Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Ethics Statement
2.3. Study Population and Data Collection
2.4. Review: Search Strategy and Selection Criteria
2.5. Definitions
- -
- Clinical: definite loss of sensation in a pale (hypopigmented) or reddish skin patch (SP) or a thickened or enlarged peripheral nerve with loss of sensation and/or weakness of the muscles supplied by that nerve.
- -
- Laboratory: demonstration of acid-fast bacilli (AFB) in a slit-skin smear (SSS) or in a skin biopsy (SB) of skin lesions with loss of sensation and/or nerve biopsy (NB) of thickened peripheral nerves.
- -
- Histopathology: evidence of granulomatous infiltrate.
- -
- Biomolecular analysis: positive polymerase chain reaction (PCR) for M. leprae and/or M. lepromatosis in nasal swab, skin or nerve biopsy.
- -
- Paucibacillary (PB) leprosy: a case of leprosy with one to five skin lesions, without a demonstrated presence of bacilli in a slit-skin smear
- -
- Multibacillary (MB) leprosy: a case of leprosy with more than five skin lesions, or with nerve involvement (pure neuritis, or any number of skin lesions plus neuritis), or with the demonstrated presence of bacilli in a SSS, irrespective of the number of skin lesions.
- -
- TT: Tuberculoid leprosy
- -
- BT: Borderline tuberculoid leprosy
- -
- BB: Mid-borderline leprosy
- -
- BL: Borderline lepromatous leprosy
- -
- LL: Lepromatous leprosy
- -
- I: Indeterminate leprosy
- -
- Multidrug Therapy (MDT): rifampicin 600 mg monthly, dapsone 100 mg daily, clofazimine 300 mg monthly, and 50 mg daily (the treatment has to be continued until a negative SSS), or
- -
- Rifampicin–Ofloxacina–Minocyclin (ROM) regimen: alternative treatment to clofazimine is based on Rifampicin 600 mg, Ofloxacin 400 mg (recently substituted by Moxifloxacin 400 mg), and Minocycline 100 mg, all once a month for 24 months.
2.6. Statistical Analysis
3. Results
3.1. Case Series
3.2. The Review
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Search Strategies (Electronic Searches)
- Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 7);
- MEDLINE (PubMed) (1966 to 14 August 2018);
- EMBASE (Embase.com) (1974 to 14 August 2018);
- Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 14 August 2018);
- ClinicalTrials.gov (www.clinicaltrials.gov);
- World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch); and
- Orphanet (www.orpha.net)
References
- Britton, W.J.; Lockwood, D.N. Leprosy. Lancet 2004, 363, 1209–1219. [Google Scholar] [CrossRef]
- WHO. Working to overcome the global impact of neglected tropical diseases—Summary. Wkly. Epidemiol Rec. 2011, 86, 113–120. [Google Scholar]
- Han, X.Y. Detection of the leprosy agent Mycobacterium lepromatosis in South America and Europe. Am. J. Trop. Med. Hyg. 2017, 96, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunzi, E.; Massone, C. Leprosy: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Noordeen, S.K. History of chemotherapy of leprosy. Clin. Dermatol. 2016, 34, 32–36. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global leprosy update, 2018: Moving towards a leprosy-free world. Wkly. Epidemiol Rec. 2019, 94, 389–412. [Google Scholar]
- Massone, C.; Nunzi, E.; Cerroni, L. Histopathologic diagnosis of leprosy in a nonendemic area. Am. J. Dermatopathol. 2010, 32, 417–419. [Google Scholar] [CrossRef]
- Aftab, H.; Nielsen, S.D.; Bygbjerg, I.C. Leprosy in Denmark 1980–2010: A review of 15 cases. BMC Res. Notes 2016, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.M.; Romero, D.; Belinchon, I. Epidemiology of Leprosy in Spain: The Role of the International Migration. PLoS Negl. Trop. Dis. 2016, 10, e0004321. [Google Scholar] [CrossRef]
- Trovato, A.; Reid, A.; Takarinda, K.C.; Montaldo, C.; Decroo, T.; Owiti, P.; Bongiorno, F.; di Carlo, S. Dangerous crossing: Demographic and clinical features of rescued sea migrants seen in 2014 at an outpatient clinic at Augusta Harbor, Italy. Confl. Health 2016, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Browne, S.G. Some aspects of the history of leprosy: The leprosie of yesterday. Proc. R. Soc. Med. 1975, 68, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Terni, M.; Signorini, F.L. The present situation of leprosy in Italy. Int. J. Lepr. 1950, 18, 519–523. [Google Scholar] [PubMed]
- Greco, D.; Galanti, M.R. Leprosy in Italy. Int. J. Lepr. Other Mycobact. Dis. 1983, 51, 495–499. [Google Scholar] [PubMed]
- Massone, C.; Brunasso, A.M.; Noto, S.; Campbell, T.M.; Clapasson, A.; Nunzi, E. Imported leprosy in Italy. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 999–1006. [Google Scholar] [CrossRef]
- Ricco, M.; Vezzosi, L.; Balzarini, F.; Mezzoiuso, A.G.; Ranzieri, S.; Vaccaro, F.G.; Odone, A.; Signorelli, C. Epidemiology of leprosy in Italy (1920–2019): A comprehensive review on existing data. Acta Biomed. 2019, 90, 7–14. [Google Scholar] [PubMed]
- Nunzi, E.; Clappason, A.; Noto, S. La Lebbra Oggi. Scuola Follereau AIFO Bologna. Available online: www.aifo.it (accessed on 1 June 2009).
- Chernet, A.; Utzinger, J.; Sydow, V.; Probst-Hensch, N.; Paris, D.H.; Labhardt, N.D.; Neumayr, A. Prevalence rates of six selected infectious diseases among African migrants and refugees: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 605–619. [Google Scholar] [CrossRef]
- Pottie, K.; Mayhew, A.D.; Morton, R.L.; Greenaway, C.; Akl, E.A.; Rahman, P.; Zenner, D.; Pareek, M.; Tugwell, P.; Welch, V.; et al. Prevention and assessment of infectious diseases among children and adult migrants arriving to the European Union/European Economic Association: A protocol for a suite of systematic reviews for public health and health systems. BMJ Open. 2017, 7, e014608. [Google Scholar] [CrossRef] [Green Version]
- WHO-SEARO. Guidelines for the Diagnosis, Treatment and Prevention of Leprosy; WHO-SEARO: New Delhi, India, 2018. [Google Scholar]
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Mycobact. Dis. 1966, 34, 255–273. [Google Scholar]
- Trave, I.; Barabino, G.; Cavalchini, A.; Parodi, A. Long-term ulcerations caused by Mycobacterium lepromatosis. Int. J. Mycobacteriol. 2020, 9, 223–225. [Google Scholar]
- Medeiros, S.; Catorze, M.G.; Vieira, M.R. Hansen’s disease in Portugal: Multibacillary patients treated between 1988 and 2003. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 29–35. [Google Scholar] [CrossRef]
- Kyriakis, K.P. Active leprosy in Greece: A 20-year survey (1988–2007). Scand. J. Infect. Dis. 2010, 42, 594–597. [Google Scholar] [CrossRef]
- Safa, G.; Coic, A.; Darrieux, L. Leprosy: A disease not to be forgotten in metropolitan France. Presse Med. 2009, 38, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Rongioletti, F.; Gallo, R.; Cozzani, E.; Parodi, A. Leprosy: A diagnostic trap for dermatopathologists in nonendemic area. Am. J. Dermatopathol. 2009, 31, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Manhas, R.; John, L.; Whittam, L.; Williamson, L. Tropical rheumatology in a UK District General Hospital: A case report of leprosy presenting as acute vasculitis. Rheumatology 2010, 49, 826–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aridon, P.; Ragonese, P.; Mazzola, M.A.; Terruso, V.; Palermo, A.; D’Amelio, M.; Savettieri, G. Leprosy: Report of a case with severe peripheral neuropathy. Neurol. Sci. 2010, 31, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Giacomet, V.; Vigano, A.; Fabiano, V.; Antinori, S.; Longhi, E.; Zuccotti, G. Leprosy: A disease not to be forgotten in the era of globalization. Pediatr. Int. 2010, 52, 849–850. [Google Scholar] [CrossRef]
- Andres, M.; Agullo, A.; Negrete, R.; Batlle, E.; Martinez, A. Lepromatous leprosy presenting as an acute polyarthritis in a Colombian immigrant in Spain. Joint Bone Spine 2012, 79, 203–204. [Google Scholar] [CrossRef]
- Pedraza Hueso, M.I.; Hinojosa Mena-Bernal, C.; Hernandez-Lain, A.; Guerrero Peral, A.L. Mononeuritis multiplex due to leprosy: A case of atypical presentation. Neurologia 2014, 29, 313–314. [Google Scholar] [CrossRef]
- Maritati, M.; Contini, C. A Case of Leprosy in Italy: A Multifaceted Disease Which Continues to Challenge Medical Doctors. J. Immigr. Minor. Health 2016, 18, 490–493. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Modarres, H.; Jaunmuktane, Z.; Brandner, S.; Rossor, A.M.; Lockwood, D.N.; Reilly, M.M.; Manji, H.; Schon, F. Leprosy in a patient infected with HIV. Pract. Neurol. 2017, 17, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Torne, K.A.-A.S.; Bailey, M.; Gupta, R.; Jester, A.; Lockwood, D.; Sunderland, L.; Wassmer, E.; Willis, T.; Vidyatharan, R. A rare case presentation of pure neural leprosy. Eur. J. Paediatr. Neurol. 2017, 21, E222–E239. [Google Scholar] [CrossRef]
- Becker, S.L.; Mawlood, D.A.; Janssen, A.; Heinricy, L.; Janssen, E.; Vogt, T.; Müller, C.S.L. Multibacillary leprosy in a migrant from Afghanistan: A disease not to be forgotten. Travel Med. Infect. Dis. 2017, 19, 66–67. [Google Scholar] [CrossRef]
- Marotta, M.; Dallolio, L.; Toni, G.; Toni, F.; Leoni, E. A case of imported leprosy in Italy: Implications for surveillance by Public Health Services of Local Health Authorities. Travel Med. Infect. Dis. 2019, 31, 101402. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, S.; Puccetti, A.; Tinazzi, E.; Codella, O.M.; Sorleto, M.; Patuzzo, G.; Colato, C.; Tessari, G.; Lunardi, C. Leprosy initially misdiagnosed as sarcoidosis, adult-onset still disease, or autoinflammatory disease. J. Clin. Rheumatol. 2011, 17, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Piras, M.A.; Are, R.; Figoni, M.; Salis, M.T.; Caddeo, A.; Fiori, M.L.; Mancini, G.G. Human Anaplasmosis and Leprosy; A Case Report; Archives of Leprosy Mailing List. Available online: http://leprosymailinglist.blogspot.com/2011/06/human-anaplasmosis-and-leprosy-case.html (accessed on 21 May 2011).
- Contreras-Steyls, M.; Lopez-Navarro, N.; Herrera-Acosta, E.; Castillo, R.; Ruiz del Portal, G.; Bosch, R.J.; Herrera, E. The current challenge of imported leprosy in Spain: A study of 7 cases. Actas Dermosifiliogr. 2011, 102, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Norman, F.F.; Fanciulli, C.; Perez-Molina, J.A.; Monge-Maillo, B.; Lopez-Velez, R. Imported and autochthonous leprosy presenting in Madrid (1989–2015): A case series and review of the literature. Travel Med. Infect. Dis. 2016, 14, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Kentikelenis, A. Intersecting crises: Migration, the economy and the right to health in Europe. Eur. J. Public Health 2018, 28, 61–62. [Google Scholar] [CrossRef]
- Rechel, B.; Mladovsky, P.; Ingleby, D.; Mackenbach, J.P.; McKee, M. Migration and health in an increasingly diverse Europe. Lancet 2013, 381, 1235–1245. [Google Scholar] [CrossRef]
- Fernandes, T.R.; Brandao, G.A.; Castro e Souza, B. Leprosy type-I reaction episode mimicking facial cellulitis--the importance of early diagnosis. An. Bras. Dermatol. 2015, 90, 73–76. [Google Scholar] [CrossRef] [Green Version]
- WHO-SEARO. Operational Manual: Global Leprosy Strategy 2016-2020: Accelerating Towards A Leprosy-Free World; WHO-SEARO: New Delhi, India, 2016. [Google Scholar]
- Lockwood, D.N.; Reid, A.J. The diagnosis of leprosy is delayed in the United Kingdom. QJM 2001, 94, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Pant, S.; Eder, B.; Vracar, A.; Mosca, D.; Orcutt, M. WHO’s global action plan to promote the health of refugees and migrants. BMJ 2019, 366, l4806. [Google Scholar] [CrossRef]
- Haefner, K.; Walther, F.; Chichava, O.A.; Ariza, L.; Alencar, C.H.; De Alencar, M.D.F.; Ramos, A.J.; Richter, J.; Heukelbach, J. High occurrence of disabilities caused by leprosy: Census from a hyperendemic area in Brazil’s savannah region. Lepr. Rev. 2017, 88, 520–532. [Google Scholar]
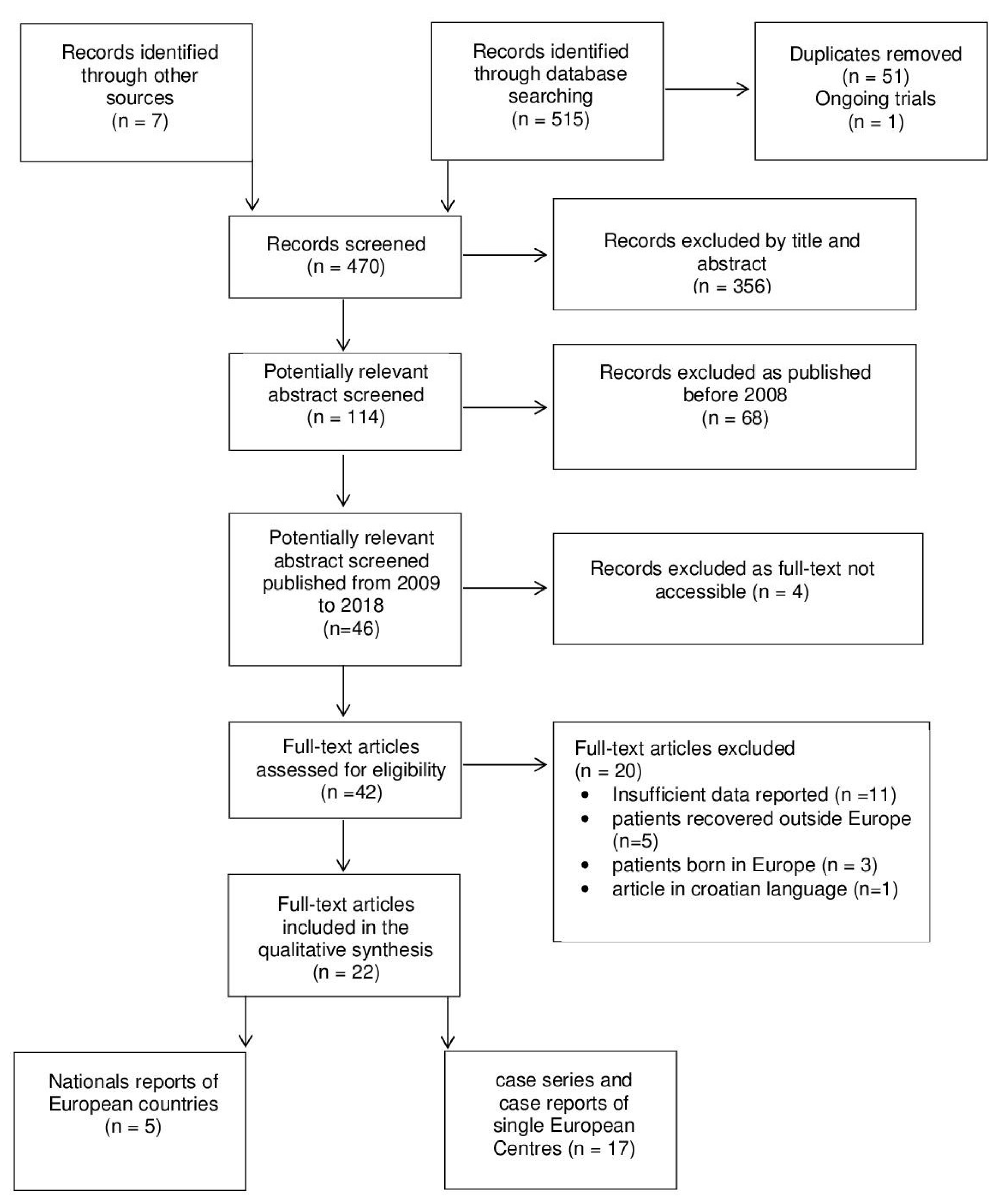
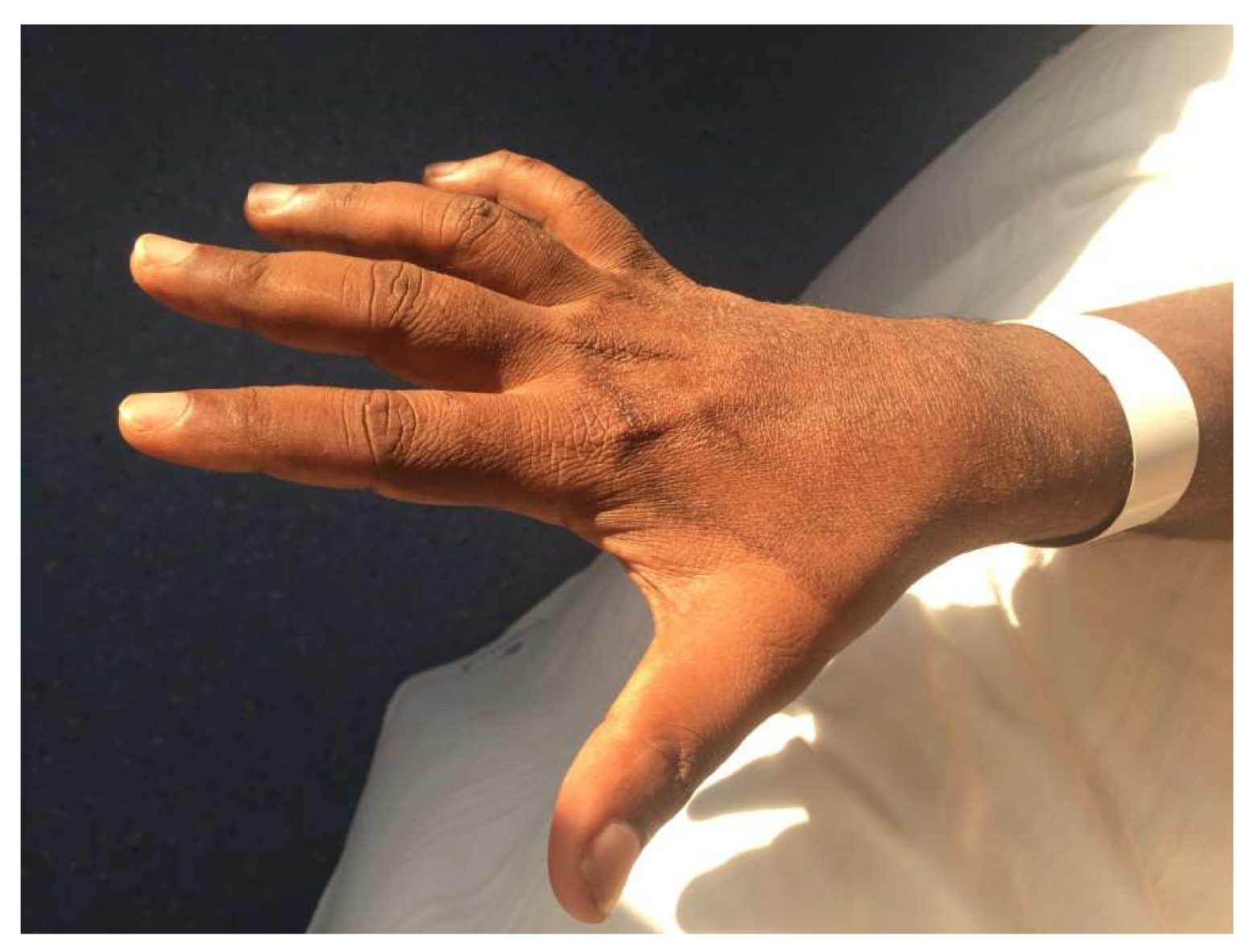
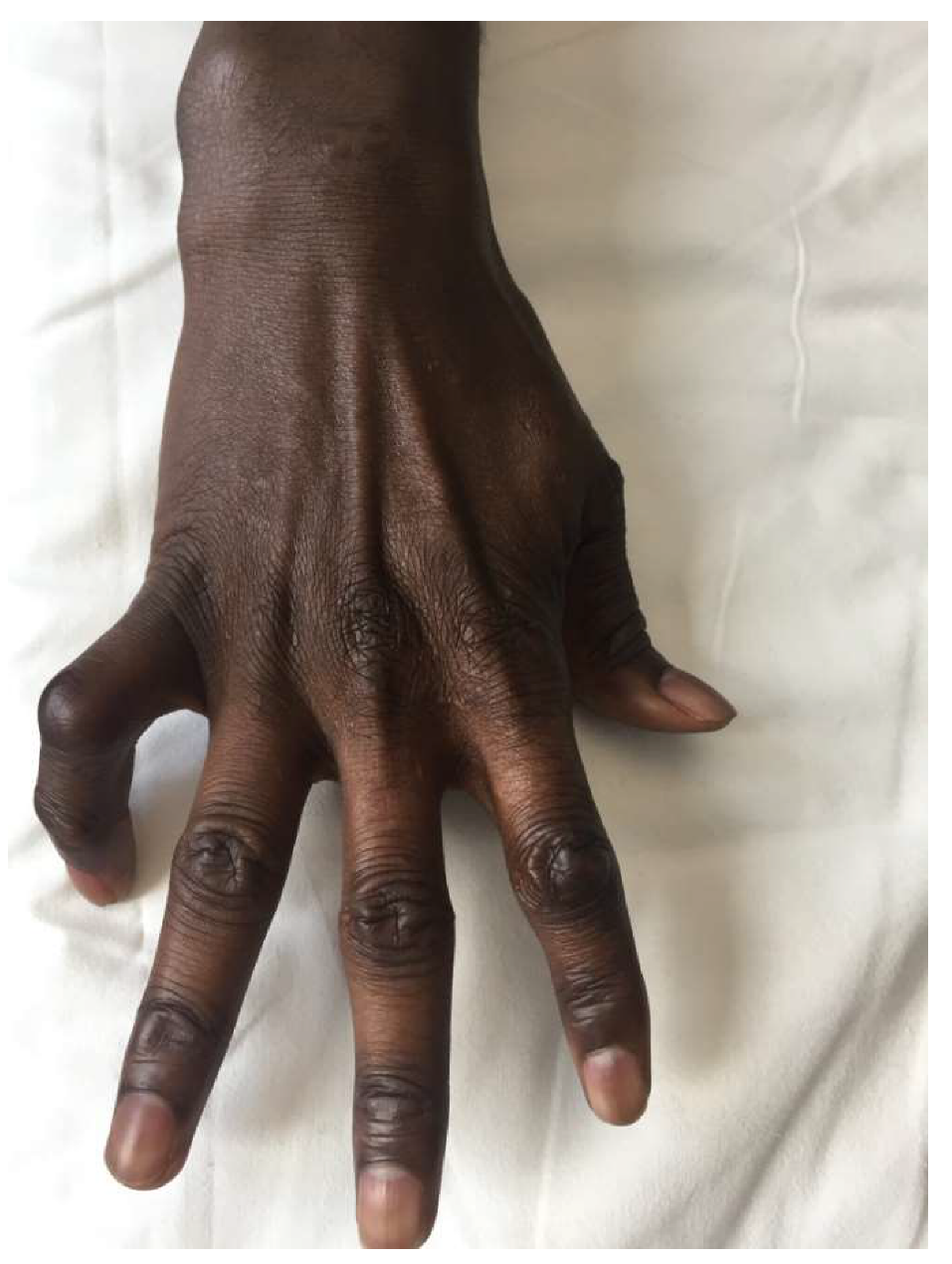

| Characteristics | Total n = 55 (%) | Africa n = 23 (%) | Asia n = 22 (%) | Latin America n = 10 (%) |
|---|---|---|---|---|
| Male | 37 (67.3) | 18 (78.3) | 17 (77.3) | 2 (20) |
| Median age (IQR), Years | 33 (28–41) | 29 (23–37) | 38 (28–43) | 34 (28–41) |
| Median duration of stay in Europe (IQR), Months | 36 (12–92) | 21 (10–82) | 55 (30–103) | 35 (7–92) |
| Previous Diagnosis of Leprosy | 19 (34.6) | 10 (43.5) | 2 (9.1) | 7 (70) |
| Median Time from Start of Symptoms and Diagnosis (IQR), Months | 12 (3–31) | 26 (3–57) | 5 (3–24) | 11 (3–25) |
| Signs and Symptoms | ||||
| Skin | 50 (90.9) | 20 (87) | 21 (95.5) | 9 (90) |
| Neuropathy | 39 (70.9) | 19 (82.6) | 14 (63.6) | 6 (60) |
| Edema | 4 (7.3) | 2 (8.7) | 2 (9.1) | 0 |
| Eye Involvement | 3 (5.5) | 1 (4.3) | 2 (9.1) | 0 |
| Fever | 3 (5.5) | 2 (8.7) | 0 | 0 |
| Arthritis | 2 (3.6) | 0 | 1 (4.5) | 1 (10) |
| Lymphadenopathy | 2 (3.6) | 1 (4.3) | 1 (4.5) | 0 |
| Diagnosis | ||||
| Nasal Swab AFB Positive (n = 54) * | 20 (37) | 8/23 (34.8) | 11/21 (52.4) | 1/10 (10) |
| SSS AFB Positive (n = 54) * | 39 (70.9) | 13/22 (59.1) | 18/22 (81.8) | 8/10 (80) |
| Granulomatous Inflammation in the Biopsy (n = 43) ^ | 21 (48.8) | 6/17 (35.3) | 13/19 (68.4) | 2/7 (28.6) |
| AFB positive in the biopsy (n = 43) ^ | 13 (30.2) | 7/17 (41.2) | 5/19 (26.3) | 1/7 (14.3) |
| PCR for M. leprae or M. lepromatosis DNA positive (n = 24) § | 17 (85) | 7/9 (77.8) | 8/9 (88.9) | 2/6 (33.3) |
| WHO classification | ||||
| PB | 19 (34.5) | 10 (43.5) | 5 (22.7) | 4 (40) |
| MB | 36 (65.5) | 13 (56.5) | 17 (77.3) | 6 (60) |
| Ridley–Jopling classification | ||||
| TT | 3 (5.4) | 2 (8.7) | 1 (4.5) | 0 |
| BT | 27 (49.1) | 12 (52.2) | 9 (40.9) | 6 (60) |
| BL | 5 (9.1) | 0 | 4 (18.2) | 1 (10) |
| LL | 17 (31) | 8 (34.8) | 6 (27.3) | 3 (30) |
| BL/LL | 3 (5.4) | 1 (4.3) | 2 (9.1) | 0 |
| Therapy | ||||
| MDT | 52 (94.6) | 20 (87) | 22 (100) | 10 (100) |
| ROM | 3 (5.4) | 3 (13) | 0 | 0 |
| Outcome | ||||
| Completed treatment | 40 (72.7) | 16 (69.6) | 16 (72.7) | 9 (90) |
| Ongoing | 7 (12.7) | 5 (21.7) | 2 (9.1) | 0 |
| Lost | 8 (14.6) | 2 (8.7) | 4 (18.2) | 1 (10) |
| Characteristic | Portugal (Medeiros S, 2009) | Greece (Kyriakis KP, 2010) | Italy (Massone C, 2010) | Denmark (Aftab H, 2016) | Spain (Ramos JM, 2016) |
|---|---|---|---|---|---|
| Years Analyzed | 1968–2003 | 1988–2007 | 2002–2008 | 1980–2010 | 2003–2013 |
| Number of Migrants/Total Patients (%) | 36/102 (35.3) | 6/33 (18.2) | 58/64 (90.6) | 15/15 (100) | 128/168 (76.2) |
| Continent of Origin, n (%) | |||||
| Africa | 20 (55.6) | 2 (33.3) | 16 (27.6) | 2 (13.3) | 27 (21.1) |
| South/Central America | 11 (30.6) | 1 (16.7) | 19 (32.7) | 0 | 92 (71.9) |
| Asia | 5 (13.8) | 3 (50) | 23 (39.7) | 13 (86.7) | 7 (5.5) |
| Not Available | 0 | 0 | 0 | 0 | 2 (1.5) |
| Reference | Country (City) | Time | Gender Age (Years) | Country of Origin | Duration of Stay in Europe (Months) | Clinical Manifestations | Methods of Diagnosis | WHO Class | R-J Class |
|---|---|---|---|---|---|---|---|---|---|
| [24] | France (Saint-Brieuc) | 2009 | M, 11 | Haiti | 36 | SNs (face), SPs (legs) | SB (AFB) | MB | LL |
| [7,25] | Italy (Genoa) | 2006 | M, 43 | Brazil | NA | anesthetic and asymmetric SPs, erythematous SNs (abdomen, things and legs) | NB (AFB) SSS (AFB) | MB | BT |
| [26] | UK (Swindon) | 2009 | F, 23 | Brazil | 48 | vascultic diffuse rash, fever, LA, SNs (face, arms and legs), polyarthritis with symmetrical synovitis (elbows, wrists, ankles, MCP joints) | SB (GI, AFB) | MB | LL, ENL |
| [27] | Italy (Palermo) | 2009 | M, 15 | Senegal | 6 | anesthetic SPs, sensory loss and motor weakness (hand, forearm), claw deformity (hand), PN (ulnar, median nerves), paraesthesia (legs) | clinical | PB | TT |
| [7] | Italy (Genoa) | 2010 | M, 28 | Nigeria | NA NA | SNs (face) | SB (AFB) | MB | LL |
| M, 22 | Colombia | SNs (extremities) | SB (AFB) | MB | NA | ||||
| M, 14 | Brazil | symmetrical SPs (entire body) | SB (AFB) | MB | NA | ||||
| [28] | Italy (Milan) | 2010 | M, 14 | Brazil | 96 | erythematous SNs (face, extremities), SPs (arms), painful edema with hypoesthesia (wrists, hands), fever, LA, weight loss | SB (AFB, PCR) | MB | LL |
| [36] | Italy (Verona) | 2006 | M, 20 | India | 36 | erythematous SNs and SPs (face, extremities), polyarthritis (wrists, ankles), fever, episcleritis | SB (MI, AFB) | MB | LL |
| [37] | Italy (Sassari) | 2011 | M, 26 | Nigeria | 12 | SPs (trunk, extremities), symmetric edema (extremities), fever, headache, LA, PN (great auricular, ulnar nervs), | SB (GI) | MB | BB/BL |
| [38] | Spain (Malaga) | 2004–2009 | F, 28 | Brazil | 8 | SP (harm) | SB (MI) | PB | I |
| M, 33 | Mali | 8 | SPs (extremities) | SB (MI) | PB | I | |||
| F, 32 | Nigeria | 48 | SP (trunk) | SB (MI) | PB | I | |||
| F, 31 | Paraguay | 36 | SPs (extremities) | SB (GI) | PB | TT | |||
| F, 26 | Brazil | 12 | SP (leg) | SB (GI) | PB | TT | |||
| M, 40 | Colombia | 12 | SPs (extremities, trunk) | SB (GI) | PB | BT | |||
| [29] | Spain (Alicante) | 2011 | M, 41 | Colombia | 108 | painful edema (extremities), synovitis (wrists, MCP joints), tenosynovitis, SNs (legs), LA, mild skin rash on trunk | SB and LB (GI, AFB) | MB | LL, ENL |
| [30] | Spain (Valladolid) | 2012 | M, 19 | Mauritania | 48 | SPs, sensory loss and motor weakness (extremities), claw deformity (hand) | SB (AFB) | MB | I |
| [31] | Italy (Ferrara) | 2015 | M, 22 | Ghana | 24 | infiltration facial skin, pain, sensory loss and motor weakness (hand), ulnar palsy, erythematous SNs (face), arthralgia, arthritis (hands, ankles, knees), hair loss and madarosis | SB (AFB, GI) | MB | BT |
| [32] | UK (London) | 2016 | M, 60 | Nigeria | 36 | facial weakness and numbness (extremities, face), thickened peripheral nerves, ulnar palsy, difficulty walking | NB (MI) | MB | PNL BT |
| [39] | Spain (Madrid) | 1989 | F, 40 | Eq. Guinea | 2 w | SP (face) | SB (GI) | PB | TT |
| 1990 | M, 30 | Philippines | 48 | SPs (face, extremities) | SSS (AFB) | MB | LL | ||
| 1994 | M, 28 | Eq. Guinea | 96 | erythematous SNs, PN | SSS (AFB) | MB | LL | ||
| 1996 | M, 48 | Colombia | 12 | hypoesthetic SPs | SSS/SB (AFB, MI) | MB | BB | ||
| 2002 | F, 65 | Colombia | 12 | infiltration facial skin, symmetrical PN | SSS/NB (AFB) | MB | LL | ||
| 2005 | M, 59 | DR | 96 | erythematous SNs | SSS (AFB) | MB | BB-BL | ||
| 2004 | F, 62 | DR | 3 | claw deformity (hands), PN legs, plantar ulcer | clinical | MB | LL | ||
| 2006 | M, 24 | Mali | 12 | SPs, asymmetric PN (left ulnar) | SB (GI) | MB | BB | ||
| 2006 | F, 28 | Brazil | 24 | infiltration facial skin, hypoesthesia legs | SSS (AFB) | MB | BL | ||
| 2007 | M, 22 | Brazil | 12 | SPs, thickening ear lobes/cheeks, fever, PN | SSS (AFB) | MB | LL | ||
| 2008 | F, 32 | Paraguay | 84 | asymmetric hypoesthetic SPs | SSS (AFB) | PB | BT | ||
| 2011 | F, 27 | Paraguay | 12 | painful SNs, fever, PN | SSS/SB (AFB, PCR) | MB | LL | ||
| 2013 | M, 40 | Venezuela | 96 | SPs, madarosis, PN | SSS (AFB) | MB | BL-LL | ||
| 2015 | F, 38 | Paraguay | 60 | SPs, PN (right ulnar) | SSS/SB (AFB, PCR) | MB | LL | ||
| [33] | UK (Birmingham) | 2017 | F, 15 | Afghanistan | 60 | left ulnar palsy | clinical | MB | PNL |
| [34] | Germany (Homburg/Saar) | 2016 | M, 28 | Afghanistan | NA | erythematous SNs and SPs (face, extremities) | SB (AFB, PCR, GI) | MB | NA |
| [35] | Italy (Rimini) | 2017 | M, 29 | Nigeria | 36 | SNs (face, hands) | SB (AFB, PCR, GI) | MB | LL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrame, A.; Barabino, G.; Wei, Y.; Clapasson, A.; Orza, P.; Perandin, F.; Piubelli, C.; Monteiro, G.B.; Longoni, S.S.; Rodari, P.; et al. Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018. Microorganisms 2020, 8, 1113. https://doi.org/10.3390/microorganisms8081113
Beltrame A, Barabino G, Wei Y, Clapasson A, Orza P, Perandin F, Piubelli C, Monteiro GB, Longoni SS, Rodari P, et al. Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018. Microorganisms. 2020; 8(8):1113. https://doi.org/10.3390/microorganisms8081113
Chicago/Turabian StyleBeltrame, Anna, Gianfranco Barabino, Yiran Wei, Andrea Clapasson, Pierantonio Orza, Francesca Perandin, Chiara Piubelli, Geraldo Badona Monteiro, Silvia Stefania Longoni, Paola Rodari, and et al. 2020. "Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018" Microorganisms 8, no. 8: 1113. https://doi.org/10.3390/microorganisms8081113






