Phenotypic Response of Wolbachia pipientis in a Cell-Free Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Trypan Blue Staining and Hemocytometer Aa23 Cell Counts
2.3. Confirmation of Wolbachia Infection Status Using PCR
2.4. Fluorescent In-Situ Hybridization
2.5. Isolation of Extracellular Wolbachia
2.6. Epifluorescence Microscopy
2.7. Phenotypic Microarray Assays
2.8. Quantitative Real-Time PCR Analysis
2.9. Wolbachia Cell Counts
2.10. Statistical Analyses
3. Results
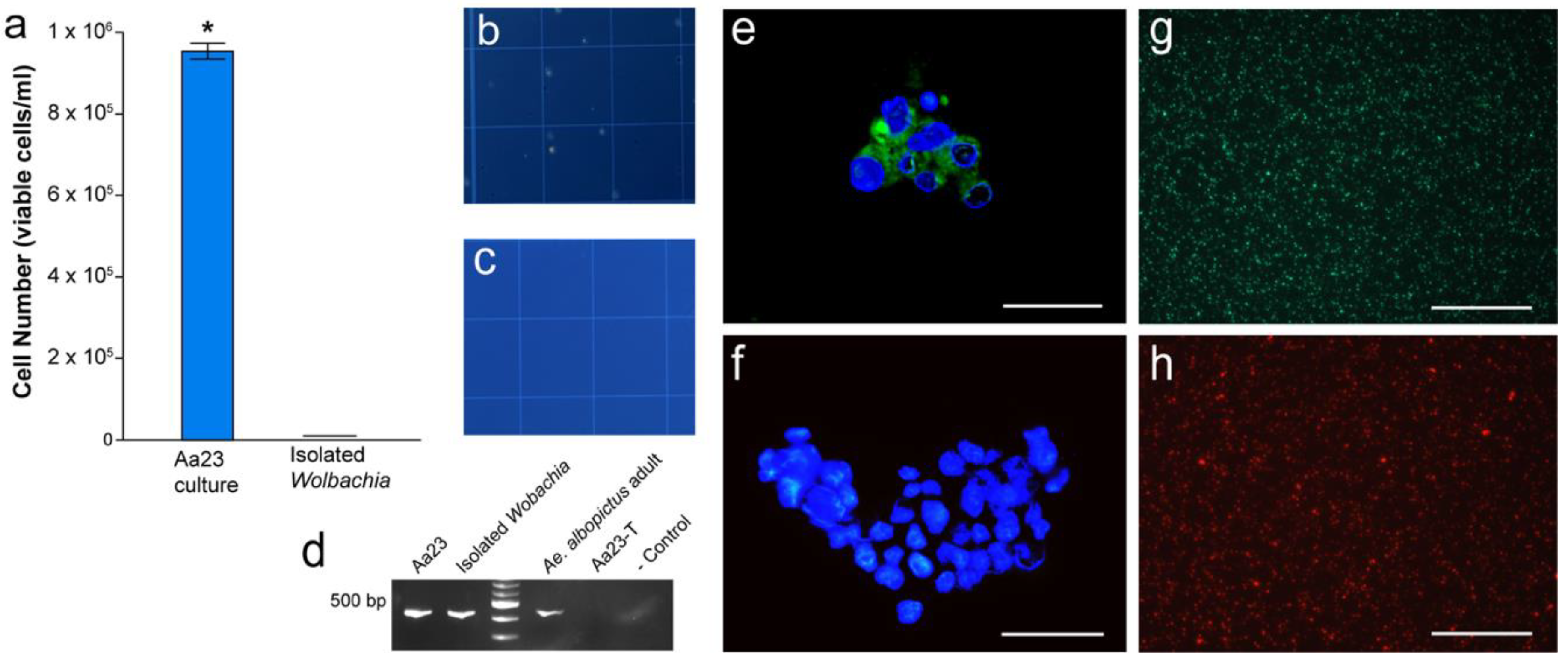
3.1. Confirmation of Wolbachia Infections and Isolation of Wolbachia
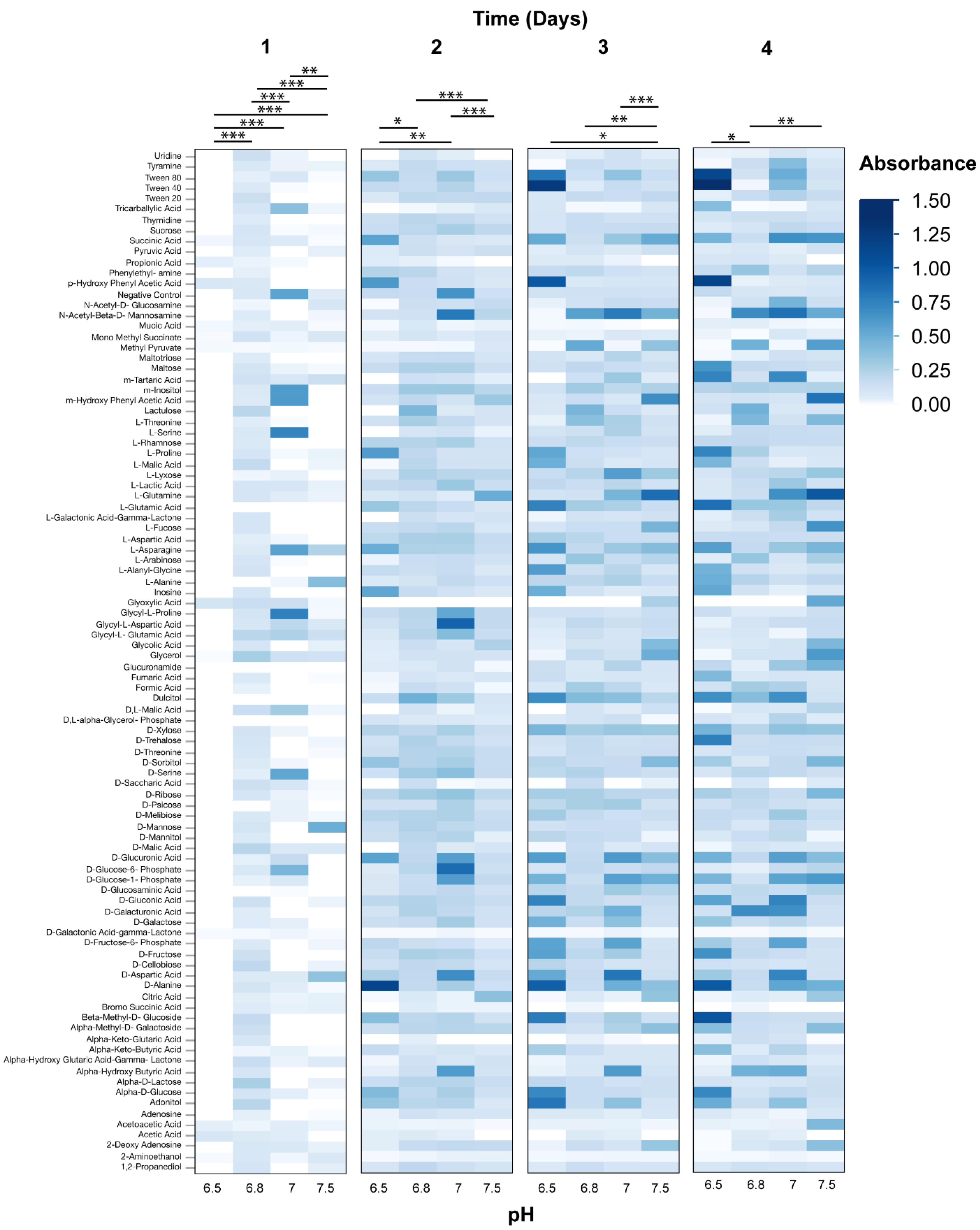
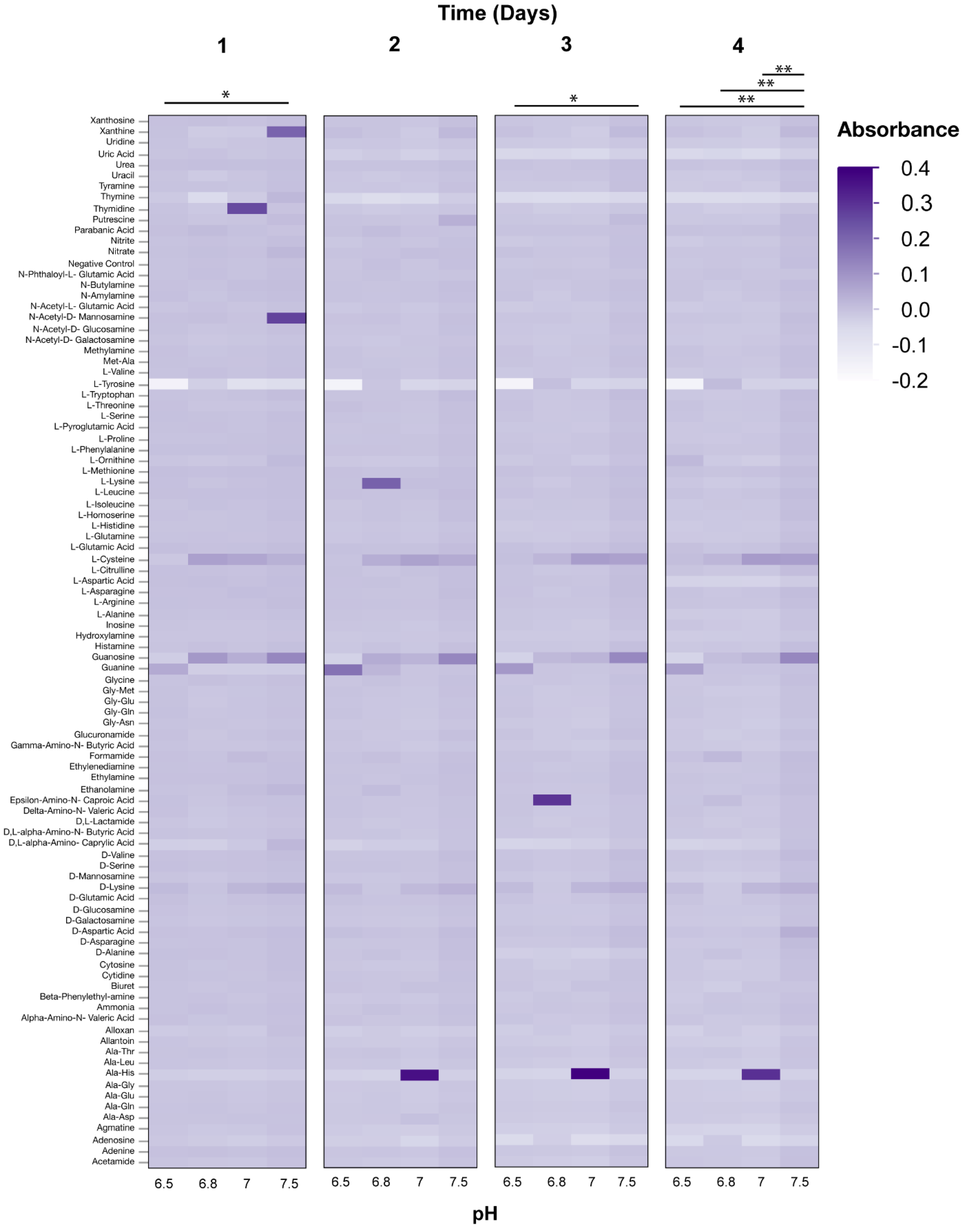
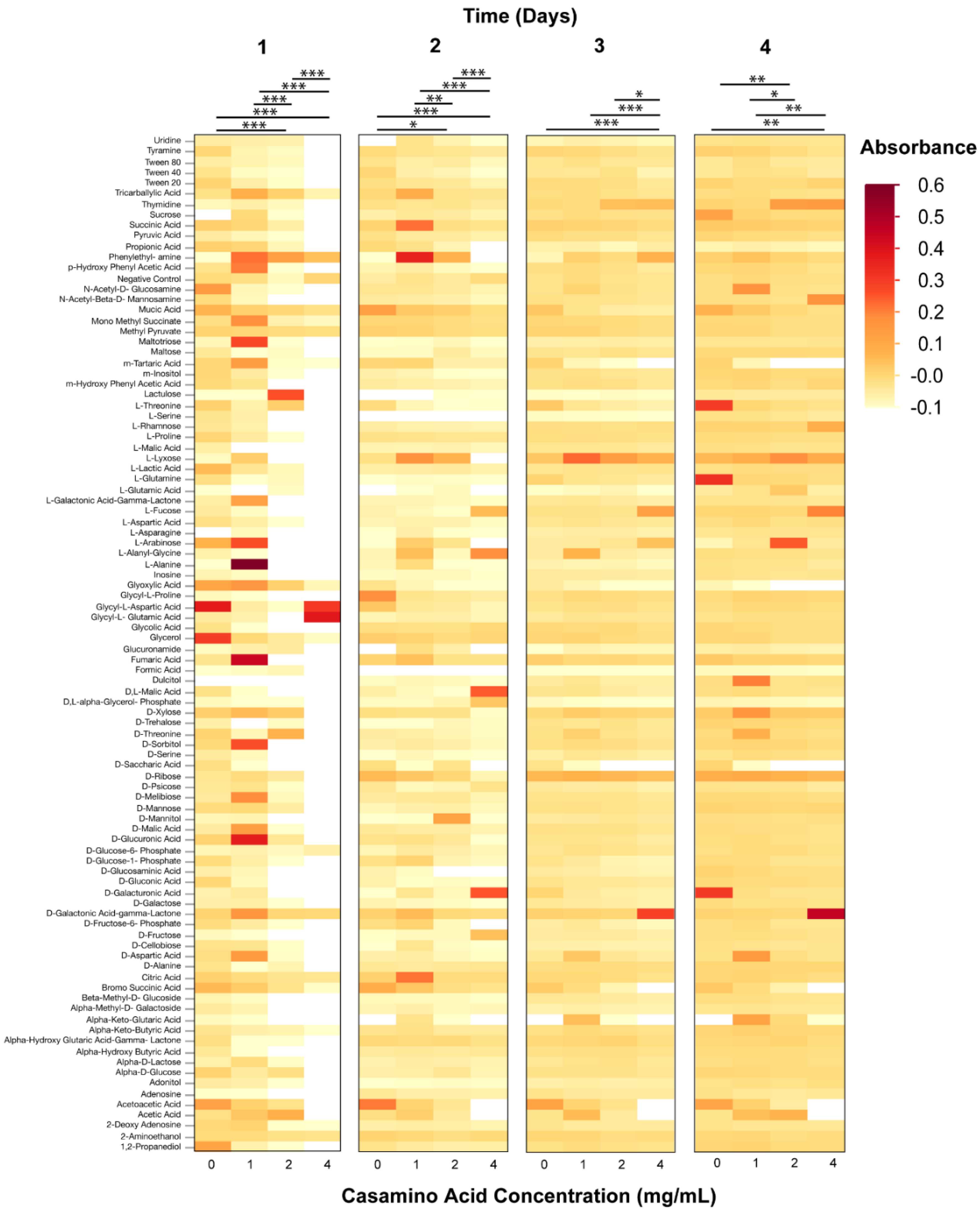
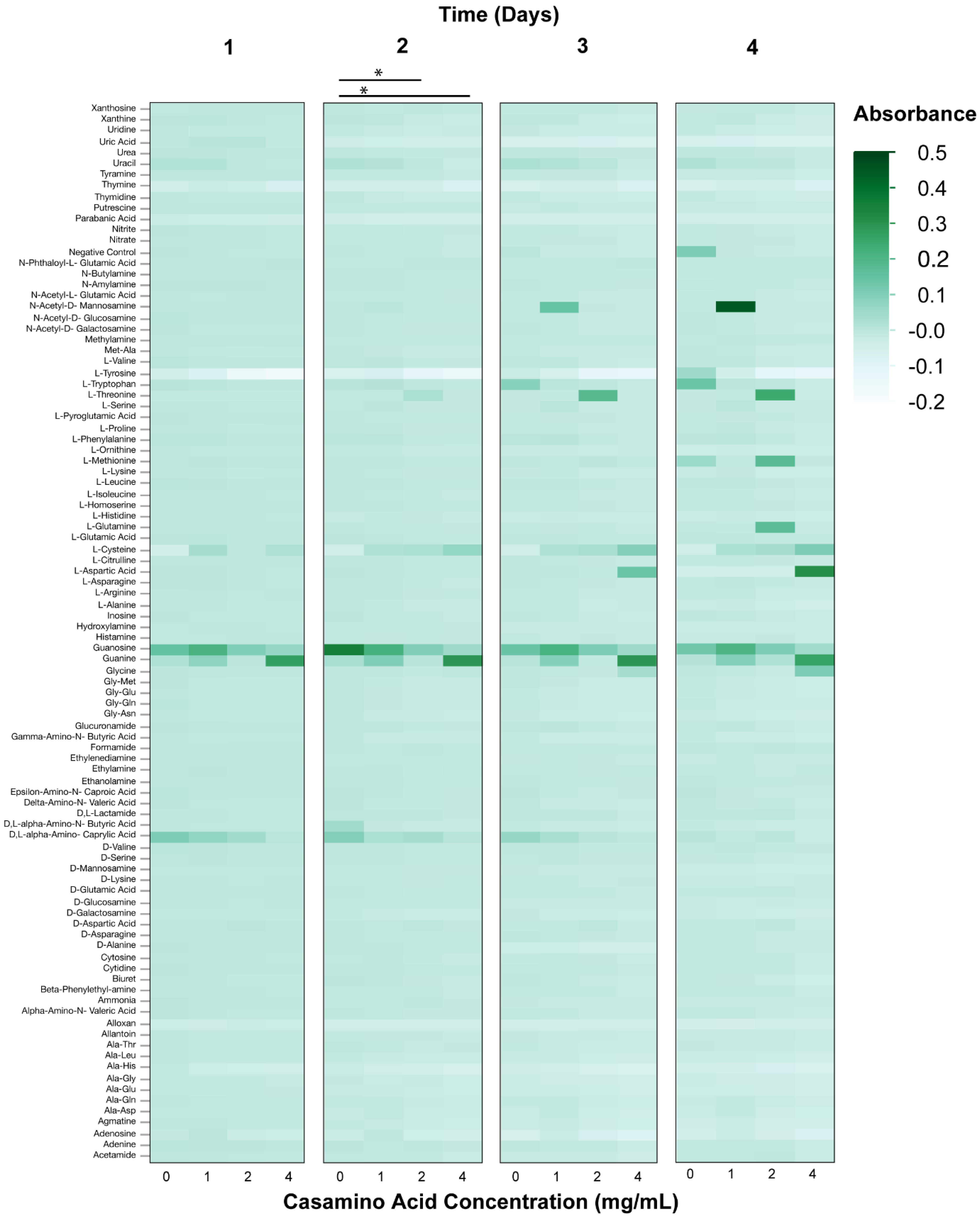
3.2. Phenotypic Microarray Assays
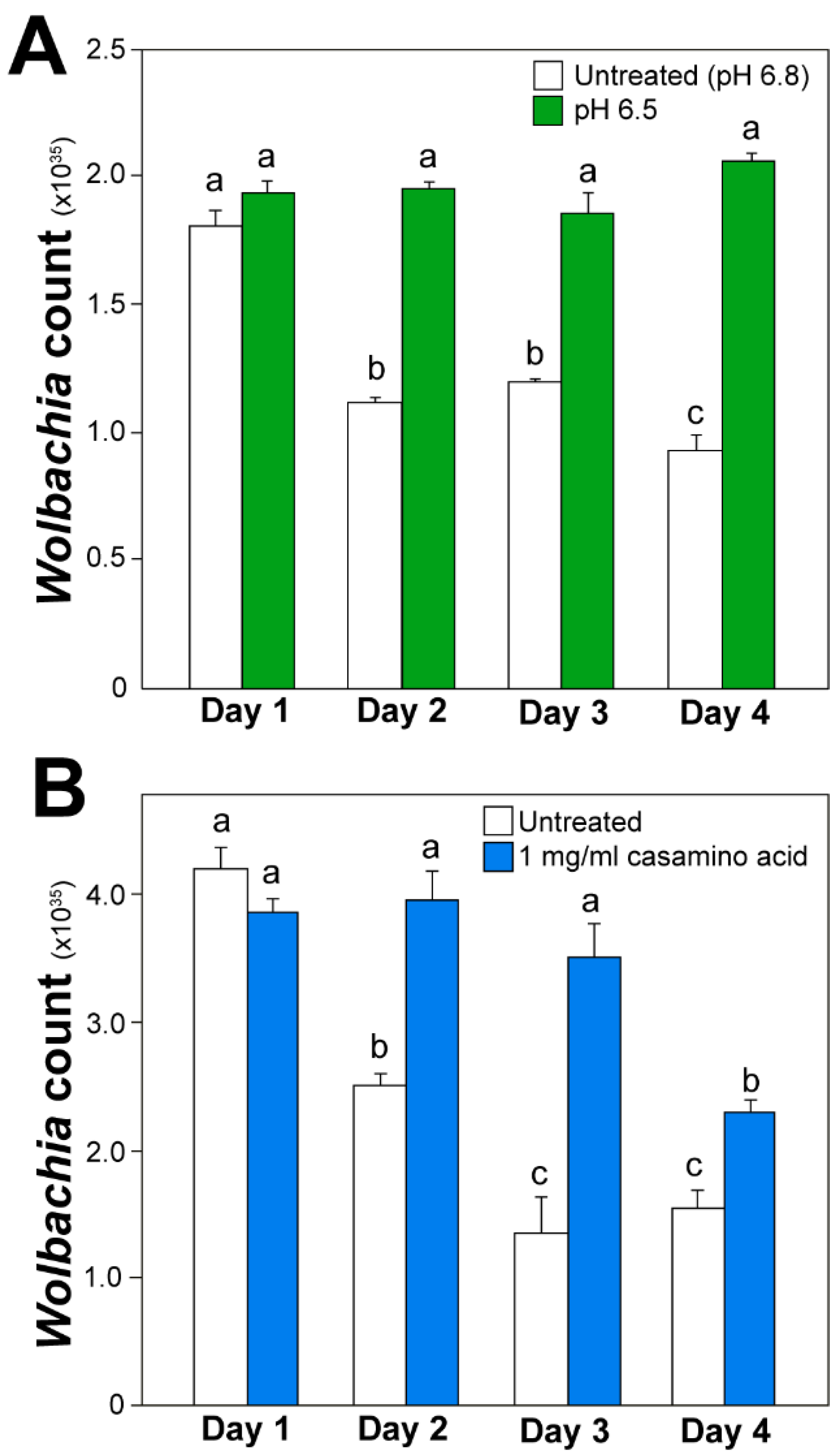
3.3. Quantitative Real-Time PCR
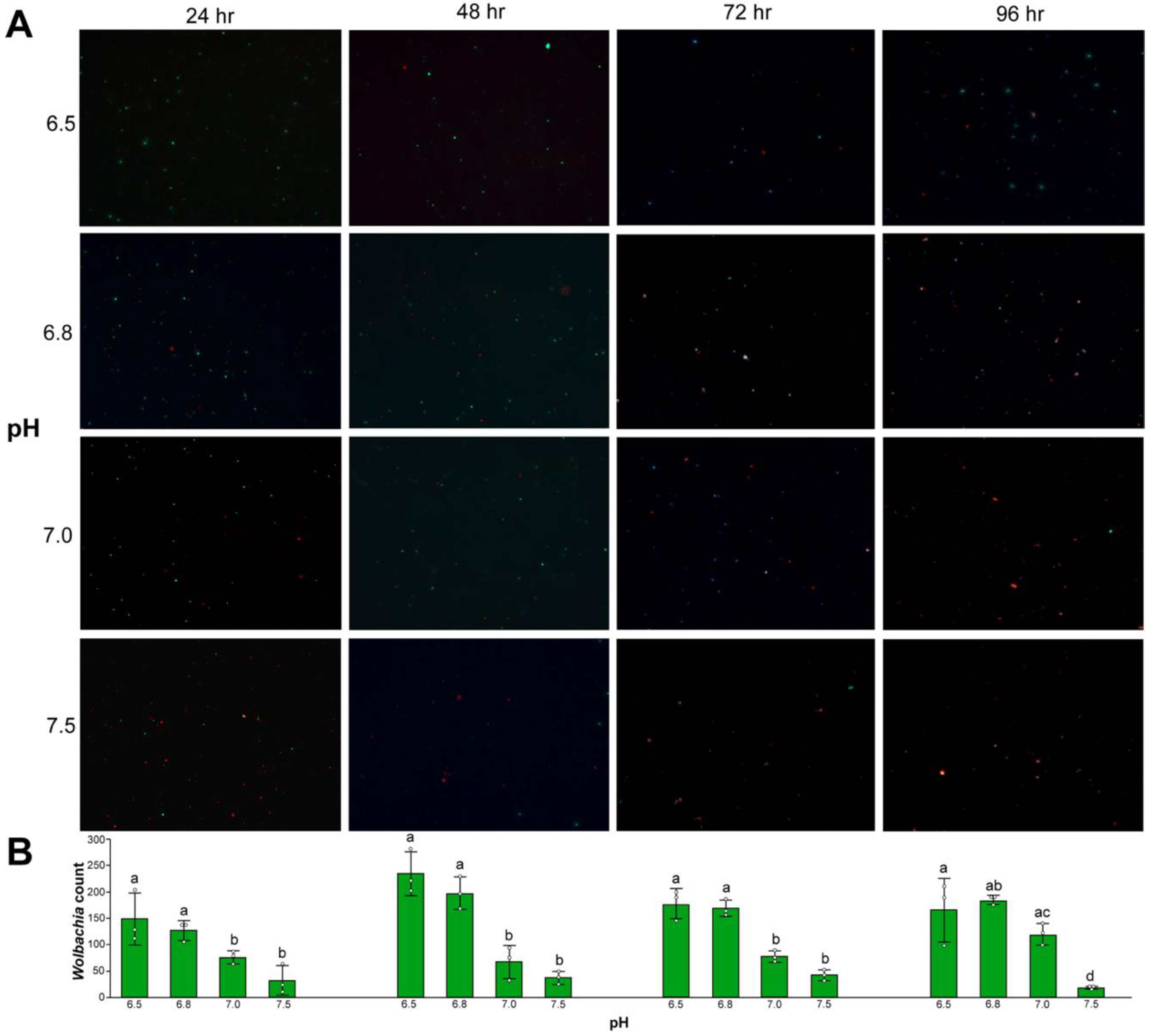
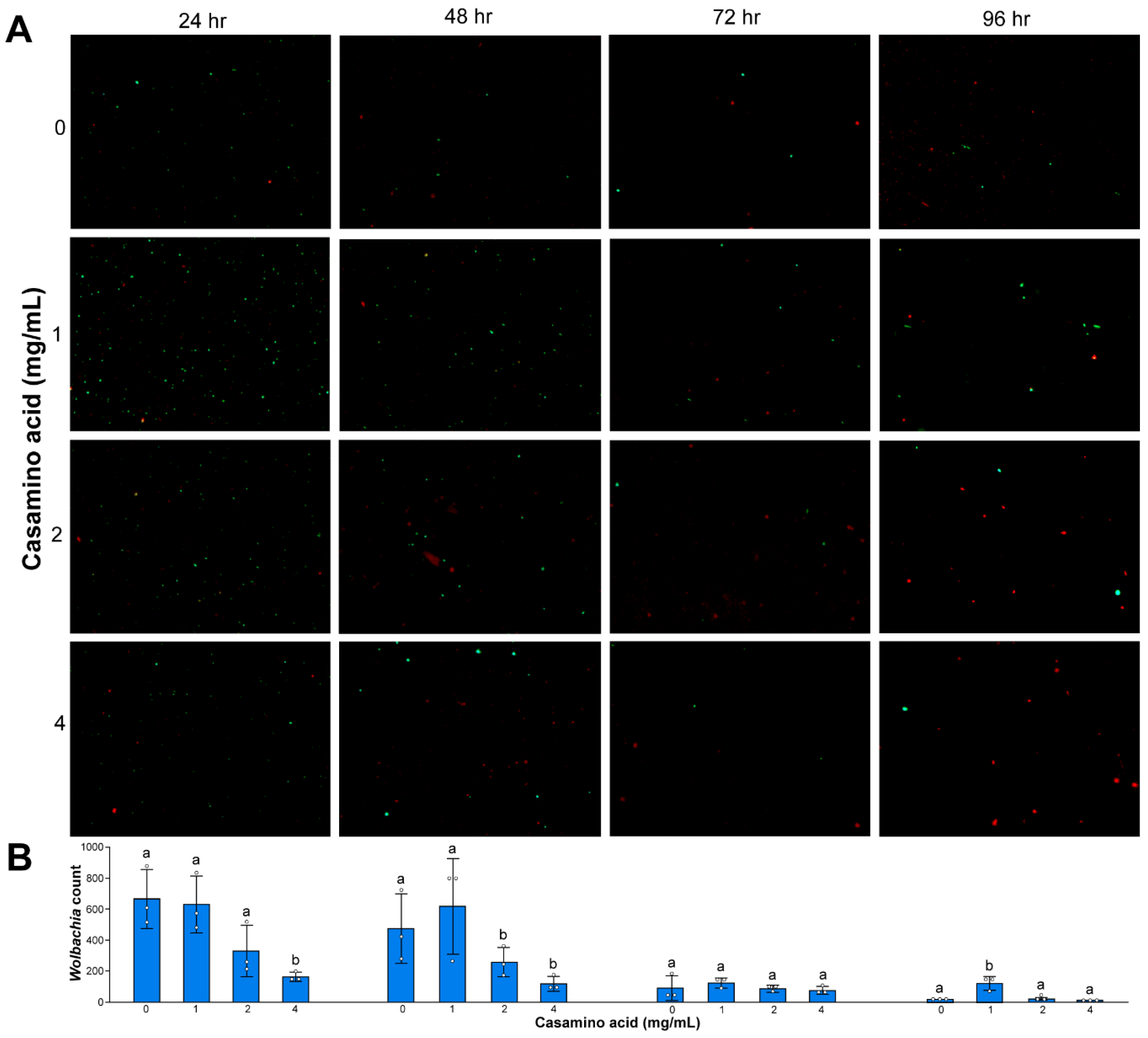
3.4. Wolbachia Cell Counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werren, J.H.; Windsor, D.M. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. Biol. Sci. 2000, 267, 1277–1285. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. Fems. Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Hurk, v.d.A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar]
- Caragata, E.P.; Dutra, H.L.C.; Moreira, L.A. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb. Cell 2016, 3, 293–295. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, P.J.; Li, M.I.; Yang, H.T.; Ng, L.C.; O’Neill, S.L. wMel limits zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS. Negl. Trop. Dis. 2017, 11, e0005496. [Google Scholar] [CrossRef]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Mains, J.W.; Brelsfoard, C.L.; Rose, R.I.; Dobson, S.L. Femal adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 2016, 6, 33846. [Google Scholar] [CrossRef]
- Aultman, K.S.; Beaty, B.J.; Walker, E.D. Genetically manipulated vectors of human disease: A practical overview. Trends Para. 2001, 17, 507–509. [Google Scholar] [CrossRef]
- Aksoy, S.; Weiss, B.; Attardo, G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. In Transgenesis and the Management of Vector-Borne Disease; Springer: New York, NY, USA, 2008; Volume 627, pp. 35–48. [Google Scholar]
- Bourtzis, K. Wolbachia-based technologies for insect pest population control. Adv. Exp. Med. Biol. 2008, 627, 104–113. [Google Scholar] [PubMed]
- Rasgon, J.L.; Gamston, C.E.; Xiaoxia, R. Survival of Wolbachia pipientis in cell-free medium. Appl. Env. Micro. 2006, 72, 6934–6937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.L.; Bourtzis, K.; Braig, H.R.; Jones, B.F.; Zhou, W.; Rousset, F.; O’Neill, S.L. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 1999, 29, 153–160. [Google Scholar] [CrossRef]
- Frydman, H.M.; Li, J.M.; Robson, D.N.; Wieschaus, E. Somatic stem cell niche tropism in Wolbachia. Nature 2006, 441, 509–512. [Google Scholar] [CrossRef]
- Gamston, C.; Rasgon, J. Maintaining Wolbachia in cell-free medium. J. Vis. Exp. 2007, 233. [Google Scholar] [CrossRef]
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Bio. 2004, 2, E69. [Google Scholar] [CrossRef]
- Brownlie, J.C.; O’Neill, S.L. Wolbachia genomes: Insights into an intracellular lifestyle. Curr. Biol. 2005, 15, R507–R509. [Google Scholar] [CrossRef]
- Klasson, L.; Westberg, J.; Sapountzis, P.; Naslund, K.; Lutnaes, Y.; Darby, A.C.; Veneti, Z.; Chen, L.; Braig, H.R.; Garrett, R.; et al. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc. Natl. Acad. Sci. USA 2009, 106, 5725–5730. [Google Scholar] [CrossRef]
- Maren Ellegaard, K.; Klasson, L.; Naslund, K.; Bourtzis, K.; Anderson, S. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 2013, 9, e1003381. [Google Scholar] [CrossRef]
- White, P.M.; Serbus, L.R.; Debec, A.; Codina, A.; Bray, W.; Guichet, A.; Lokey, R.S.; Sullivan, W. Reliance of Wolbachia on High Rates of Host Proteolysis Revealed by a Genome-Wide RNAi Screen of Drosophila Cells. Genetics 2017, 205, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Verhoeve, V.I.; Guillotte, M.L.; Lehman, S.S.; Rennoll, S.A.; Beier-Sexton, M.; Rahman, M.S.; Azad, A.F.; Gillespie, J.J. Wholly Rickettsia! Reconstructed Metabolic Profile of the Quintessential Bacterial Parasite of Eukaryotic Cells. MBio 2017, 8, e00859-17. [Google Scholar] [CrossRef] [PubMed]
- Marmion, B.P.; Storm, P.A.; Ayres, J.G.; Semendric, L.; Mathews, L.; Winslow, W.; Turra, M.; Harris, R.J. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 2005, 98, 7–20. [Google Scholar] [CrossRef]
- Vlak, J.M.; de Gooijer, C.D.; Tramper, J.; Miltenburger, H.G. Insect Cell Cultures: Fundamental and Applied Aspects; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Medina, M.; Lopez-Rivas, A.; Zuidema, D.; Belsham, G.J.; Domingo, E.; Vlak, J.M. Strong buffering capacity of insect cells. Implications for the baculovirus expression system. Cytotechnology 1995, 17, 21–26. [Google Scholar] [CrossRef]
- Beare, P.A.; Jeffrey, B.M.; Long, C.M.; Martens, C.M.; Heinzen, R.A. Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation. PLoS Pathog. 2018, 14, e1006922. [Google Scholar] [CrossRef]
- Beare, P.A.; Larson, C.L.; Gilk, S.D.; Heinzen, R.A. Two systems for targeted gene deletion in Coxiella burnetii. Appl. Env. Microbiol. 2012, 78, 4580–4589. [Google Scholar] [CrossRef]
- Clay, K.A.; Hartley, M.G.; Russell, P.; Norville, I.H. Use of axenic media to determine antibiotic efficacy against Coxiella burnetii. Int. J. Antimicrob. Agents 2018, 51, 806–808. [Google Scholar] [CrossRef]
- Omsland, A.; Cockrell, D.C.; Fischer, E.R.; Heinzen, R.A. Sustained axenic metabolic activity by the obligate intracellular bacterium Coxiella burnetii. J. Bacteriol. 2008, 190, 3203–3212. [Google Scholar] [CrossRef][Green Version]
- Sanchez, S.E.; Vallejo-Esquerra, E.; Omsland, A. Use of Axenic Culture Tools to Study Coxiella burnetii. Curr. Protoc. Microbiol. 2018, 50, e52. [Google Scholar] [CrossRef]
- Sandoz, K.M.; Popham, D.L.; Beare, P.A.; Sturdevant, D.E.; Hansen, B.; Nair, V.; Heinzen, R.A. Transcriptional Profiling of Coxiella burnetii Reveals Extensive Cell Wall Remodeling in the Small Cell Variant Developmental Form. PLoS ONE 2016, 11, e0149957. [Google Scholar] [CrossRef] [PubMed]
- Pontes, M.H.; Dale, C. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 2006, 14, 406–412. [Google Scholar] [CrossRef]
- Omsland, A.; Cockrell, D.C.; Howe, D.; Fischer, E.R.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; Heinzen, R.A. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. USA 2009, 106, 4430–4434. [Google Scholar] [CrossRef]
- Omsland, A.; Hackstadt, T.; Heinzen, R.A. Bringing culture to the uncultured: Coxiella burnetii and lessons for obligate intracellular bacterial pathogens. PLoS Pathog. 2013, 9, e1003540. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.W.; Chevignon, G.; Oliver, K.M.; Strand, M.R. Culture of an aphid heritable symbiont demonstrates its direct role in defence against parasitoids. Proc. Biol. Sci. 2017, 284, 20171925. [Google Scholar] [CrossRef] [PubMed]
- Matthew, C.Z.; Darby, A.C.; Young, S.A.; Hume, L.H.; Welburn, S.C. The rapid isolation and growth dynamics of the tsetse symbiont Sodalis glossinidius. Fems. Microbiol. Lett. 2005, 248, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Masson, F.; Calderon Copete, S.; Schupfer, F.; Garcia-Arraez, G.; Lemaitre, B. In Vitro Culture of the Insect Endosymbiont Spiroplasma poulsonii Highlights Bacterial Genes Involved in Host-Symbiont Interaction. MBio 2018, 9, e00024-18. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Pettigrew, M.M.; Sinkins, S.P.; Braig, H.R.; Andreadis, T.G.; Tesh, R.B. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol. Biol. 1997, 6, 33–39. [Google Scholar] [CrossRef]
- McGraw, E.A.; Merritt, D.J.; Droller, J.N.; O’Neill, S.L. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. Biol. Sci. 2001, 268, 2565–2570. [Google Scholar] [CrossRef]
- Minard, G.; Tran, F.H.; Raharimalala, F.N.; Hellard, E.; Ravelonandro, P.; Mavingui, P.; Valiente Moro, C. Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. Fems. Microbiol. Ecol. 2013, 83, 63–73. [Google Scholar] [CrossRef]
- Mühling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. Isme. J. 2008, 2, 379. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.; Tran-Van, V.; Potier, P.; Mavingui, P. Transinfection and growth discrepancy of Drosophila Wolbachia strain wMel in cell lines of the mosquito Aedes albopictus. J. Appl. Microbiol. 2010, 108, 2133–2141. [Google Scholar] [PubMed]
- Mackie, A.M.; Hassan, K.A.; Paulsen, I.T.; Tetu, S.G. Biolog Phenotype Microarrays for phenotypic characterization of microbial cells. Methods Mol. Biol. 2014, 1096, 123–130. [Google Scholar] [PubMed]
- Mee, P.T.; Weeks, A.R.; Walker, P.J.; Hoffmann, A.A.; Duchemin, J.-B. Detection of Low-Level Cardinium and Wolbachia Infections in Culicoides. Appl. Env. Microbiol. 2015, 81, 6177–6188. [Google Scholar] [CrossRef] [PubMed]
- Turelli, M.; Hoffman, A.A. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 1999, 8, 243–255. [Google Scholar] [CrossRef]
- Coutinho-Abreu, I.V.; Zhu, K.Y.; Ramalho-Ortigao, M. Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitol. Int. 2010, 59, 1–8. [Google Scholar] [CrossRef]
- Kriesner, P.; Hoffmann, A.A.; Lee, S.F.; Turelli, M.; Weeks, A.R. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 2013, 9, e1003607. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef]
- Cho, E.A.; Lee, D.W.; Cha, Y.H.; Lee, S.J.; Jung, H.C.; Pan, J.G.; Pyun, Y.R. Characterization of a novel D-lyxose isomerase from Cohnella laevoribosii RI-39 sp. nov. J. Bacteriol. 2007, 189, 1655–1663. [Google Scholar] [CrossRef][Green Version]
- Darby, A.C.; Armstrong, S.D.; Bah, G.S.; Kaur, G.; Hughes, M.A.; Kay, S.M.; Koldkjaer, P.; Rainbow, L.; Radford, A.D.; Blaxter, M.L.; et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012, 22, 2467–2477. [Google Scholar] [CrossRef]
- Grote, A.; Voronin, D.; Ding, T.; Twaddle, A.; Unnasch, T.R.; Lustigman, S.; Ghedin, E. Defining Brugia malayi and Wolbachia symbiosis by stage-specific dual RNA-seq. PLoS Negl. Trop. Dis. 2017, 11, e0005357. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Ye, Y.H.; Turner, K.; Eyles, D.W.; McGraw, E.A.; O’Neill, S.L. The wMelPop strain of Wolbachia interferes with dopamine levels in Aedes aegypti. Parasit. Vectors 2011, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Rohrscheib, C.E.; Bondy, E.; Josh, P.; Riegler, M.; Eyles, D.; van Swinderen, B.; Weible, M.W.; Brownlie, J.C. Wolbachia Influences the Production of Octopamine and Affects Drosophila Male Aggression. Appl. Env. Microbiol. 2015, 81, 4573–4580. [Google Scholar]
- Valentine, R.C.; Bojanowski, R.; Gaudy, E.; Wolfe, R.S. Mechanism of the allantoin fermentation. J. Biol. Chem. 1962, 237, 2271–2277. [Google Scholar]
- Beckmann, J.F.; Ronau, J.A.; Hochstrasser, M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2017, 2, 17007. [Google Scholar] [CrossRef]
- Zhang, G.; Hussain, M.; Asgari, S. Regulation of arginine methyltransferase 3 by a Wolbachia-induced microRNA in Aedes aegypti and its effect on Wolbachia and dengue virus replication. Insect Biochem. Mol. Biol. 2014, 53, 81–88. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafsur, A.M.; Ghosh, A.; Brelsfoard, C.L. Phenotypic Response of Wolbachia pipientis in a Cell-Free Medium. Microorganisms 2020, 8, 1060. https://doi.org/10.3390/microorganisms8071060
Krafsur AM, Ghosh A, Brelsfoard CL. Phenotypic Response of Wolbachia pipientis in a Cell-Free Medium. Microorganisms. 2020; 8(7):1060. https://doi.org/10.3390/microorganisms8071060
Chicago/Turabian StyleKrafsur, Alyssa M., Arnab Ghosh, and Corey L. Brelsfoard. 2020. "Phenotypic Response of Wolbachia pipientis in a Cell-Free Medium" Microorganisms 8, no. 7: 1060. https://doi.org/10.3390/microorganisms8071060
APA StyleKrafsur, A. M., Ghosh, A., & Brelsfoard, C. L. (2020). Phenotypic Response of Wolbachia pipientis in a Cell-Free Medium. Microorganisms, 8(7), 1060. https://doi.org/10.3390/microorganisms8071060




