. 2012, 4, 6. [Google Scholar] [CrossRef]
- Ren, Z.; Turton, J.; Borody, T.; Pang, G.; Clancy, R. Selective Th2 pattern of cytokine secretion in Mycobacterium avium subsp. paratuberculosis infected Crohn’s disease. J. Gastroenterol. Hepatol. 2008, 23, 310–314. [Google Scholar] [CrossRef]
- Click, R.E. A 60-day probiotic protocol with Dietzia subsp. C79793-74 prevents development of Johne’s disease parameters after in utero and/or neonatal MAP infection. Virulence 2011, 2, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Stempak, J.M.; Grist, J.; Bressler, B.; Silverberg, M.S.; Bach, H. Effect of inflammatory bowel disease therapies on immunogenicity of Mycobacterium paratuberculosis proteins. Scand. J. Gastroenterol. 2014, 49, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.C.; Day, A.S.; Pearson, J.; Barclay, M.L.; Gearry, R.B.; Roberts, R.L.; Bentley, R.W. SLC11A1 polymorphisms in inflammatory bowel disease and Mycobacterium avium subspecies paratuberculosis status. World J. Gastroenterol. WJG 2010, 16, 5727. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.A.; Schwarz, D.G.G.; Pietralonga, P.A.G.; Faria, A.C.S.; Braga, I.F.E.; Carvalho, G.D.; Valente, F.L.; Machado, J.P.; Guimarães, L.M.P.; Ferrari, M.D.L.A.; et al. Presence of Mycobacterium avium subsp. paratuberculosis (MAP) in Brazilian patients with inflammatory bowel diseases and in controls. Sao Paulo Med. J. 2016, 134, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.; Ren, Z.; Turton, J.; Pang, G.; Wettstein, A. Molecular evidence for Mycobacterium avium subspecies paratuberculosis (MAP) in Crohn’s disease correlates with enhanced TNF-α secretion. Digest. Liver Dis. 2007, 39, 445–451. [Google Scholar] [CrossRef]
- Al-Shamali, M.; Khan, I.; Al-Nakib, B.; Al-Hassan, F.; Mustafa, A.S. A multiplex polymerase chain reaction assay for the detection of Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Scand. J. Gastroenterol. 1997, 32, 819–823. [Google Scholar] [CrossRef]
- Momotani, E.; Romona, N.M.; Yoshihara, K.; Momotani, Y.; Hori, M.; Ozaki, H.; Eda, S.; Ikegami, M. Molecular pathogenesis of bovine paratuberculosis and human inflammatory bowel diseases. Vet. Immunol. Immunopathol. 2012, 148, 55–68. [Google Scholar] [CrossRef]
- Arru, G.; Caggiu, E.; Paulus, K.; Sechi, G.P.; Mameli, G.; Sechi, L.A. Is there a role for Mycobacterium avium subspecies paratuberculosis in Parkinson’s disease? J. Neuroimmunol. 2016, 293, 86–90. [Google Scholar] [CrossRef]
- Dow, C.T. M. paratuberculosis and Parkinson’s diseases—Is this a trigger. Med. Hypotheses 2014, 83, 709–712. [Google Scholar] [CrossRef]
- Naser, A.; Odeh, A.K.; Sharp, R.C.; Qasem, A.; Beg, S.; Naser, S.A. Polymorphisms in TNF receptor superfamily 1B (TNFRSF1B: rs3397) are linked to Mycobacterium avium paratuberculosis infection and osteoporosis in rheumatoid arthritis. Microorganisms 2019, 7, 646. [Google Scholar] [CrossRef]
- Bo, M.; Erre, G.L.; Niegowska, M.; Piras, M.; Taras, L.; Longu, M.G.; Passiu, G.; Sechi, L.A. Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis: Investigating a mechanism of molecular mimicry. Clin. Exp. Rheumatol. 2018, 36, 376–381. [Google Scholar] [PubMed]
- Dudemaine, P.L.; Fecteau, G.; Lessard, M.; Labrecque, O.; Roy, J.P.; Bissonnette, N. Increased blood-circulating interferon-γ, interleukin-17, and osteopontin levels in bovine paratuberculosis. J. Dairy Sci. 2014, 97, 3382–3393. [Google Scholar] [CrossRef] [PubMed]
- Karcher, E.L.; Johnson, C.S.; Beitz, D.C.; Stabel, J.R. Osteopontin immunoreactivity in the ileum and ileocecal lymph node of dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 2008, 126, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Karcher, E.L.; Bayles, D.O.; Bannantine, J.P.; Beitz, D.C.; Stabel, J.R. Osteopontin: A novel cytokine involved in the regulation of Mycobacterium avium subspecies paratuberculosis infection in periparturient dairy cattle. J. Dairy Sci. 2008, 91, 3079–3091. [Google Scholar] [CrossRef]
- De Laat, M.; Lally, V.; Lipponen, L.; Simons, R.J. Investigating patterns of interaction in networked learning and computer-supported collaborative learning: A role for Social Network Analysis. Int. J. Comput. Supported Collab. Learn. 2007, 2, 87–103. [Google Scholar] [CrossRef]
- McCulloh, I.; Armstrong, H.; Johnson, A. Social Network Analysis with Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1 1118169476. ISBN 2 9781118169476. [Google Scholar]
- Bill, H. Network Diameter and Emotional Values in the Global Brain. Available online: http://www.ssec.wisc.edu/~billh/gbrain0.html (accessed on 28 March 2020).
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, N. Analysis of average shortest-path length of scale-free network. J. Appl. Math. 2013, 2013, 865643. [Google Scholar] [CrossRef]




| Attributes | Counts/Rates |
|---|---|
| Documents | 3889 |
| Sources | 581 |
| Keywords Plus | 5175 |
| Author’s Keywords | 3110 |
| Average citations/documents | 20.65 |
| Authors | 6662 |
| Author Appearances | 18,666 |
| Authors of single-authored documents | 217 |
| Authors of multi-authored documents | 6445 |
| Single-authored documents | 348 |
| Documents/ Author | 0.584 |
| Authors/Document | 1.71 |
| Co-Authors/Documents | 4.8 |
| Collaboration Index | 1.82 |
| Document types | |
| Article | 3737 |
| Article, book chapter | 38 |
| Article, early access | 3 |
| Article, proceedings paper | 111 |
| R | Authors | Articles | % of 3889 | AGR | ADY | PDLY | h-Index |
|---|---|---|---|---|---|---|---|
| 1 | Whittington, R.J. | 143 | 3.68 | 0 | 4.3 | 10.8 | 34 |
| 2 | Collins, M.T. | 130 | 3.34 | 30 | 1.7 | 5.6 | 35 |
| 3 | Stabel, J.R. | 125 | 3.21 | −100 | 2.7 | 8.3 | 30 |
| 4 | Singh, S.V. | 111 | 2.85 | −170 | 6 | 27.7 | 26 |
| 5 | Bannantine, J.P. | 109 | 2.80 | 30 | 4.7 | 14.4 | 29 |
| 6 | Whitlock, R.H. | 84 | 2.16 | −30 | 0 | 0 | 28 |
| 7 | Sohal, J.S. | 71 | 1.83 | −80 | 2.7 | 17.4 | 25 |
| 8 | Juste, R.A. | 64 | 1.65 | −30 | 2.3 | 14.3 | 21 |
| 9 | Nielsen, S.S. | 64 | 1.65 | −130 | 0.7 | 3.5 | 23 |
| 10 | Pavlik, I. | 60 | 1.54 | 0 | 0 | 0 | 22 |
| 11 | Sechi, L.A. | 59 | 1.52 | −170 | 3.3 | 18.9 | 19 |
| 12 | Singh, P.K. | 58 | 1.49 | 0 | 0 | 0 | 25 |
| 13 | Stevenson, K. | 57 | 1.47 | −100 | 1 | 6.5 | 24 |
| 14 | Singh, A.V. | 55 | 1.41 | 0 | 0 | 0 | 25 |
| 15 | Barkema, H.W. | 54 | 1.39 | −200 | 3.3 | 20.4 | 19 |
| 16 | Begg, D.J. | 54 | 1.39 | 0 | 3 | 20.9 | 15 |
| 17 | De Buck, J. | 52 | 1.34 | −200 | 3 | 22 | 15 |
| 18 | Koets, A.P. | 51 | 1.31 | 0 | 0.3 | 3.7 | 15 |
| 19 | Garrido, J.M. | 50 | 1.29 | 30 | 2 | 14.3 | 19 |
| 20 | Grant, I.R. | 49 | 1.26 | −70 | 1.7 | 12.2 | 27 |
| 21 | Schukken, Y.H. | 49 | 1.26 | −100 | 1.7 | 12.8 | 19 |
| 22 | Wells, S.J. | 49 | 1.26 | 30 | 1 | 7.7 | 21 |
| S/n | Author | Paper | Reference | TC | TCperYear |
|---|---|---|---|---|---|
| 1 | Ott et al. | “Herd-level economic losses associated with Johne’s disease on US dairy operations” | [6] | 439 | 20.9 |
| 2 | Naser et al. | “Culture of Mycobacterium avium subspecies Para tuberculosis from the blood of patients with Crohn’s disease” | [31] | 434 | 27.12 |
| 3 | Green et al. | “Sequence and characteristics or IS 900, an insertion element identified in a human Crohn’s disease isolate or Mycobacterium Para tuberculosis” | [32] | 370 | 11.94 |
| 4 | Sanderson et al. | “Mycobacterium Para tuberculosis DNA in Crohn’s disease tissue” | [33] | 322 | 11.5 |
| 5 | Li et al. | “The complete genome sequence of Mycobacterium avium subspecies Para tuberculosis” | [34] | 316 | 21.07 |
| 6 | Sweeney et al. | “Mycobacterium Para tuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows” | [35] | 315 | 11.25 |
| 7 | Bull et al. | “Detection and verification of Mycobacterium avium subsp. Para tuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease” | [29] | 307 | 18.06 |
| 8 | Vary et al. | “Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium Para tuberculosis in Johne’s disease” | [36] | 305 | 10.17 |
| 9 | Sweeney et al. | “Mycobacterium Para tuberculosis isolated from fetuses of infected cows not manifesting signs of the disease” | [37] | 299 | 10.68 |
| R | Country | Articles | % of 3889 | Freq (%) | SCA | SCA% of 3889 | SCA/Articles (%) | MCA | % of 3889 | MCA/Articles (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | USA | 887 | 22.81 | 26.20 | 726 | 18.67 | 81.85 | 161 | 4.14 | 18.15 |
| 2 | Australia | 236 | 6.07 | 6.97 | 209 | 5.37 | 88.56 | 27 | 0.69 | 11.44 |
| 3 | India | 204 | 5.25 | 6.02 | 180 | 4.63 | 88.24 | 24 | 0.62 | 11.77 |
| 4 | Canada | 199 | 5.12 | 5.88 | 159 | 4.09 | 79.90 | 40 | 1.03 | 20.10 |
| 5 | United Kingdom | 192 | 4.94 | 5.67 | 148 | 3.81 | 77.08 | 44 | 1.13 | 22.92 |
| 6 | Germany | 183 | 4.71 | 5.40 | 155 | 3.99 | 84.70 | 28 | 0.72 | 15.30 |
| 7 | Italy | 125 | 3.21 | 3.69 | 91 | 2.34 | 72.80 | 34 | 0.87 | 27.2 |
| 8 | Spain | 113 | 2.91 | 3.34 | 88 | 2.26 | 77.88 | 25 | 0.64 | 22.12 |
| 9 | Ireland | 105 | 2.70 | 3.10 | 80 | 2.06 | 76.19 | 25 | 0.64 | 23.81 |
| 10 | New Zealand | 94 | 2.42 | 2.78 | 74 | 1.90 | 78.72 | 20 | 0.51 | 21.28 |
| 11 | Netherlands | 91 | 2.34 | 2.69 | 64 | 1.65 | 70.33 | 27 | 0.69 | 29.67 |
| 12 | Denmark | 82 | 2.11 | 2.42 | 72 | 1.85 | 87.80 | 10 | 0.26 | 12.20 |
| 13 | France | 73 | 1.88 | 2.16 | 55 | 1.41 | 75.34 | 18 | 0.46 | 24.66 |
| 14 | Brazil | 66 | 1.70 | 1.95 | 62 | 1.59 | 93.94 | 4 | 0.10 | 6.06 |
| 15 | Czech Republic | 61 | 1.57 | 1.80 | 49 | 1.26 | 80.33 | 12 | 0.31 | 19.67 |
| 16 | Iran | 50 | 1.29 | 1.48 | 44 | 1.13 | 88.00 | 6 | 0.15 | 12.00 |
| 17 | Japan | 50 | 1.29 | 1.48 | 36 | 0.93 | 72.00 | 14 | 0.36 | 28.00 |
| 18 | Norway | 44 | 1.13 | 1.30 | 38 | 0.98 | 86.36 | 6 | 0.15 | 13.64 |
| 19 | Austria | 43 | 1.11 | 1.27 | 32 | 0.82 | 74.42 | 11 | 0.28 | 25.58 |
| 20 | Chile | 43 | 1.11 | 1.27 | 22 | 0.57 | 51.16 | 21 | 0.54 | 48.84 |
| 21 | Belgium | 41 | 1.05 | 1.21 | 35 | 0.90 | 85.37 | 6 | 0.15 | 14.63 |
| 22 | Argentina | 40 | 1.03 | 1.18 | 34 | 0.87 | 85.00 | 6 | 0.15 | 15.00 |
| 23 | Korea | 38 | 0.98 | 1.12 | 32 | 0.82 | 84.21 | 6 | 0.15 | 15.79 |
| 24 | Switzerland | 26 | 0.67 | 0.77 | 19 | 0.49 | 73.08 | 7 | 0.18 | 26.92 |
| 25 | Turkey | 26 | 0.67 | 0.77 | 26 | 0.67 | 100.00 | 0 | 0.00 | 0 |
| 26 | Greece | 25 | 0.64 | 0.74 | 15 | 0.39 | 60.00 | 10 | 0.26 | 40.00 |
| 27 | Poland | 25 | 0.64 | 0.74 | 23 | 0.59 | 92.00 | 2 | 0.05 | 8.00 |
| 28 | Portugal | 21 | 0.54 | 0.62 | 16 | 0.41 | 76.19 | 5 | 0.13 | 23.81 |
| 29 | Colombia | 19 | 0.49 | 0.56 | 15 | 0.39 | 78.95 | 4 | 0.10 | 21.05 |
| 30 | Hungary | 19 | 0.49 | 0.56 | 14 | 0.36 | 73.68 | 5 | 0.13 | 26.32 |
| 31 | Mexico | 19 | 0.49 | 0.56 | 17 | 0.44 | 89.47 | 2 | 0.05 | 10.53 |
| 32 | Saudi Arabia | 19 | 0.49 | 0.56 | 14 | 0.36 | 73.68 | 5 | 0.13 | 26.32 |
| 33 | Sweden | 16 | 0.41 | 0.47 | 10 | 0.26 | 62.50 | 6 | 0.15 | 37.5 |
| 34 | China | 15 | 0.39 | 0.44 | 13 | 0.33 | 86.67 | 2 | 0.05 | 13.33 |
| 35 | Pakistan | 11 | 0.28 | 0.32 | 11 | 0.28 | 100.00 | 0 | 0.00 | 0 |
| 36 | Egypt | 9 | 0.23 | 0.27 | 8 | 0.21 | 88.89 | 1 | 0.03 | 11.11 |
| 37 | Israel | 9 | 0.23 | 0.27 | 4 | 0.10 | 44.44 | 5 | 0.13 | 55.56 |
| 38 | Iceland | 6 | 0.15 | 0.18 | 5 | 0.13 | 83.33 | 1 | 0.03 | 16.67 |
| 39 | Jordan | 6 | 0.15 | 0.18 | 5 | 0.13 | 83.33 | 1 | 0.03 | 16.67 |
| 40 | Slovakia | 6 | 0.15 | 0.18 | 4 | 0.10 | 66.67 | 2 | 0.05 | 33.33 |
| 41 | Slovenia | 4 | 0.10 | 0.12 | 4 | 0.10 | 100.00 | 0 | 0.00 | 0 |
| 42 | South Africa | 4 | 0.10 | 0.12 | 4 | 0.10 | 100.00 | 0 | 0.00 | 0 |
| 43 | Sudan | 4 | 0.10 | 0.12 | 4 | 0.10 | 100.00 | 0 | 0.00 | 0 |
| 44 | Uganda | 3 | 0.08 | 0.09 | 1 | 0.03 | 33.33 | 2 | 0.05 | 66.67 |
| 45 | Venezuela | 3 | 0.08 | 0.09 | 3 | 0.08 | 100.00 | 0 | 0.00 | 0 |
| 46 | Bulgaria | 2 | 0.05 | 0.06 | 2 | 0.05 | 100.00 | 0 | 0.00 | 0 |
| 47 | Morocco | 2 | 0.05 | 0.06 | 1 | 0.03 | 50.00 | 1 | 0.03 | 50.00 |
| 48 | Peru | 2 | 0.05 | 0.06 | 2 | 0.05 | 100.00 | 0 | 0.00 | 0 |
| 49 | Philippines | 2 | 0.05 | 0.06 | 2 | 0.05 | 100.00 | 0 | 0.00 | 0 |
| 50 | Taiwan | 2 | 0.05 | 0.06 | 1 | 0.03 | 50.00 | 1 | 0.03 | 50.00 |
| 51 | Albania | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 52 | Algeria | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 53 | Bhutan | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 54 | Cameroon | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 55 | Costa Rica | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 56 | Croatia | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 57 | Cyprus | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 58 | Finland | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 59 | Ghana | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 60 | Indonesia | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 61 | Iraq | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 62 | Kuwait | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 63 | Nepal | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 64 | Oman | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 65 | Panama | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 66 | Russia | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 67 | Singapore | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 68 | Thailand | 1 | 0.03 | 0.03 | 1 | 0.03 | 100.00 | 0 | 0.00 | 0 |
| 69 | U Arab Emirates | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| 70 | Uruguay | 1 | 0.03 | 0.03 | 0 | 0.00 | 0.00 | 1 | 0.03 | 100.00 |
| R | Sources | Articles | % of 3889 | AGR | ADY | PDLY | h-Index | IJCR(SJR) |
|---|---|---|---|---|---|---|---|---|
| 1 | Veterinary Microbiology | 186 | 4.78 | −70 | 2 | 3.5 | 44 | 2.791(1.17) |
| 2 | Journal of Dairy Science | 162 | 4.17 | 0 | 13.3 | 25.3 | 33 | |
| 3 | Preventive Veterinary Medicine | 147 | 3.78 | −100 | 4.3 | 9.5 | 34 | 2.302(1.1) |
| 4 | Veterinary Immunology and Immunopathology | 137 | 3.52 | −30 | 4 | 10.3 | 25 | |
| 5 | American Journal of Veterinary Research | 133 | 3.42 | 0 | 0 | 0 | 30 | 1.070(0.63) |
| 6 | Journal of Clinical Microbiology | 110 | 2.83 | −30 | 0 | 0 | 49 | 4.959(2.31) |
| 7 | Journal of Veterinary Diagnostic Investigation | 85 | 2.19 | 0 | 1.7 | 6.3 | 25 | 1.174(0.6) |
| 8 | Australian Veterinary Journal | 82 | 2.11 | −30 | 0 | 0 | 25 | 0.887(0.41) |
| 9 | Applied and Environmental Microbiology | 72 | 1.85 | −70 | 0 | 0 | 38 | 4.077(1.66) |
| 10 | Infection and Immunity | 72 | 1.85 | 0 | 0.7 | 3 | 35 | 3.160(1.59) |
| 11 | Plos One | 68 | 1.75 | −300 | 4.3 | 19.1 | 22 | 2.776(1.1) |
| 12 | Journal of The American Veterinary Medical Association | 62 | 1.59 | 0 | 0 | 0 | 30 | |
| 13 | Veterinary Record | 59 | 1.52 | 70 | 2.3 | 11.7 | 18 | |
| 14 | Veterinary Research | 52 | 1.34 | −100 | 2.7 | 19.5 | 15 | |
| 15 | Journal of Comparative Pathology | 43 | 1.11 | 0 | 0 | 0 | 17 | |
| 16 | Clinical and Vaccine Immunology | 41 | 1.05 | 0 | 0.7 | 5.1 | 17 | 3.233(1.4) |
| 17 | Berliner Und Munchener Tierarztliche Wochenschrift | 39 | 1.00 | −30 | 2.7 | 27.6 | 8 | |
| 18 | Small Ruminant Research | 36 | 0.93 | 0 | 1.3 | 12.1 | 11 | |
| 19 | Pesquisa Veterinaria Brasileira | 34 | 0.87 | −30 | 0.7 | 9.5 | 8 | |
| 20 | Acta Veterinaria Scandinavica | 33 | 0.85 | 0 | 0 | 0 | 11 |
| Topic | No. Articles | % of 3889 | AGR | ADY | PDLY | h-Index |
|---|---|---|---|---|---|---|
| Livestock | 634 | 16.30 | −30.00 | 27 | 12.8 | 51 |
| Milk | 203 | 5.22 | −130.00 | 12.7 | 18.7 | 35 |
| Dairy | 154 | 3.96 | 0.00 | 8.3 | 16.2 | 28 |
| Feces | 57 | 1.47 | −70.00 | 2.3 | 12.3 | 18 |
| Antibody | 43 | 1.11 | 0.00 | 2 | 14 | 15 |
| Wildlife | 43 | 1.11 | 30.00 | 1.3 | 9.3 | 13 |
| Environments | 32 | 0.82 | 100.00 | 3.7 | 34.4 | 12 |
| Virulence | 14 | 0.36 | 70.00 | 1.3 | 28.6 | 7 |
| Water | 12 | 0.31 | −30.00 | 1 | 25 | 6 |
| Resistance | 8 | 0.21 | 100.00 | 1 | 37.5 | 5 |
| Meat and meat products | 6 | 0.15 | 30.00 | 0.3 | 16.7 | 5 |
| Wastewaters | 4 | 0.10 | 30.00 | 0.7 | 50 | 2 |
| Feed | 3 | 0.08 | 0.00 | 0.3 | 33.3 | 2 |
| Microbiota | 3 | 0.08 | −30.00 | 0 | 0 | 3 |
| Paediatric | 3 | 0.08 | 0.00 | 0 | 0 | 3 |
| Anaerobic digestion | 2 | 0.05 | 30.00 | 0.7 | 100 | 1 |
| Manure | 0 | 0.00 | 0.00 | 0 | 0 | 0 |
| Pasture/pasteurisation | 0 | 0.00 | 0.00 | 0 | 0 | 0 |
| Sewage | 0 | 0.00 | 0.00 | 0 | 0 | 0 |
| Urine | 0 | 0.00 | 0.00 | 0 | 0 | 0 |
| Disorder | Total | AGR | ADY | PDLY | h-Index |
|---|---|---|---|---|---|
| Ulcerative colitis | 13 | −30 | 0 | 0 | 9 |
| Multiple sclerosis | 10 | 0 | 0.7 | 20 | 6 |
| Resistance (disease) | 8 | 100 | 1 | 37.5 | 5 |
| Type 1 diabetes | 6 | 70 | 1 | 50 | 4 |
| Osteopontin | 5 | 70 | 0.7 | 40 | 2 |
| Rheumatoid arthritis | 4 | 100 | 1.3 | 100 | 1 |
| Sarcoidosis | 2 | 0 | 0.3 | 50 | 1 |
| Thyroid disorders | 2 | 0 | 0.7 | 100 | 2 |
| Parkinson’s disease | 1 | −30 | 0 | 0 | 1 |
| transthyretin | 1 | 0 | 0 | 0 | 1 |
| Psoriasis | 0 | 0 | 0 | 0 | 0 |
| Lupus | 0 | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekundayo, T.C.; Okoh, A.I. Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms 2020, 8, 1212. https://doi.org/10.3390/microorganisms8081212
Ekundayo TC, Okoh AI. Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms. 2020; 8(8):1212. https://doi.org/10.3390/microorganisms8081212
Chicago/Turabian StyleEkundayo, Temitope C., and Anthony I. Okoh. 2020. "Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases" Microorganisms 8, no. 8: 1212. https://doi.org/10.3390/microorganisms8081212
APA StyleEkundayo, T. C., & Okoh, A. I. (2020). Systematic Assessment of Mycobacterium avium Subspecies Paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms, 8(8), 1212. https://doi.org/10.3390/microorganisms8081212





