Mouse Abdominal Fat Depots Reduced by Butyric Acid-Producing Leuconostoc mesenteroides
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
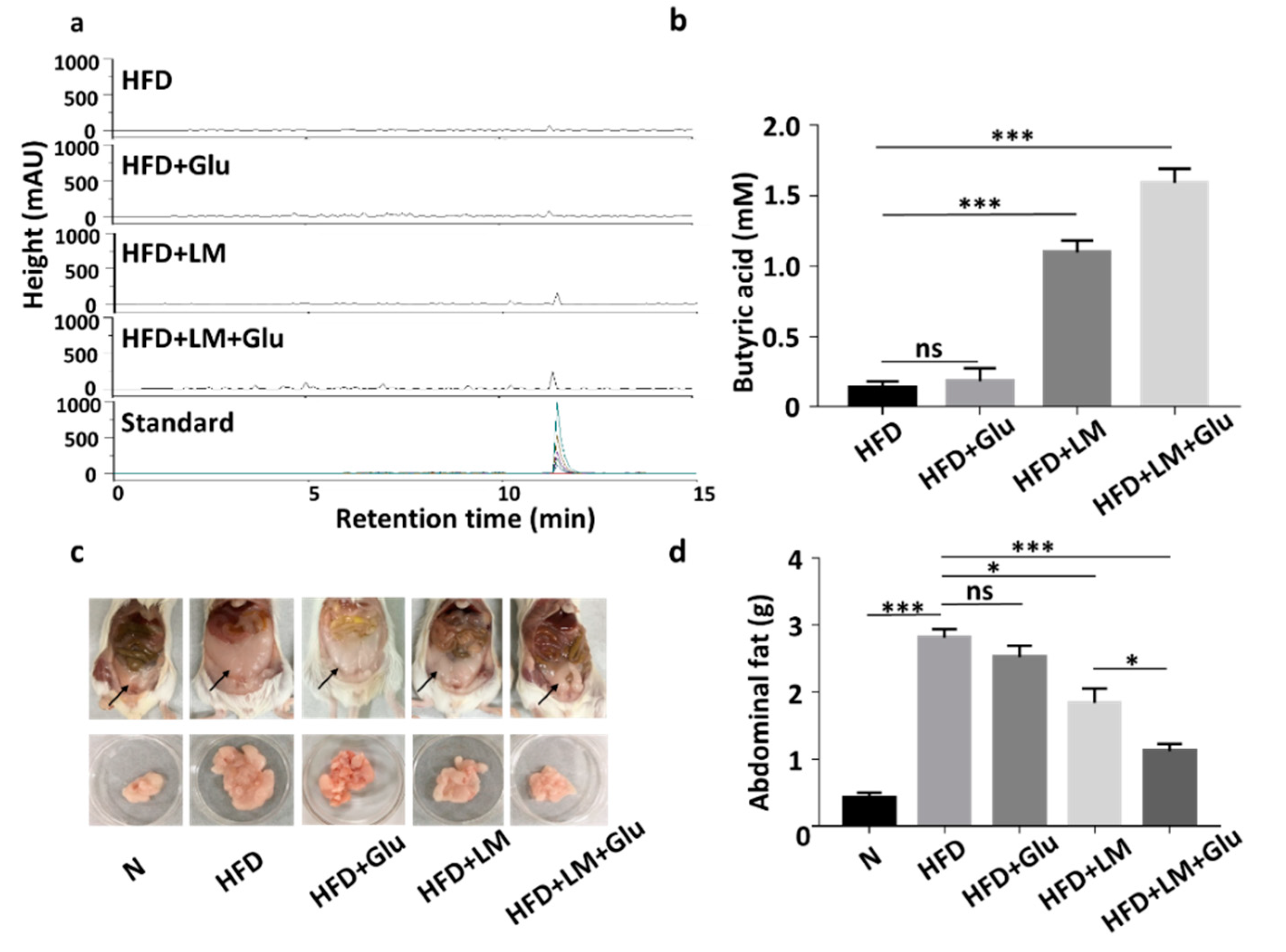
2.2. Glucose Fermentation of L. mesenteroides EH-1
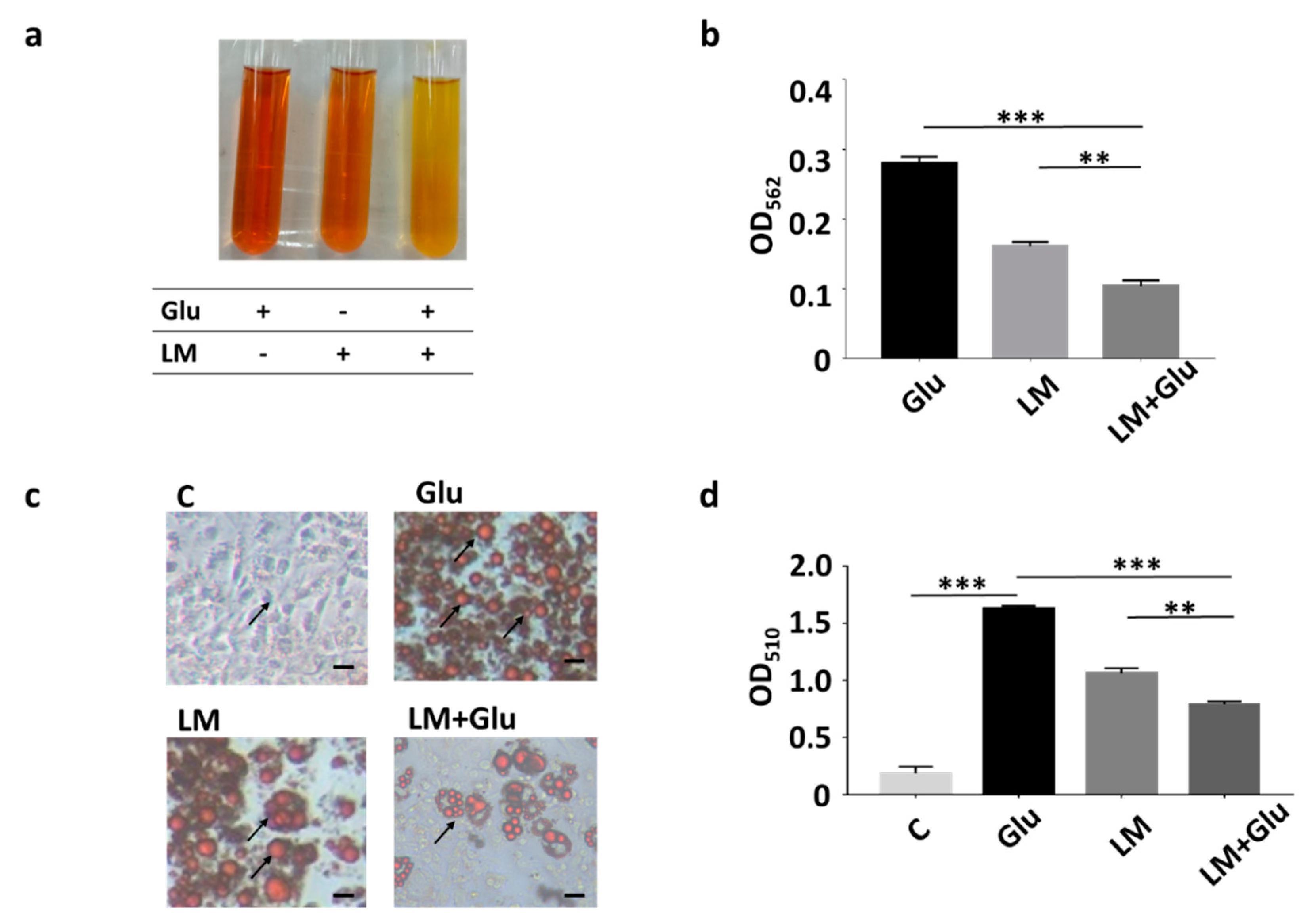
2.3. T3-L1 Cell Differentiation and Oil Red O Staining
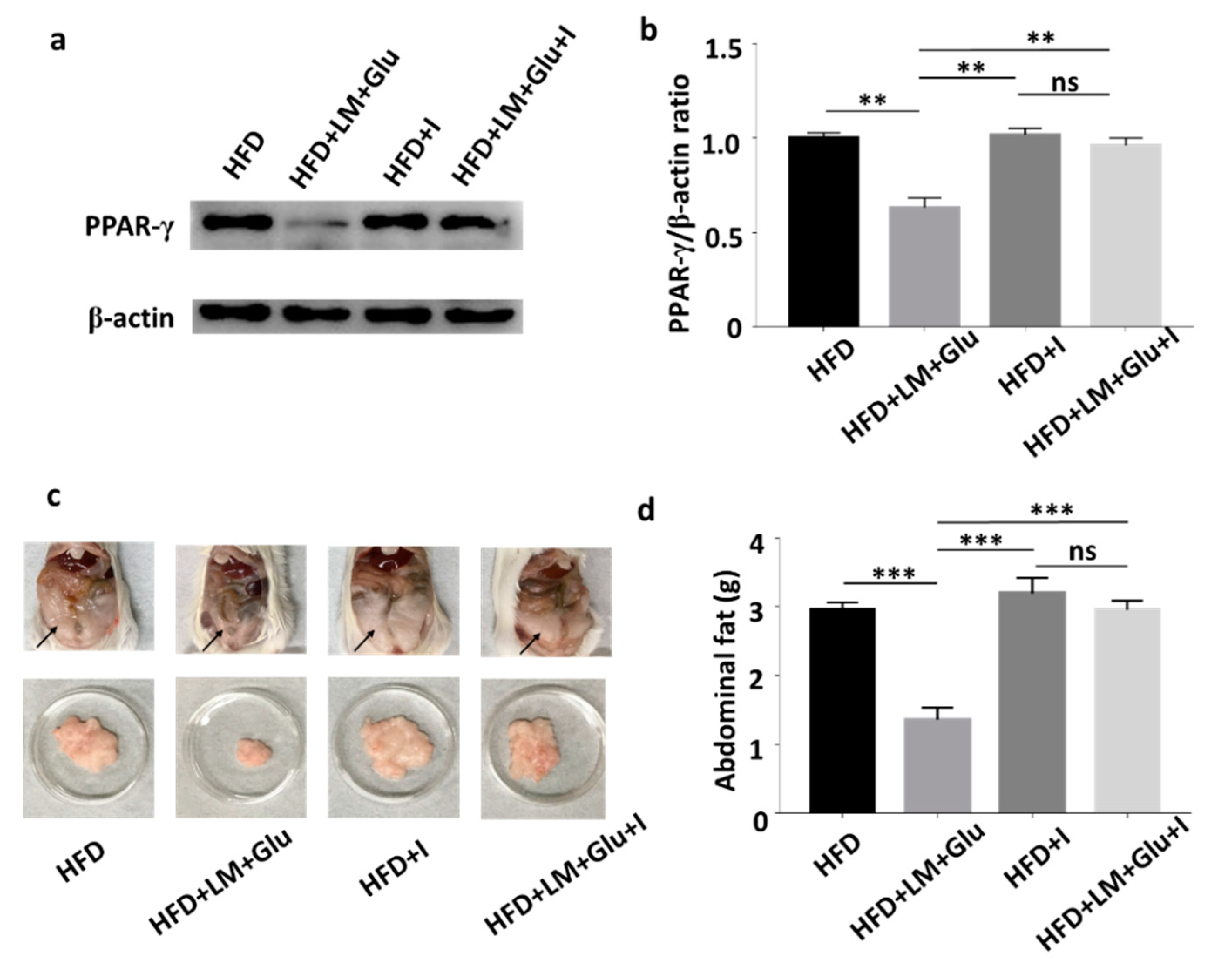
2.4. Feeding Mice with L. mesenteroides EH-1 and Ffar2 Inhibition
2.5. Butyric Acid Detection by High-Performance Liquid Chromatography (HPLC)
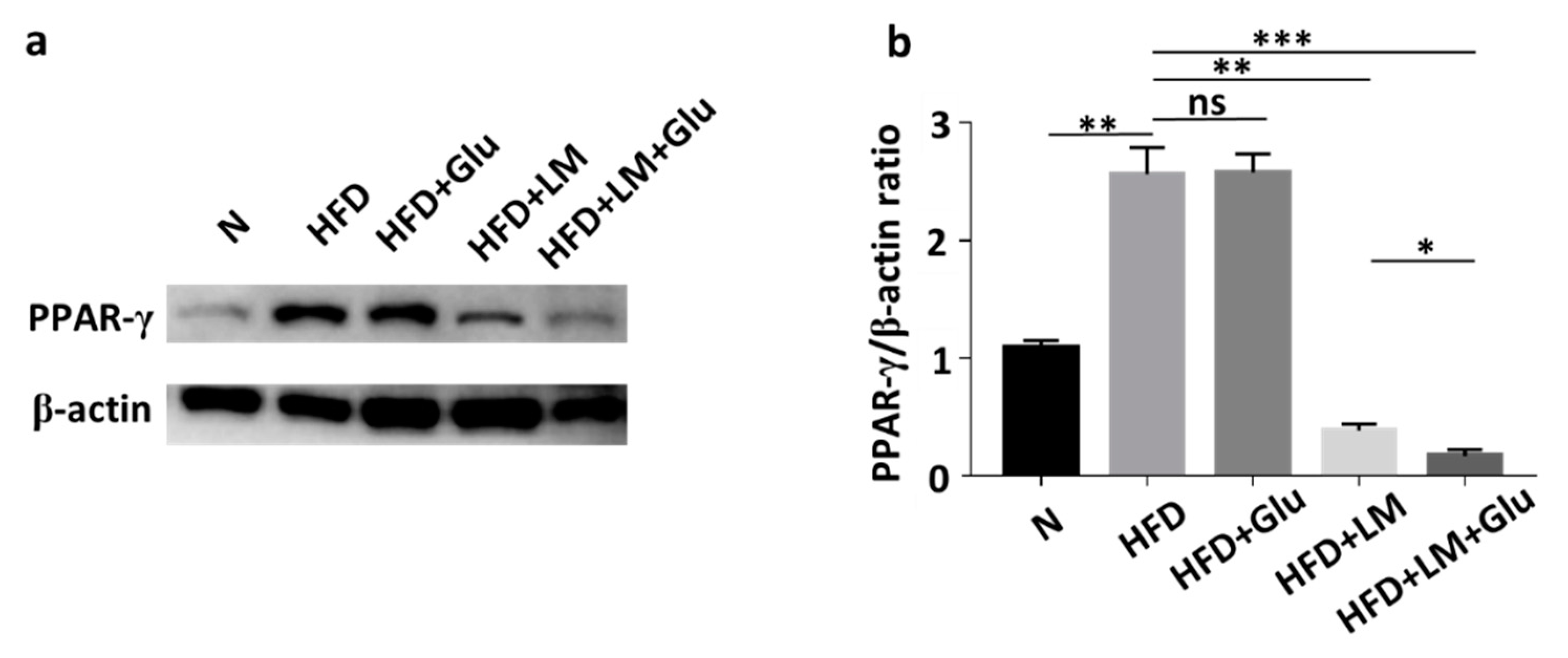
2.6. PPAR-γ Expression
2.7. Statistical Analysis
3. Results
3.1. Reduction of Lipid Droplets in Differentiated 3T3-L1 Adipocytes by L. mesenteroides EH-1
3.2. In Vivo Production of Butyric Acid and Reduction of HFD-Induced Abdominal Fats by L. mesenteroides EH-1
3.3. Suppression of HFD-Induced PPAR-γ Upregulation by L. mesenteroides EH-1
3.4. Effects of Ffar2 Inhibitor Administration with L. mesenteroides EH-1 Probiotic Diet on PPAR-γ Level in High-Fat Diet Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, U.A.; Fitch, M.D.; Beyl, R.A.; Hellerstein, M.K.; Ravussin, E. Differences in in vivo cellular kinetics in abdominal and femoral subcutaneous adipose tissue in women. Diabetes 2016, 65, 1642–1647. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Nakamura, T.; Shimomura, I.; Kotani, K. Visceral fat accumulation and cardiovascular disease. Obes. Res. 1995, 3, 645S–647S. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ohno, T.; Hada, N.; Fujiyoshi, M.; Kuga, M.; Nishimura, M.; Murai, A.; Horio, F. Genetic analysis of abdominal fat distribution in SM/J and A/J mice. J. Lipid Res. 2010, 51, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, K. Cold Spring Harbor Perspect. Biol 2012, 4, 1485–1495. [Google Scholar]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Scott, J.M.; Graham, A.; Chen, Y.; Deuster, P.A. Control diet in a high-fat diet study in mice: Regular chow and purified low-fat diet have similar effects on phenotypic, metabolic, and behavioral outcomes. Nutr. Neurosci. 2019, 22, 19–28. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hållenius, F.F.; Nyman, M. Monobutyrin reduces liver cholesterol and improves intestinal barrier function in rats fed high-fat diets. Nutrients 2019, 11, 308. [Google Scholar] [CrossRef]
- Iván, J.; Major, E.; Sipos, A.; Kovács, K.; Horváth, D.; Tamás, I.; Bay, P.; Dombrádi, V.; Lontay, B. The short-chain fatty acid propionate inhibits adipogenic differentiation of human chorion-derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef]
- Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. 2012, 3, 111. [Google Scholar] [CrossRef]
- Jocken, J.W.; González Hernández, M.A.; Hoebers, N.T.; van der Beek, C.M.; Essers, Y.P.; Blaak, E.E.; Canfora, E.E. Short-chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front. Endocrinol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Hague, A.; Butt, A.J.; Paraskeva, C. The role of butyrate in human colonic epithelial cells: An energy source or inducer of differentiation and apoptosis? Proc. Nutr. Soc. 1996, 55, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Liu, L.; Liu, C.; Han, X. Ophiopogonin D alleviates high-fat diet–induced metabolic syndrome and changes the structure of gut microbiota in mice. FASEB J. 2018, 32, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, W.; Schwengers, D.; Buchholz, K.; Vandamme, E. A wide range of carbohydrate modifications by a single micro-organism: Leuconostoc mesenteroides. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 10, pp. 351–358. [Google Scholar]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Traisaeng, S.; Batsukh, A.; Chuang, T.-H.; Herr, D.R.; Huang, Y.-F.; Chimeddorj, B.; Huang, C.-M. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Akiba, Y.; Maruta, K.; Narimatsu, K.; Said, H.; Kaji, I.; Kuri, A.; Iwamoto, K.-i.; Kuwahara, A.; Kaunitz, J.D. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am. J. Physiol. Gastrl. 2017, 313, G117–G128. [Google Scholar] [CrossRef]
- Sikder, K.; Shukla, S.K.; Patel, N.; Singh, H.; Rafiq, K. High fat diet upregulates fatty acid oxidation and ketogenesis via intervention of PPAR-γ. Cell. Physiol. Biochem. 2018, 48, 1317–1331. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Johanningsmeier, S.; McFeeters, R.F.; Fleming, H.P.; Thompson, R.L. Effects of Leuconostoc mesenteroides starter culture on fermentation of cabbage with reduced salt concentrations. J. Food Sci. 2007, 72, M166–M172. [Google Scholar] [CrossRef]
- Liu, S.; Ren, F.; Zhao, L.; Jiang, L.; Hao, Y.; Jin, J.; Zhang, M.; Guo, H.; Lei, X.; Sun, E. Starch and starch hydrolysates are favorable carbon sources for Bifidobacteria in the human gut. BMC Microbiol. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology e-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Nelson, D.L.; Cox, M.M.; Lehninger, A.L. Principles of Biochemistry; Freeman New York: New York, NY, USA, 2008. [Google Scholar]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Hickey, M.; Carey, J.; Azevedo, J.; Houmard, J.; Pories, W.; Israel, R.; Dohm, G. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E453–E457. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Pace, B.S.; White, G.L.; Dover, G.J.; Boosalis, M.S.; Faller, D.V.; Perrine, S.P. Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood J. Am. Soc. Hematol. 2002, 100, 4640–4648. [Google Scholar] [CrossRef]
- Matheus, V.; Monteiro, L.; Oliveira, R.; Maschio, D.; Collares-Buzato, C. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp. Biol. Med. 2017, 242, 1214–1226. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Khan, S.; Jena, G. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: Study in juvenile diabetic rat. Chem. Biol. Interact. 2014, 213, 1–12. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T. PPARγ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Poirier, H.; Rouault, C.; Clement, L.; Niot, I.; Monnot, M.-C.; Guerre-Millo, M.; Besnard, P. Hyperinsulinaemia triggered by dietary conjugated linoleic acid is associated with a decrease in leptin and adiponectin plasma levels and pancreatic beta cell hyperplasia in the mouse. Diabetologia 2005, 48, 1059–1065. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Hara, K.; Yamauchi, T.; Terauchi, Y.; Tobe, K.; Nagai, R. Molecular mechanism of insulin resistance and obesity. Exp. Biol. Med. 2003, 228, 1111–1117. [Google Scholar] [CrossRef]
- Roberts, L.D.; Murray, A.J.; Menassa, D.; Ashmore, T.; Nicholls, A.W.; Griffin, J.L. The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011, 12, R75. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.-L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 2014, 5, e01011–e01014. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.J.; Pham, M.T.; Rahim, A.R.; Chuang, T.-H.; Hsieh, M.-F.; Huang, C.-M. Mouse Abdominal Fat Depots Reduced by Butyric Acid-Producing Leuconostoc mesenteroides. Microorganisms 2020, 8, 1180. https://doi.org/10.3390/microorganisms8081180
Yang JJ, Pham MT, Rahim AR, Chuang T-H, Hsieh M-F, Huang C-M. Mouse Abdominal Fat Depots Reduced by Butyric Acid-Producing Leuconostoc mesenteroides. Microorganisms. 2020; 8(8):1180. https://doi.org/10.3390/microorganisms8081180
Chicago/Turabian StyleYang, John Jackson, Minh Tan Pham, Adelia Riezka Rahim, Tsung-Hsien Chuang, Ming-Fa Hsieh, and Chun-Ming Huang. 2020. "Mouse Abdominal Fat Depots Reduced by Butyric Acid-Producing Leuconostoc mesenteroides" Microorganisms 8, no. 8: 1180. https://doi.org/10.3390/microorganisms8081180
APA StyleYang, J. J., Pham, M. T., Rahim, A. R., Chuang, T.-H., Hsieh, M.-F., & Huang, C.-M. (2020). Mouse Abdominal Fat Depots Reduced by Butyric Acid-Producing Leuconostoc mesenteroides. Microorganisms, 8(8), 1180. https://doi.org/10.3390/microorganisms8081180





