Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurement of Physical and Biogeochemical Parameters
2.3. DNA Extraction, 16S rRNA Gene Amplification, and Sequencing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics and Bacterial Community Composition
3.2. Environmental Factors Determining Bacterial Community Compositions
3.3. Implication for the Surface Marian Cove Bacterial Community Change
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sigman, D.M.; Boyle, E.A. Glacial/Interglacial Variations in Atmospheric Carbon Dioxide. Nature 2000, 407, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, K.R.; van Dijken, G.; Long, M. Coastal Southern Ocean: A Strong Anthropogenic CO2 Sink. Geophys. Res. Lett. 2008, 35, L21602. [Google Scholar] [CrossRef]
- Cavicchioli, R. Microbial Ecology of Antarctic Aquatic Systems. Nat. Rev. Microbiol. 2015, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, S.; Heywood, K.J.; Thompson, A.F.; Aoki, S. Multidecadal Warming of Antarctic Waters. Science 2014, 346, 1227–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, A.J.; Holland, P.R.; Meredith, M.P.; Murray, T.; Luckman, A.; Vaughan, D.G. Ocean Forcing of Glacier Retreat in the Western Antarctic Peninsula. Science 2016, 353, 283–286. [Google Scholar] [CrossRef] [Green Version]
- Stenni, B.; Curran, M.A.; Abram, N.; Orsi, A.; Goursaud, S.; Masson-Delmotte, V.; Neukom, R.; Goosse, H.; Divine, D.; Van Ommen, T. Antarctic Climate Variability on Regional and Continental Scales Over the Last 2000 Years. Clim. Past 2017, 13, 1609–1634. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.; Kyung Lee, M.; Il Yoon, H.; Il Lee, Y.; Yoon Kang, C. Hydrography of Marian Cove, King George Island, West Antarctica: Implications for Ice-Proximal Sedimentation during Summer. Antarct. Sci. 2015, 27, 185–196. [Google Scholar] [CrossRef]
- Cook, A.J.; Fox, A.J.; Vaughan, D.G.; Ferrigno, J.G. Retreating Glacier Fronts on the Antarctic Peninsula Over the Past Half-Century. Science 2005, 308, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.; Overland, J.E.; Walsh, J.E. An Arctic and Antarctic Perspective on Recent Climate Change. Int. J. Climatol. 2007, 27, 277–293. [Google Scholar] [CrossRef]
- Meredith, M.P.; King, J.C. Rapid Climate Change in the Ocean West of the Antarctic Peninsula during the Second Half of the 20th Century. Geophys. Res. Lett. 2005, 32, L19604. [Google Scholar] [CrossRef]
- Vaughan, D.G. Recent Trends in Melting Conditions on the Antarctic Peninsula and their Implications for Ice-Sheet Mass Balance and Sea Level. Arct. Antarct. Alp. Res. 2006, 38, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2011, 4, 11–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holben, W.E.; Harris, D. DNA-Based Monitoring of Total Bacterial Community Structure in Environmental Samples. Mol. Ecol. 1995, 4, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kim, K.; Lee, S.; Back, J. The Community Structure of Meiofauna in Marian Cove, King George Island, Antarctica. Ocean Polar Res. 2011, 33, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Ahn, I.; Moon, H.; Jeon, M.J.; Kang, S. First Record of Massive Blooming of Benthic Diatoms and their Association with Megabenthic Filter Feeders on the Shallow Seafloor of an Antarctic Fjord: Does Glacier Melting Fuel the Bloom? Ocean Sci. J. 2016, 51, 273–279. [Google Scholar] [CrossRef]
- Torsvik, V.; Øvreås, L. Microbial Diversity and Function in Soil: From Genes to Ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Logue, J.; Findlay, S.E.G.; Comte, J. Editorial: Microbial Responses to Environmental Changes. Front. Microbiol. 2015, 6, 1364. [Google Scholar] [CrossRef]
- Lee, W.; Kang, S.; Paul, A.M.; Kwak, I. Temporal Dynamics and Patterning of Meiofauna Community by Self-Organizing Artificial Neural Networks. Ocean Polar Res. 2003, 25, 237–247. [Google Scholar] [CrossRef]
- Moreno-Pino, M.; De la Iglesia, R.; Valdivia, N.; Henríquez-Castilo, C.; Galán, A.; Díez, B.; Trefault, N. Variation in Coastal Antarctic Microbial Community Composition at Sub-Mesoscale: Spatial Distance or Environmental Filtering? FEMS Microbiol. Ecol. 2016, 92, fiw088. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, Y.; Qiao, Z.; Jin, H.; Li, H. Diversity of Bacterioplankton in Coastal Seawaters of Fildes Peninsula, King George Island, Antarctica. Arch. Microbiol. 2014, 196, 137–147. [Google Scholar] [CrossRef]
- Yoon, H.I.; Han, M.W.; Park, B.; Oh, J.; Chang, S. Glaciomarine Sedimentation and Palaeo-Glacial Setting of Maxwell Bay and its Tributary Embayment, Marian Cove, South Shetland Islands, West Antarctica. Mar. Geol. 1997, 140, 265–282. [Google Scholar] [CrossRef]
- Yoo, K.; Yoon, H.; Oh, J.; Kim, Y.; Kang, C. Water Column Properties and Dispersal Pattern of Suspended Particulate Matter (SPM) of Marian Cove during Austral Summer, King George Island, West Antarctica. Sea 1999, 4, 266–274. [Google Scholar]
- Herlemann, D.P.R.; Labrenz, M.; Jurgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in Bacterial Communities Along the 2000 km Salinity Gradient of the Baltic Sea. Isme J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast Clustering Algorithms for Metagenomic Sequence Analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, K.R. The Biplot Graphic Display of Matrices with Application to Principal Component Analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER V6: User Manual-Tutorial; Plymouth Marine Laboratory: Plymouth, UK, 2006; pp. 1–190. [Google Scholar]
- Hamady, M.; Lozupone, C.; Knight, R. Fast UniFrac: Facilitating High-Throughput Phylogenetic Analyses of Microbial Communities Including Analysis of Pyrosequencing and PhyloChip Data. Isme J. 2009, 4, 17–27. [Google Scholar] [CrossRef]
- Wilkinson, L.; Friendly, M. The History of the Cluster Heat Map. Am. Stat. 2009, 63, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Bonett, D.G.; Wright, T.A. Sample Size Requirements for Estimating Pearson, Kendall and Spearman Correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Dongen, S.v.; Enright, A.J. Metric Distances Derived from Cosine Similarity and Pearson and Spearman Correlations. arXiv 2012, arXiv:1208.3145. [Google Scholar]
- Methé, B.A.; Hiorns, W.D.; Zehr, J.P. Contrasts between Marine and Freshwater Bacterial Community Composition: Analyses of Communities in Lake George and Six Other Adirondack Lakes. Limnol. Oceanogr. 1998, 43, 368–374. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Kirchman, D.L. Community Composition of Marine Bacterioplankton Determined by 16S rRNA Gene Clone Libraries and Fluorescence in Situ Hybridization. Appl. Environ. Microbiol. 2000, 66, 5116–5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, C.L.; Larsson, J.; Yooseph, S.; Ininbergs, K.; Goll, J.; Asplund-Samuelsson, J.; McCrow, J.P.; Celepli, N.; Allen, L.Z.; Ekman, M.; et al. Functional Tradeoffs Underpin Salinity-Driven Divergence in Microbial Community Composition. PLoS ONE 2014, 9, e89549. [Google Scholar] [CrossRef]
- Yew, W.C.; Pearce, D.A.; Dunn, M.J.; Samah, A.A.; Convey, P. Bacterial Community Composition in Adélie (Pygoscelis Adeliae) and Chinstrap (Pygoscelis Antarctica) Penguin Stomach Contents from Signy Island, South Orkney Islands. Polar Biol. 2017, 40, 2517–2530. [Google Scholar] [CrossRef]
- Thouzeau, C.; Froget, G.; Monteil, H.; Le Maho, Y.; Harf-Monteil, C. Evidence of Stress in Bacteria Associated with Long-Term Preservation of Food in the Stomach of Incubating King Penguins (Aptenodytes Patagonicus). Polar Biol. 2003, 26, 115–123. [Google Scholar] [CrossRef]
- Curson, A.R.J.; Rogers, R.; Todd, J.D.; Brearley, C.A.; Johnston, A.W.B. Molecular Genetic Analysis of a Dimethylsulfoniopropionate Lyase that Liberates the Climate-Changing Gas Dimethylsulfide in several Marine A-Proteobacteria and Rhodobacter Sphaeroides. Environ. Microbiol. 2008, 10, 757–767. [Google Scholar] [CrossRef]
- Higuchi, T.; Agostini, S.; Casareto, B.E.; Yoshinaga, K.; Suzuki, T.; Nakano, Y.; Fujimura, H.; Suzuki, Y. Bacterial Enhancement of Bleaching and Physiological Impacts on the Coral Montipora Digitata. J. Exp. Mar. Biol. Ecol. 2013, 440, 54–60. [Google Scholar] [CrossRef]
- Lipson, D.A. Relationships between Temperature Responses and Bacterial Community Structure Along Seasonal and Altitudinal Gradients. FEMS Microbiol. Ecol. 2007, 59, 418–427. [Google Scholar] [CrossRef]
- Campbell, B.J.; Kirchman, D.L. Bacterial Diversity, Community Structure and Potential Growth Rates Along an Estuarine Salinity Gradient. Isme J. 2012, 7, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.S. Nutrients, Chlorophyll-a and Primary Productivity in Maxwell Bay, King George Island, Antarctica. KOREAN J. Polar Res. 1990, 1, 11–18. [Google Scholar]
- Lee, J.; Jin, Y.K.; Hong, J.K.; Yoo, H.J.; Shon, H. Simulation of a Tidewater Glacier Evolution in Marian Cove, King George Island, Antarctica. Geosci. J. 2008, 12, 33–39. [Google Scholar] [CrossRef]
- Ahn, I.; Chung, K.H.; Choi, H.J. Influence of Glacial Runoff on Baseline Metal Accumulation in the Antarctic Limpet Nacella Concinna from King George Island. Mar. Pollut. Bull. 2004, 49, 119–127. [Google Scholar] [CrossRef]
- Moon, H.; Wan Hussin, W.M.R.; Kim, H.; Ahn, I. The Impacts of Climate Change on Antarctic Nearshore Mega-Epifaunal Benthic Assemblages in a Glacial Fjord on King George Island: Responses and Implications. Ecol. Indic. 2015, 57, 280–292. [Google Scholar] [CrossRef]
- Vaughan, D.G.; Marshall, G.J.; Connolley, W.M.; King, J.C.; Mulvaney, R. Devil in the Detail. Science 2001, 293, 1777–1779. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, D.G.; Marshall, G.J.; Connolley, W.M.; Parkinson, C.; Mulvaney, R.; Hodgson, D.A.; King, J.C.; Pudsey, C.J.; Turner, J. Recent Rapid Regional Climate Warming on the Antarctic Peninsula. Clim. Chang. 2003, 60, 243–274. [Google Scholar] [CrossRef]
- Park, B.; Chang, S.; Yoon, H.I.; Chung, H. Recent Retreat of Ice Cliffs, King George Island, South Shetland Islands, Antarctic Peninsula. Ann. Glaciol. 1998, 27, 633–635. [Google Scholar] [CrossRef] [Green Version]
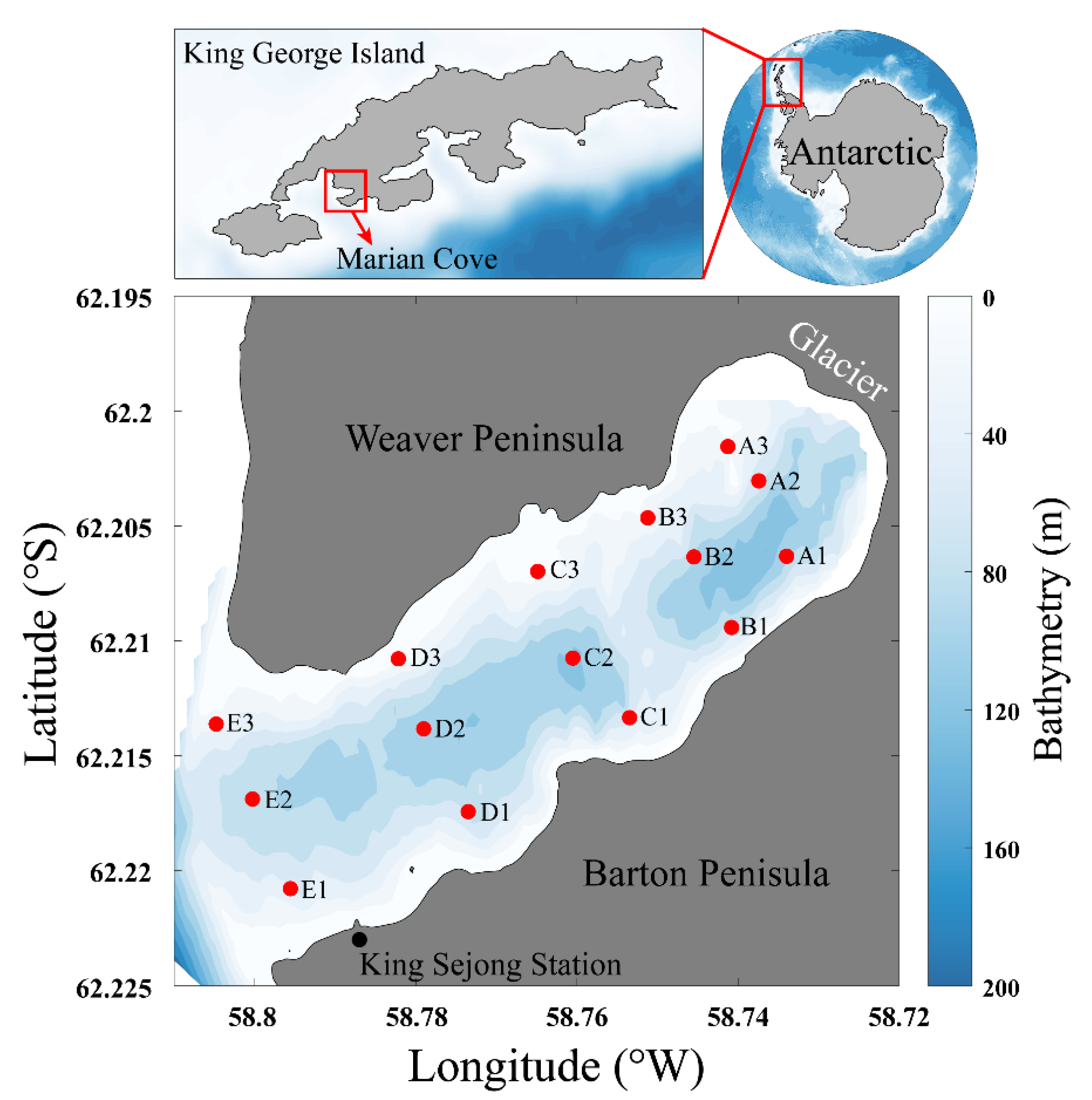

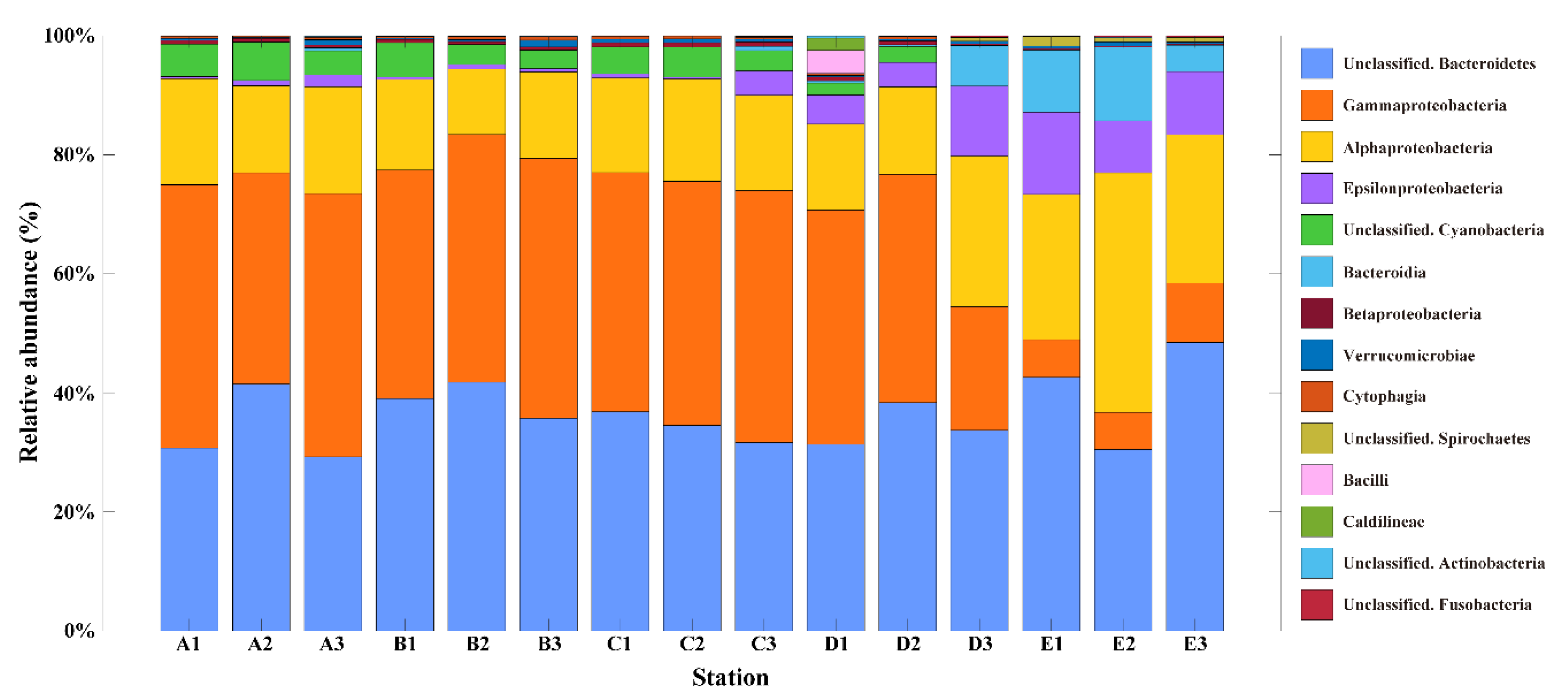

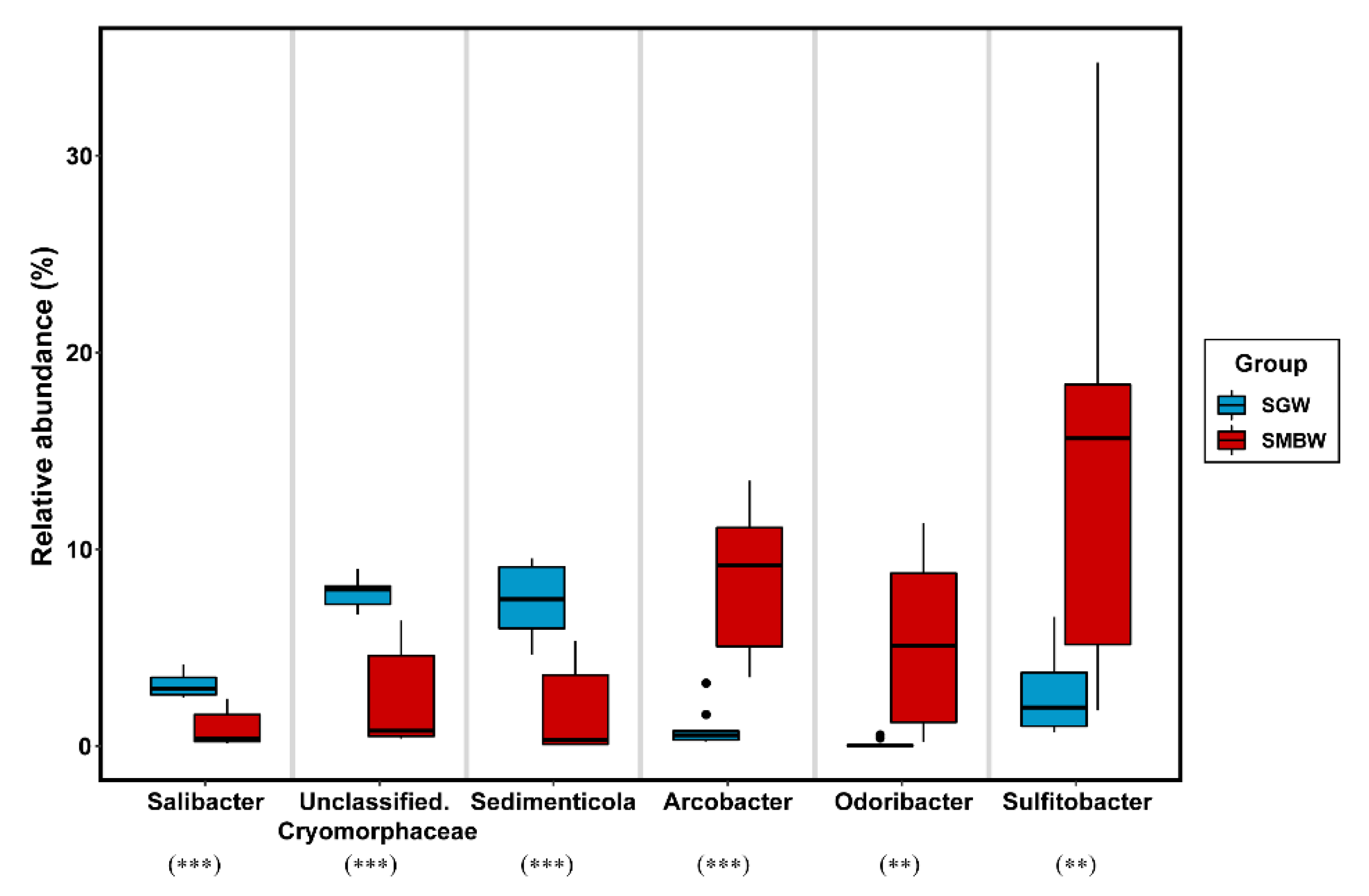

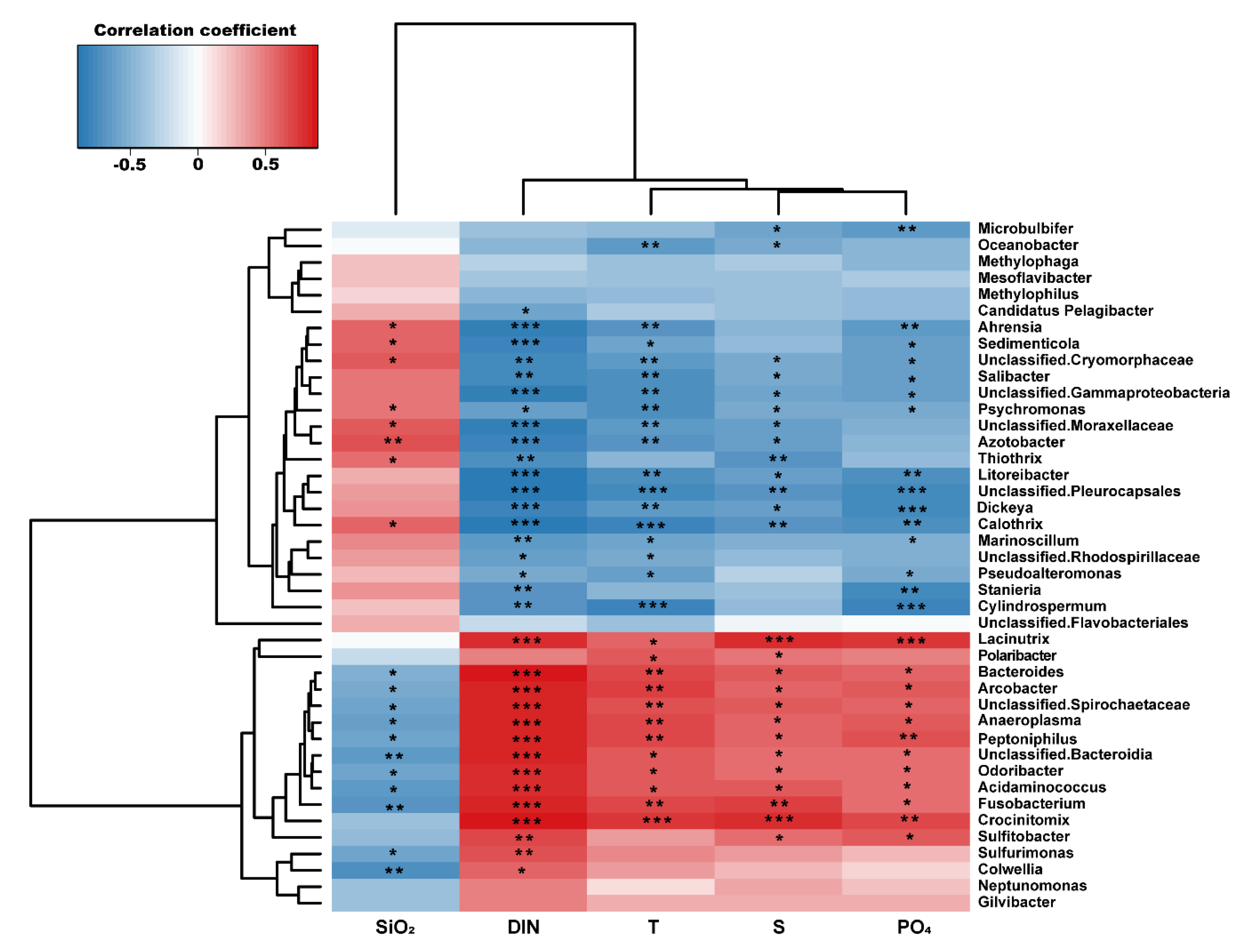
| Station | Latitude (°S) | Longitude (°W) | T (°C) | S (psu) | DIN (μM) | PO4 (μM) | SiO2 (μM) | Read Counts | OTU | Chao1 | Shannon |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 62.2063 | 58.7340 | 0.28 | 33.20 | 17.01 | 1.71 | 64.44 | 39,598 | 235 | 269.2 | 4.62 |
| A2 | 62.2030 | 58.7374 | 0.62 | 32.95 | 19.07 | 1.71 | 63.55 | 49,976 | 241 | 296.1 | 4.40 |
| A3 | 62.2016 | 58.7413 | 0.58 | 31.44 | 19.29 | 1.61 | 53.67 | 35,442 | 252 | 290.3 | 4.75 |
| B1 | 62.2094 | 58.7408 | 1.30 | 33.04 | 18.11 | 1.61 | 61.48 | 40,813 | 228 | 299.9 | 4.24 |
| B2 | 62.2064 | 58.7455 | 1.41 | 33.35 | 19.32 | 1.71 | 63.88 | 41,502 | 223 | 262.0 | 4.44 |
| B3 | 62.2047 | 58.7512 | 0.73 | 33.01 | 19.18 | 1.74 | 63.40 | 33,969 | 227 | 280.1 | 4.53 |
| C1 | 62.2133 | 58.7535 | 1.66 | 33.20 | 18.61 | 1.65 | 62.45 | 33,642 | 243 | 284.8 | 4.52 |
| C2 | 62.2108 | 58.7605 | 1.16 | 33.20 | 19.96 | 1.74 | 63.28 | 45,573 | 233 | 258.2 | 4.49 |
| C3 | 62.2070 | 58.7649 | 0.73 | 33.35 | 23.25 | 1.71 | 59.24 | 44,372 | 255 | 298.7 | 4.78 |
| D1 | 62.2174 | 58.7735 | 2.48 | 33.14 | 25.21 | 1.74 | 62.33 | 26,430 | 300 | 343.6 | 5.16 |
| D2 | 62.2138 | 58.7790 | 1.80 | 33.32 | 21.04 | 1.74 | 57.88 | 44,444 | 254 | 296.0 | 4.50 |
| D3 | 62.2108 | 58.7822 | 1.95 | 33.35 | 24.79 | 1.74 | 52.78 | 53,793 | 246 | 299.0 | 3.76 |
| E1 | 62.2208 | 58.7956 | 2.78 | 33.45 | 32.82 | 1.82 | 62.75 | 47,913 | 248 | 303.3 | 3.62 |
| E2 | 62.2169 | 58.8003 | 2.32 | 33.36 | 30.86 | 1.79 | 62.12 | 49,610 | 223 | 271.4 | 3.41 |
| E3 | 62.2136 | 58.8048 | 2.08 | 33.25 | 31.21 | 1.76 | 61.28 | 63,410 | 233 | 296.2 | 3.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, J.-H.; Lim, J.-H.; Jeong, J.-H.; Heo, J.-M.; Kim, I.-N. Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018. Microorganisms 2020, 8, 1115. https://doi.org/10.3390/microorganisms8081115
Kim S, Kim J-H, Lim J-H, Jeong J-H, Heo J-M, Kim I-N. Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018. Microorganisms. 2020; 8(8):1115. https://doi.org/10.3390/microorganisms8081115
Chicago/Turabian StyleKim, Soyeon, Ju-Hyoung Kim, Jae-Hyun Lim, Jin-Hyun Jeong, Jang-Mu Heo, and Il-Nam Kim. 2020. "Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018" Microorganisms 8, no. 8: 1115. https://doi.org/10.3390/microorganisms8081115
APA StyleKim, S., Kim, J.-H., Lim, J.-H., Jeong, J.-H., Heo, J.-M., & Kim, I.-N. (2020). Distribution and Control of Bacterial Community Composition in Marian Cove Surface Waters, King George Island, Antarctica during the Summer of 2018. Microorganisms, 8(8), 1115. https://doi.org/10.3390/microorganisms8081115






