Yarrowia lipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Selection of the Yeast Strains as Lipase and Protease Producers
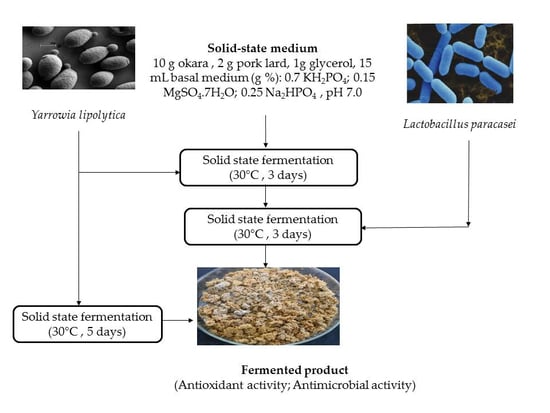
2.3. Inoculum Preparation and Solid State Fermentation (SSF) Fermentation
2.3.1. SSF Fermentation with the Monoculture of Y. lipolytica
2.3.2. SSF Fermentation with the Co-Culture of Y. lipolytica and L. paracasei
2.4. Identifying the Significant Variables of the Biotechnological Process by Using the Plackett–Burman Design
2.5. Antimicrobial Activity
2.6. Antioxidant Capacity
2.7. Statistical Analysis
3. Results
3.1. Screening of the Yeast Strains that Are Able to Produce Hydrolases
3.2. Antimicrobial Activity
3.3. Antioxidant Activity
3.4. Screening of the Fermentation Parameters Using Plackett–Burman Design
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, Y.; Wang, J.; Wu, J.; Wang, L.; Rui, X.; Xing, G.; Dong, M. Enhancing the functional properties of soymilk residues (okara) by solid-state fermentation with Actinomucor elegans. CyTA J. Food 2016, 15, 155–163. [Google Scholar] [CrossRef][Green Version]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of by-products from soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef] [PubMed]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Okara: A Nutritionally valuable by-product able to stabilize lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017, 8, 641. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S. The effects of carbohydrase, probiotic Lactobacillus paracasei and yeast Lindnera saturnus on the composition of a novel okara (soybean residue) functional beverage. LWT 2019, 100, 196–204. [Google Scholar] [CrossRef]
- Filho, M.M.; Busanello, M.; Garcia, S. Optimization of the fermentation parameters for the growth of Lactobacillus in soymilk with okara flour. LWT 2016, 74, 456–464. [Google Scholar] [CrossRef]
- Szotkowski, M.; Byrtusova, D.; Haronikova, A.; Vysoka, M.; Rapta, M.; Shapaval, V.; Marova, I. Study of metabolic adaptation of red yeasts to waste animal fat substrate. Microorganism 2019, 7, 578. [Google Scholar] [CrossRef]
- Yamsub, A.; Kaewchada, A.; Jaree, A. Pork lard conversion to biodiesel using a microchannel reactor. Korean J. Chem. Eng. 2014, 31, 2170–2176. [Google Scholar] [CrossRef]
- Lopes, M.; Gomes, A.C.; Silva, C.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef]
- Tzirita, M.; Papanikolaou, S.; Chatzifragkou, A.; Quilty, B. Waste fat biodegradation and biomodification by Yarrowia lipolytica and a bacterial consortium composed of Bacillus spp. and Pseudomonas putida. Eng. Life Sci. 2018, 18, 932–942. [Google Scholar] [CrossRef]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolyticaand Its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.O.; Farias, M.A.; Belo, I.; Coelho, M. Nitrogen sources on tpomw valorization through solid state fermentation performed by Yarrowia lipolytica. Braz. J. Chem. Eng. 2016, 33, 261–270. [Google Scholar] [CrossRef]
- Lanka, S.; Latha, J.N.L. A short review on various screening methods to isolate potential lipase producers: lipases-the present and future enzymes of biotech industry. Int. J. Boil. Chem. 2015, 9, 207–219. [Google Scholar] [CrossRef]
- Ramnath, L.; Sithole, B.; Govinden, R. Identification of lipolytic enzymes isolated from bacteria indigenous to Eucalyptus wood species for application in the pulping industry. Biotechnol. Rep. 2017, 15, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Parfene, G.; Horincar, V.B.; Bahrim, G. Preliminary study regarding the use of some Yarrowia lipolytica strains for solid state hydrolysis of crude coconut fat. St. Cerc. St. CICBI. 2012, 13, 187–194. [Google Scholar]
- Ozturkoglu-Budak, S.; Wiebenga, A.; Bron, P.A.; De Vries, R.P. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int. J. Food Microbiol. 2016, 237, 17–27. [Google Scholar] [CrossRef]
- Cotarlet (Scântee), M.; Bahrim, G.; Negoită, T.; Stougaard, P. Screening of polar streptomycetes able to produce cold-active hydrolytic enzymes using common and chromogenic substrates. Rom. Biotechnol. Lett. 2008, 13, 69–80. [Google Scholar]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; Garcia, H. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturică, M.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleț, M.; Stănciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO 2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.J. Production and properties of health-promoting proteins and peptides from bovine colostrum and milk. Cell. Mol. Boil. 2013, 59, 26–38. [Google Scholar]
- Marova, I.; Szotkowski, M.; Vanek, M.; Rapta, M.; Byrtusova, D.; Mikheichyk, N.; Haronikova, A.; Čertík, M.; Shapaval, V. Utilization of animal fat waste as carbon source by carotenogenic yeasts—A screening study. EuroBiotech J. 2017, 1, 310–318. [Google Scholar] [CrossRef]
- Cotârleț, M.; Vasile, A.M.; Cantaragiu, A.M.; Gaspar-Pintiliescu, A.; Craciunescu, O.; Oancea, A.; Moraru, A.; Moraru, I.; Bahrim, G.-E. Colostrum-derived bioactive peptides obtained by fermentation with kefir grains enriched with selected yeasts. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2019, 43, 54–68. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Oancea, A.; Cotarlet, M.; Vasile, A.M.; Bahrim, G.E.; Shaposhnikov, S.; Craciunescu, O.; Oprita, E.I. Angiotensin-converting enzyme inhibition, antioxidant activity and cytotoxicity of bioactive peptides from fermented bovine colostrum. Int. J. Dairy Technol. 2019, 73, 108–116. [Google Scholar] [CrossRef]
- Cotârleţ, M.; Vasile, A.M.; Gaspar-Pintiliescu, A.; Oancea, A.; Bahrim, G.-E. Tribiotication strategy for the functionalization of bovine colostrum through the biochemical activities of artisanal and selected starter cultures. CyTA J. Food 2020, 18, 274–280. [Google Scholar] [CrossRef]
- Gdula, A.; Chrzanowska, J.; Szoltysik, M.; Wojtatowicz, M.; Guerzoni, M.E. Enzymatic abilities and comparison of lipolytic activity of two Yarrowia lipolytica strains. Biotechnologia 2003, 2, 83–89. [Google Scholar]
- Muhialdin, B.J.; Hassan, Z.; Sadon, S.K. Biopreservation of food by lactic acid bacteria against spoilage fungi. Ann. Food Sci. Technol. 2011, 12, 44–57. [Google Scholar]
- Mok, W.K.; Tan, Y.X.; Lee, J.J.L.; Kim, J.; Chen, W.N. A metabolomic approach to understand the solid-state fermentation of okara using Bacillus subtilis WX-17 for enhanced nutritional profile. AMB Express 2019, 9, 60. [Google Scholar] [CrossRef]
- Patrignani, F.; Vannini, L.; Gardini, F.; Guerzoni, M.E.; Lanciotti, R. Variability of the lipolytic activity and volatile molecules production by a strain of Yarrowia lipolytica in pork fat and its dependence on environmental conditions. Meat Sci. 2011, 89, 21–26. [Google Scholar] [CrossRef] [PubMed]

| No. | Strain Code | Protease Assay * | Lipase Assay * |
|---|---|---|---|
| 1 | Y. lipolytica MIUG D5 | 1.30 | 1.20 |
| 2 | Y. lipolytica MIUG D6 | −** | − |
| 3 | Y. lipolytica MIUG D7 | − | − |
| 4 | C. lipolytica MIUG 106 | 1.16 | 1.08 |
| 5 | C. lipolytica MIUG D67 | − | − |
| 6 | C. lipolytica MIUG D69 | − | − |
| 7 | C. lipolytica MIUG D96 | 1.05 | 1.03 |
| 8 | C. lipolytica MIUG D98 | 1.13 | 1.05 |
| 9 | C. lipolytica MIUG D99 | − | 1.03 |
| 10 | C. lipolytica MIUG D100 | 1.15 | 1.08 |
| 11 | C. lipolytica MIUG D101 | 1.10 | 1.05 |
| 12 | C. lipolytica MIUG D111 | 1.05 | 1.03 |
| 13 | C. lipolytica MIUG D73 | − | nd |
| 14 | C. colliculosa MIUG D108 | − | nd |
| 15 | C. krusei MIUG D97 | − | nd |
| 16 | C. colliculosa MIUG D102 | − | nd |
| 17 | C. colliculosa MIUG D115 | − | nd |
| 18 | C. krusei MIUG D74 | − | nd |
| 19 | Y. lipolytica ATCC 18942 | 1.65 | 1.60 |
| Sample | Inhibition Ratio % | |
|---|---|---|
| A. niger MIUG M5 | B. subtilis MIUG B1 | |
| Fermented product with Y. lipolytica MIUG D5 | 6 | 16 |
| Fermented product with Y. lipolytica ATCC 18942 | 9 | 23 |
| Fermented product with co-culture of Y. lipolytica MIUG D5 and L. paracasei MIUG BL20 | 0 * | 29 |
| Fermented product with co-culture of Y. lipolytica ATCC 18942 and L. paracasei MIUG BL20 | 0 | 33 |
| Sample | Antioxidant Activity, mM TE/g |
|---|---|
| Control | 6.86 ± 1.07 * |
| Fermented product with Y. lipolytica MIUG D5 | 18.49 ± 0.37 a |
| Fermented product with Y. lipolytica ATCC 18942 | 18.06 ± 0.55 ab |
| Fermented product with co-culture of Y. lipolytica MIUG D5 and L. paracasei MIUG BL20 | 17.27 ± 0.75 bc |
| Fermented product with co-culture of Y. lipolytica ATCC 18942 and L. paracasei MIUG BL20 | 14.60 ± 0.30 c |
| Run | Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A * | B | C | D | E | F | G | Antioxidant Activity, mM TE/g | Antimicrobial Activity% | |
| 1 | 20.00 | 3.00 | 1.50 | 1 × 105 | 48.00 | 1.00 | 72.00 | 13.09 | 44 |
| 2 | 20.00 | 1.00 | 0.50 | 1 x 105 | 96.00 | 1.00 | 72.00 | 6.77 | 28 |
| 3 | 10.00 | 3.00 | 1.50 | 1 × 105 | 96.00 | 2.00 | 72.00 | 15.17 | 44 |
| 4 | 10.00 | 1.00 | 0.50 | 1 × 107 | 48.00 | 2.00 | 72.00 | 10.75 | 36 |
| 5 | 10.00 | 3.00 | 0.50 | 1 x 107 | 96.00 | 1.00 | 72.00 | 17.78 | 44 |
| 6 | 10.00 | 1.00 | 1.50 | 1 × 105 | 96.00 | 2.00 | 24.00 | 14.06 | 24 |
| 7 | 20.00 | 1.00 | 1.50 | 1 × 107 | 96.00 | 1.00 | 24.00 | 13.45 | 32 |
| 8 | 10.00 | 1.00 | 0.50 | 1 × 105 | 48.00 | 1.00 | 24.00 | 11.28 | 40 |
| 9 | 20.00 | 3.00 | 0.50 | 1 × 105 | 48.00 | 2.00 | 24.00 | 8.95 | 40 |
| 10 | 20.00 | 3.00 | 0.50 | 1 × 107 | 96.00 | 2.00 | 24.00 | 13.36 | 44 |
| 11 | 20.00 | 1.00 | 1.50 | 1 × 107 | 48.00 | 2.00 | 72.00 | 13.31 | 64 |
| 12 | 10.00 | 3.00 | 1.50 | 1 × 107 | 48.00 | 1.00 | 24.00 | 12.11 | 34 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 507.00 | 1 | 507.00 | 7.85 | 0.0187 |
| E-time for yeast fermentation | 507.00 | 1 | 507.00 | 7.85 | 0.0187 |
| Residual | 646.00 | 10 | 64.60 | / | / |
| Cor Total | 1153.00 | 11 | / | / | / |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 50.44 | 2 | 25.22 | 5.71 | 0.0251 |
| B-pork lard | 38.52 | 1 | 38.52 | 8.71 | 0.0162 |
| F-LAB inoculum | 11.92 | 1 | 11.92 | 2.70 | 0.1350 |
| Residual | 39.78 | 9 | 4.42 | / | / |
| Cor Total | 90.23 | 11 | / | / | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotârleț, M.; Stănciuc, N.; Bahrim, G.E. Yarrowia lipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation. Microorganisms 2020, 8, 1098. https://doi.org/10.3390/microorganisms8081098
Cotârleț M, Stănciuc N, Bahrim GE. Yarrowia lipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation. Microorganisms. 2020; 8(8):1098. https://doi.org/10.3390/microorganisms8081098
Chicago/Turabian StyleCotârleț, Mihaela, Nicoleta Stănciuc, and Gabriela Elena Bahrim. 2020. "Yarrowia lipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation" Microorganisms 8, no. 8: 1098. https://doi.org/10.3390/microorganisms8081098
APA StyleCotârleț, M., Stănciuc, N., & Bahrim, G. E. (2020). Yarrowia lipolytica and Lactobacillus paracasei Solid State Fermentation as a Valuable Biotechnological Tool for the Pork Lard and Okara’s Biotransformation. Microorganisms, 8(8), 1098. https://doi.org/10.3390/microorganisms8081098