Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains Against Phytophthora infestans
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Media
2.2. Generation of Δhcn Strains
2.3. Growth Curves
2.4. Cyanide Detection Assay
2.5. Dual Assays
2.6. Zoospore Germination Experiment
2.7. Leaf Disk Experiment
2.8. Fatty Acid Methyl Esters Analysis (FAMEs)
3. Results
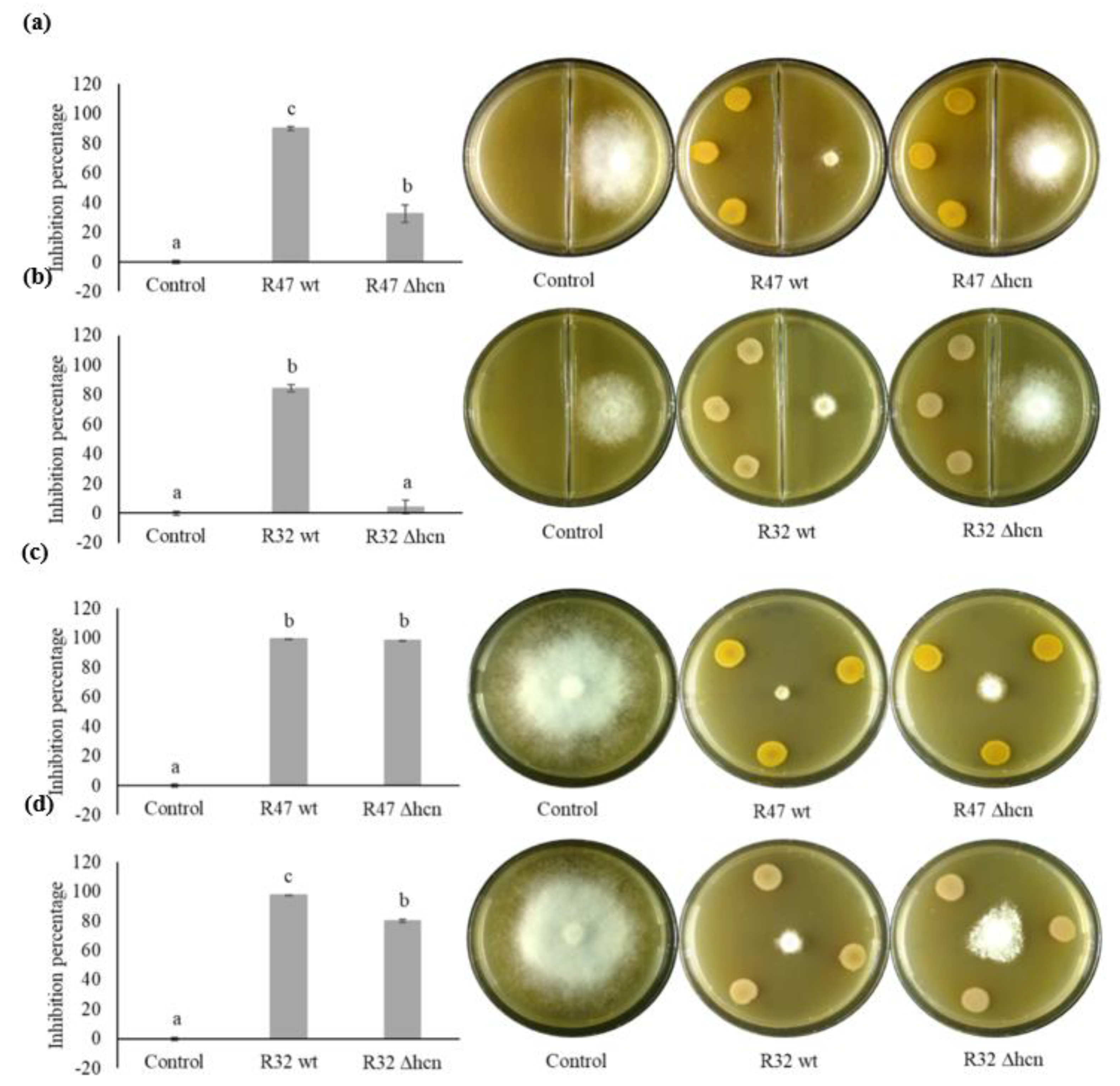
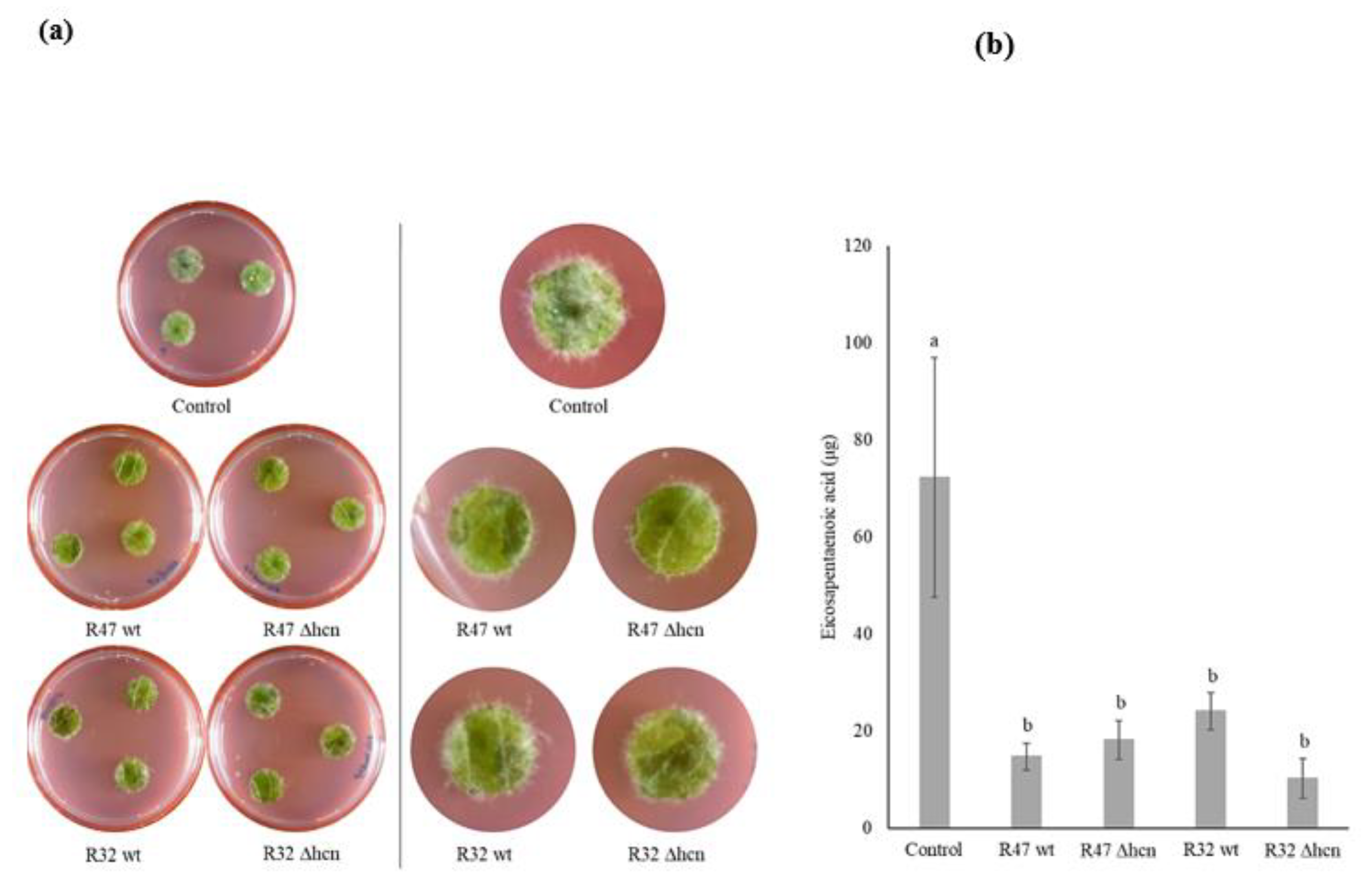
3.1. Contribution of HCN to the Inhibition of P. infestans Mycelium Growth
3.2. Contribution of HCN to the Inhibition of P. infestans Zoospore Germination
3.3. Contribution of HCN to the Inhibition of Infection by P. infestans on Potato Leaf Disks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fry, W. Phytophthora infestans: The plant (and R gene) destroyer. Mol. Plant. Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Visser, R.G.F.; Van Der Vossen, E.A.G. Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Res. 2008, 51, 47–57. [Google Scholar] [CrossRef]
- Cooke, L.R.; Schepers, H.T.A.M.; Hermansen, A.; Bain, R.A.; Bradshaw, N.J.; Ritchie, F.; Shaw, D.S.; Evenhuis, A.; Kessel, G.J.T.; Wander, J.G.N.; et al. Epidemiology and Integrated Control of Potato Late Blight in Europe. Potato Res. 2011, 54, 183–222. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Coffey, A.; Guo, J.; Waters, D.M.; Arendt, E.K. Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: A review. Appl. Microbiol. Biotechnol. 2012, 96, 37–48. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; De Vos, P.; Kromann, P.; Declerck, S.; Prestwich, B.D. Biological control agents: From field to market, problems, and challenges. Trends Biotechnol. 2014, 32, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, L.; Bönisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 2015, 81, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S.; Sheikh, I.H.; Khan, A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World J. Microbiol. Biotechnol. 2006, 22, 641–650. [Google Scholar] [CrossRef]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 is a key aphicidal metabolite. Can. J. Microbiol. 2019. [Google Scholar] [CrossRef]
- Flury, P.; Vesga, P.; Péchy-Tarr, M.; Aellen, N.; Dennert, F.; Hofer, N.; Kupferschmied, K.P.; Kupferschmied, P.; Metla, Z.; Ma, Z.; et al. Antimicrobial and insecticidal: Cyclic lipopeptides and hydrogen cyanide produced by plant-beneficial Pseudomonas strains CHA0, CMR12a and PCL1391 contribute to insect killing. Front. Microbiol. 2017, 8, 100. [Google Scholar] [CrossRef]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis O6 exhibits nematicidal activity against meloidogyne hapla. Plant. Pathol. J. 2018. [Google Scholar] [CrossRef]
- Stutz, E.W. Naturally Occurring Fluorescent Pseudomonads Involved in Suppression of Black Root Rot of Tobacco. Phytopathology 1986. [Google Scholar] [CrossRef]
- Ahl, P.; Voisard, C.; Défago, G. Iron Bound-Siderophores, Cyanic Acid, and Antibiotics Involved in Suppression of Thielaviopsis basicola by a Pseudomonas fluorescens Strain. J. Phytopathol. 1986. [Google Scholar] [CrossRef]
- Laville, J.; Blumer, C.; Von Schroetter, C.; Gaia, V.; Défago, G.; Keel, C.; Haas, D. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J. Bacteriol. 1998, 180, 3187–3196. [Google Scholar] [CrossRef] [PubMed]
- Zdor, R.E. Bacterial cyanogenesis: Impact on biotic interactions. J. Appl. Microbiol. 2015, 118, 267–274. [Google Scholar] [CrossRef]
- Bröckner, A.; Raspotnig, G.; Wehner, K.; Meusinger, R.; Norton, R.A.; Heethoff, M. Storage and release of hydrogen cyanide in a chelicerate (Oribatula tibialis). Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Frangipani, E.; Pérez-Martínez, I.; Williams, H.D.; Cherbuin, G.; Haas, D. A novel cyanide-inducible gene cluster helps protect Pseudomonas aeruginosa from cyanide. Environ. Microbiol. Rep. 2014. [Google Scholar] [CrossRef]
- Cunningham, L.; Pitt, M.; Williams, H.D. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Mol. Microbiol. 1997. [Google Scholar] [CrossRef]
- Cipollone, R.; Frangipani, E.; Tiburzi, F.; Imperi, F.; Ascenzi, P.; Visca, P. Involvement of Pseudomonas aeruginosa rhodanese in protection from cyanide toxicity. Appl. Environ. Microbiol. 2007. [Google Scholar] [CrossRef][Green Version]
- De Vrieze, M.; Varadarajan, A.R.; Schneeberger, K.; Bailly, A.; Ahrens, C.H.; Weisskopf, L. Linking comparative genomics of nine potato-associated Pseudomonas isolates with their differing biocontrol potential against late blight. Front. Microbiol. 2020, 11, 857. [Google Scholar] [CrossRef]
- Guyer, A.; De Vrieze, M.; Bönisch, D.; Gloor, R.; Musa, T.; Bodenhausen, N.; Bailly, A.; Weisskopf, L. The anti-phytophthora effect of selected potato-associated Pseudomonas strains: From the laboratory to the field. Front. Microbiol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, M.; Germanier, F.; Vuille, N.; Weisskopf, L. Combining Different Potato-Associated Pseudomonas Strains for Improved Biocontrol of Phytophthora infestans. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, E.; de Lorenzo, V. Engineering multiple genomic deletions in Gram-negative bacteria: Analysis of the multi-resistant antibiotic profile of Pseudomonas putida KT2440. Environ. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Cernava, T.; Aschenbrenner, I.A.; Grube, M.; Liebminger, S.; Berg, G. A novel assay for the detection of bioactive volatiles evaluated by screening of lichen-associated bacteria. Front. Microbiol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Bruisson, S.; Meyer, S.; Zühlke, D.; Hirschfeld, C.; Joller, C.; L’Haridon, F.; Mène-Saffrané, L.; Riedel, K.; Weisskopf, L. A sulfur-containing volatile emitted by potato-associated bacteria confers protection against late blight through direct anti-oomycete activity. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.E. Phytophthora infestans: New Tools (and Old Ones) Lead to New Understanding and Precision Management. Annu. Rev. Phytopathol. 2016, 54, 529–547. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Flier, W.G. The Biology of Phytophthora infestans at Its Center of Origin. Annu. Rev. Phytopathol. 2005, 43, 171–190. [Google Scholar] [CrossRef]
- Walsh, U.F.; Morrissey, J.P.; O’Gara, F. Pseudomonas for biocontrol of phytopathogens: From functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 2001, 12, 289–295. [Google Scholar] [CrossRef]
- Morrison, C.K.; Arseneault, T.; Novinscak, A.; Filion, M. Phenazine-1-carboxylic acid production by Pseudomonas fluorescens LBUM636 alters Phytophthora infestans’ growth and late blight development. Phytopathology 2016, 107, 273–279. [Google Scholar] [CrossRef]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 2015, 6, 1295. [Google Scholar] [CrossRef]
- De Vrieze, M.; Gloor, R.; Massana Codina, J.; Torriani, S.; Gindro, K.; L’Haridon, F.; Bailly, A.; Weisskopf, L. Biocontrol activity of three Pseudomonas on a newly assembled collection of Phytophthora infestans isolates. Phytopathology 2019, 109, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Leongt, J.; Teintzet, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, A.; Chinchilla, D.; Tan, C.; Mène-Saffrané, L.; L’Haridon, F.; Weisskopf, L. Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains Against Phytophthora infestans . Microorganisms 2020, 8, 1144. https://doi.org/10.3390/microorganisms8081144
Anand A, Chinchilla D, Tan C, Mène-Saffrané L, L’Haridon F, Weisskopf L. Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains Against Phytophthora infestans . Microorganisms. 2020; 8(8):1144. https://doi.org/10.3390/microorganisms8081144
Chicago/Turabian StyleAnand, Abhishek, Delphine Chinchilla, Christopher Tan, Laurent Mène-Saffrané, Floriane L’Haridon, and Laure Weisskopf. 2020. "Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains Against Phytophthora infestans " Microorganisms 8, no. 8: 1144. https://doi.org/10.3390/microorganisms8081144
APA StyleAnand, A., Chinchilla, D., Tan, C., Mène-Saffrané, L., L’Haridon, F., & Weisskopf, L. (2020). Contribution of Hydrogen Cyanide to the Antagonistic Activity of Pseudomonas Strains Against Phytophthora infestans . Microorganisms, 8(8), 1144. https://doi.org/10.3390/microorganisms8081144






