Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa
Abstract
1. Introduction
2. Pathogen Biology
3. Host Diversity
4. Ecology of MAP Infection
5. Paratuberculosis in Livestock and Wildlife
6. The Role of MAP in Idiopathic Inflammatory Bowel Disease and Related Diseases
7. Current Status of Idiopathic Inflammatory Bowel Disease and Related Diseases in Africa
8. Socio-Economic Impact of MAP in Africa
9. Control and Management
10. Future Research on MAP in Africa
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Grohn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne’s Disease. Front. Vet. Sci. 2017, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W. Pathogenesis of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 537–546. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Whyte, P.; More, S.J.; Green, M.J.; O’Grady, L.; Garcia, A.; Doherty, M.L. The effect of paratuberculosis on milk yield--A systematic review and meta-analysis. J. Dairy Sci. 2016, 99, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.; Willemsen, P.T.; van Zijderveld, F.G. Paratuberculosis recognized as a problem at last: A review. Vet. Q. 2000, 22, 200–204. [Google Scholar] [CrossRef]
- Stabel, J.R. Johne’s disease: A hidden threat. J. Dairy Sci. 1998, 81, 283–288. [Google Scholar] [CrossRef]
- Whitlock, B.K.; Welborn, M.; Prado, M.; Plummer, A. Faculty Publications and Other Works—Large Animal Clinical Sciences. Available online: https://trace.tennessee.edu/utk_largpubs/19/ (accessed on 15 June 2020).
- Collins, M.T. Paratuberculosis: Review of present knowledge. Acta Vet. Scand. 2003, 44, 217–221. [Google Scholar]
- Lombard, J.E.; Gardner, I.A.; Jafarzadeh, S.R.; Fossler, C.P.; Harris, B.; Capsel, R.T.; Wagner, B.A.; Johnson, W.O. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013, 108, 234–238. [Google Scholar] [CrossRef]
- Windsor, P. Challenges of managing paratuberculosis: Australian perspectives. In Proceedings of the 28th World Buiatrics Congress, Cairns, Australia, 27 July–1 August 2014. [Google Scholar]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Saez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef]
- Okuni, J. Occurence of paratuberculosis in African countries: A review. J. Vet. Adv. 2013, 3, 1–8. [Google Scholar]
- Omega, J.A.; Musalia, L.M.; Kuria, J.K. Knowledge, Attitude and Practices towards Paratuberculosis in Cattle and Sheep in Kericho County and Konoin Sub-County, Kenya. Afr. J. Educ. Sci. Technol. 2019, 5, 76–86. [Google Scholar]
- WHO. Neglected tropical diseases. Available online: https://www.who.int/neglected_diseases/diseases/en/ (accessed on 25 June 2020).
- Policap, K.; Victor, N.; Patricia, M. Paratuberculosis and route in cattle in the three (3) Northern regions of Cameroon. In Proceedings of the 11th International Colloquium on Paratuberculosis; Saxmose Nielsen, S., Ed.; Curran Associates, Inc.: Red Hook, NY, USA, 2012; Volume 19. [Google Scholar]
- Amin, A.S.; Hsu, C.Y.; Darwish, S.F.; Ghosh, P.; AbdEl-Fatah, E.M.; Behour, T.S.; Talaat, A.M. Ecology and genomic features of infection with Mycobacterium avium subspecies paratuberculosis in Egypt. Microbiology 2015, 161, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Zeid, A.A.; Hassan, D.; El-Sayed, A.; Zschoeck, M. Studies on Johne’s disease in Egyptian cattle. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, M. Investigation on the occurrence and pathology of paratuberculosis (Johne’s disease) in apparently healthy cattle slaughtered at Elfora export abattoir Bishoftu, Ethiopia. Addis Ababa Institutional Repository. Available online: http://etd.aau.edu.et/handle/123456789/21779 (accessed on 15 June 2020).
- Vanleeuwen, J.A.; Tolosa, T.; Nemera, M.; Belaineh, B. Seroprevalence of Mycobacterium avium SSP paratuberculosis infection in Ethiopian dairy farms. Bull. Anim. Health Prod. Afr. 2014, 62, 95–100. [Google Scholar]
- Otchere, I.D.; Asante-Poku, A.; Osei-Wusu, S.; Aboagye, S.Y.; Yeboah-Manu, D. Isolation and characterization of nontuberculous mycobacteria from patients with pulmonary tuberculosis in Ghana. Int. J. Mycobacteriol. 2017, 6, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gossler, R.; Leyk, W.; Hunermund, G. [Serological studies in cattle in the Kabete area (Kenya). 1. Occurrence of antibodies against para influenza-3-,IBR-,BDD-virus, chlamydia and Coxiella burneti]. Berl Munch Tierarztl. Wochenschr. 1973, 86, 164–166. [Google Scholar] [PubMed]
- Omega, J.A.; Kuria, J.K.; Musalia, L.M. Prevalence of Bovine and Ovine Paratuberculosis in Kericho County and Konoin Sub County, Kenya. Bull. Anim. Health Prod. Afr. 2019, 67, 181–189. [Google Scholar]
- Paling, R.W.; Waghela, S.; Macowan, K.J.; Heath, B.R. The occurrence of infectious diseases in mixed farming of domesticated wild herbivores and livestock in Kenya. II. Bacterial diseases. J. Wildl. Dis. 1988, 24, 308–316. [Google Scholar] [CrossRef]
- Jori, F.; Godfroid, J.; Michel, A.L.; Potts, A.D.; Jaumally, M.R.; Sauzier, J.; Roger, M. An assessment of zoonotic and production limiting pathogens in rusa deer (Cervus timorensis rusa) from Mauritius. Transbound. Emerg. Dis. 2014, 61 (Suppl. 1), 31–42. [Google Scholar] [CrossRef]
- Bauerfeind, R.; Benazzi, S.; Weiss, R.; Schliesser, T.; Willems, H.; Baljer, G. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. J. Clin. Microbiol. 1996, 34, 1617–1621. [Google Scholar] [CrossRef]
- Benazzi, S.; el Hamidi, M.; Schliesser, T. Paratuberculosis in sheep flocks in Morocco: A serological, microscopical and cultural survey. Zentralbl Veterinarmed B. 1996, 43, 213–219. [Google Scholar] [CrossRef]
- Benazzi, S.; Berrada, J.; Schliesser, T. First report of paratuberculosis (Johne’s disease) in sheep in Morocco. Zentralbl Veterinarmed B. 1995, 42, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Konte, M. [Paratuberculosis. Diagnosis of the first case in an imported cattle in Senegal]. Rev. Elev Med. Vet. Pays Trop. 1988, 41, 147–148. [Google Scholar] [PubMed]
- Fechner, K.; Schafer, J.; Munster, P.; Ternes, K.; Doring, S.; Volkel, I.; Kaup, F.J.; Czerny, C.P. Detection of Mycobacterium Avium Subspecies Paratuberculosis in Rock Hyraxes (Procavia Capensis) Imported from South Africa. J. Zoo Wildl. Med. 2017, 48, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Davey, S.C.; Van Helden, L.S.; Kettner, F.; May, S.M.; Last, R.; Grewar, J.D.; Botha, L.; Van Helden, P.D. Paratuberculosis in a domestic dog in South Africa. J. S. Afr. Vet. Assoc. 2017, 88, e1–e5. [Google Scholar] [CrossRef]
- Michel, A.L.; Bastianello, S.S. Paratuberculosis in sheep: An emerging disease in South Africa. Vet. Microbiol. 2000, 77, 299–307. [Google Scholar] [CrossRef]
- Chaudhary, A.Q.; Fawi, M.T.; Obeid, H.M. Johne’s disease in goats in the Sudan. Vet. Rec. 1964, 76, 246–247. [Google Scholar]
- Abbas, B.; Idris, S.E.O.; Burhan, A. Isolation of M. paratuberculosis from goats in Sudan. Sudan J. Vet. Sci. Anim. Husb. 1986, 25, 81–86. [Google Scholar]
- Aradaib, I.E.; Abbas, Z.A.; Abbas, B.; El Sanousi, S.M. Evaluation of conventional methods and nested PCR (nPCR) for detection of paratuberculosis in goats. Vet. Res. Commun. 2005, 29, 381–385. [Google Scholar] [CrossRef]
- Abubakr, A.M.; El Sanousi, S.M. A survey of Johne’s disease in Khartoum. Vet. Rec. 1978, 18, 94–95. [Google Scholar]
- Fawi, M.T.; Obeid, H.M. A note on Johne’s disease among cattle in the Sudan. Bull. Epizoot. Dis. Afri. 1964, 12, 437. [Google Scholar] [CrossRef]
- Mongash, B.M. Diagnosis of Johne’s Disease in Cattle. Master’s Thesis, University of Khartoum, Khartoum North, Sudan, 1986. [Google Scholar]
- Mohammed, K.B.; El-Eragi, A.M.S.; Zakia, A.M. Seroprevalence of Bovine Paratuberculosis Specific Antibodies in Khartoum and Al-Jazeera States, Sudan. J. Anim. Vet. Adv. 2010, 9, 2098–2101. [Google Scholar] [CrossRef]
- Mohammed, K.B.; Mohamed, Z.A. Clinico-pathological studies of cattle naturally infected with Mycobacterium avium subspecies paratuberculosis in Khartoum State, Sudan. Vet. World 2012, 3, 69–74. [Google Scholar] [CrossRef]
- Mpenda, F.; Buza, J. Seroprevalence of Paratuberculosis in Goats and Sheep in Arusha, Northern Tanzania. Intl. J. Sci. Res. (IJSR) 2014, 3, 541–545. [Google Scholar]
- Okuni, J.B.; Reinacher, M.; Loukopoulos, P.; Ojok, L. Prevalence and spectrum of Johne’s disease lesions in cattle slaughtered at two abattoirs in Kampala, Uganda. Trop. Anim. Health Prod. 2013, 45, 1197–1202. [Google Scholar] [CrossRef]
- Okuni, B.J.; Oyo, T.; Kisekka, M.; Ochwo, S.; Kalenzi Atuhaire, D.; Afayoa, M.; Olaho-Mukani, W.; Ojok, L. Detection of Antibodies and Confirmation of Mycobacterium avium Subspecies paratuberculosis Using Nested PCR in Bulk Milk Samples from Nakasongola and Sembabule Districts, Uganda. ISRN Vet. Sci. 2013, 2013, 369730. [Google Scholar] [CrossRef][Green Version]
- Okuni, J.B.; Loukopoulos, P.; Reinacher, M.; Ojok, L. Seroprevalence of Mycobacterium avium Subspecies Paratuberculosis Antibodies in Cattle from Wakiso, Mpigi and Luwero Districts in Uganda. Int. J. Anim. Vet. Adv. 2011, 3, 156–160. [Google Scholar]
- Pandey, G.S.; Shimizu, K.; Orino, K.; Schneebeli, M. Preliminary observations on ovine paratuberculosis (Johne’s disease) in Zambia. Rev. Elev. Med. Vet. Pays. Trop. 1990, 42, 515–516. [Google Scholar]
- Tortoli, E. Impact of genotypic studies on mycobacterial taxonomy: The new mycobacteria of the 1990s. Clin. Microbiol. Rev. 2003, 16, 319–354. [Google Scholar] [CrossRef]
- Janagama, H.K.; Senthilkumar; Bannantine, J.P.; Kugadas, A.; Jagtap, P.; Higgins, L.; Witthuhn, B.; Sreevatsan, S. Iron-sparing response of Mycobacterium avium subsp. paratuberculosis is strain dependent. BMC Microbiol. 2010, 10, 268. [Google Scholar] [CrossRef]
- De Juan, L.; Alvarez, J.; Romero, B.; Bezos, J.; Castellanos, E.; Aranaz, A.; Mateos, A.; Dominguez, L. Comparison of four different culture media for isolation and growth of type II and type I/III Mycobacterium avium subsp. paratuberculosis strains isolated from cattle and goats. Appl. Environ. Microbiol. 2006, 72, 5927–5932. [Google Scholar] [CrossRef]
- Chern, E.C.; King, D.; Haugland, R.; Pfaller, S. Evaluation of quantitative polymerase chain reaction assays targeting Mycobacterium avium, M. intracellulare, and M. avium subspecies paratuberculosis in drinking water biofilms. J. Water Health 2015, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- White, C.I.; Birtles, R.J.; Wigley, P.; Jones, P.H. Mycobacterium avium subspecies paratuberculosis in free-living amoebae isolated from fields not used for grazing. Vet. Rec. 2010, 166, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.J. Mycobacterium avium subspecies paratuberculosis: A review of current knowledge. J. Zoo Wildl. Med. 2001, 32, 293–304. [Google Scholar] [CrossRef]
- Lovell, R.; Levi, M.; Francis, J. Studies on the survival of Johne’s bacilli. J. Comp. Pathol. Ther. 1944, 54, 120–129. [Google Scholar] [CrossRef]
- Wang, J.; Moolji, J.; Dufort, A.; Staffa, A.; Domenech, P.; Reed, M.B.; Behr, M.A. Iron Acquisition in Mycobacterium avium subsp. paratuberculosis. J. Bacteriol. 2015, 198, 857–866. [Google Scholar] [CrossRef]
- Davis, W.C. On deaf ears, Mycobacterium avium paratuberculosis in pathogenesis Crohn’s and other diseases. World J. Gastroenterol. 2015, 21, 13411–13417. [Google Scholar] [CrossRef]
- Tavasoly, A.; Moosavi, Z.; Taghipour Bazargani, T.; Bazargani, M. Accidental self-inoculation in a veterinarian with attenuated vaccine of bovine Johne’s disease. Iran. J. Vet. Res. 2007, 8, 374–376. [Google Scholar] [CrossRef]
- Richter, E.; Wessling, J.; Lugering, N.; Domschke, W.; Rusch-Gerdes, S. Mycobacterium avium subsp. paratuberculosis infection in a patient with HIV, Germany. Emerg. Infect. Dis. 2002, 8, 729–731. [Google Scholar] [CrossRef]
- Dhand, N.K.; Toribio, J.A.; Whittington, R.J. Adsorption of Mycobacterium avium subsp. paratuberculosis to soil particles. Appl. Environ. Microbiol. 2009, 75, 5581–5585. [Google Scholar] [CrossRef]
- Johnson-Ifearulundu, Y.J.; Kaneene, J.B. Relationship between soil type and Mycobacterium paratuberculosis. J. Am. Vet. Med. Assoc. 1997, 210, 1735–1740. [Google Scholar] [PubMed]
- Sung, N.; Collins, M.T. Variation in resistance of Mycobacterium paratuberculosis to acid environments as a function of culture medium. Appl. Environ. Microbiol. 2003, 69, 6833–6840. [Google Scholar] [CrossRef] [PubMed]
- Dhand, N.K.; Eppleston, J.; Whittington, R.J.; Toribio, J.A. Association of farm soil characteristics with ovine Johne’s disease in Australia. Prev. Vet. Med. 2009, 89, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Hermon-Taylor, J.; El-Zaatari, F.A.K. The Mycobacterium avium subspecies paratuberculosis problem and its relation to the causation of Crohn disease. In Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management; Bartram, J., Cotruvo, J., Dufour, A., Rees, G., Pedley, S., Eds.; IWA Publishing: London, UK, 2004; pp. 74–94. [Google Scholar]
- Whan, L.B.; Grant, I.R.; Ball, H.J.; Scott, R.; Rowe, M.T. Bactericidal effect of chlorine on Mycobacterium paratuberculosis in drinking water. Lett. Appl. Microbiol. 2001, 33, 227–231. [Google Scholar] [CrossRef]
- Pickup, R.W.; Rhodes, G.; Bull, T.J.; Arnott, S.; Sidi-Boumedine, K.; Hurley, M.; Hermon-Taylor, J. Mycobacterium avium subsp. paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: Diverse opportunities for environmental cycling and human exposure. Appl. Environ. Microbiol. 2006, 72, 4067–4077. [Google Scholar] [CrossRef]
- Cook, K.L.; Britt, J.S.; Bolster, C.H. Survival of Mycobacterium avium subsp. paratuberculosis in biofilms on livestock watering trough materials. Vet. Microbiol. 2010, 141, 103–109. [Google Scholar] [CrossRef]
- Pillars, R.B.; Grooms, D.L.; Kaneene, J.B. Longitudinal study of the distribution of Mycobacterium avium subsp. paratuberculosis in the environment of dairy herds in the Michigan Johne’s disease control demonstration herd project. Can. Vet. J. 2009, 50, 1039–1046. [Google Scholar]
- Lund, B.M.; Gould, G.W.; Rampling, A.M. Pasteurization of milk and the heat resistance of Mycobacterium avium subsp. paratuberculosis: A critical review of the data. Int. J. Food Microbiol. 2002, 77, 135–145. [Google Scholar] [CrossRef]
- Grant, I.R.; Ball, H.J.; Rowe, M.T. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows’ milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 2002, 68, 2428–2435. [Google Scholar] [CrossRef]
- Grant, I.R. Does Mycobacterium paratuberculosis survive current pasteurization conditions? Appl. Environ. Microbiol. 1998, 64, 2760–2761. [Google Scholar]
- Grant, I.R. Zoonotic potential of Mycobacterium avium ssp. paratuberculosis: The current position. J. Appl. Microbiol. 2005, 98, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R.; Foddai, A.C.G.; Tarrant, J.C.; Kunkel, B.; Hartmann, F.A.; McGuirk, S.; Hansen, C.; Talaat, A.M.; Collins, M.T. Viable Mycobacterium avium ssp. paratuberculosis isolated from calf milk replacer. J. Dairy Sci. 2017, 100, 9723–9735. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.S. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis...and colorectal cancer? Infect. Agent Cancer 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Uzoigwe, J.C.; Khaitsa, M.L.; Gibbs, P.S. Epidemiological evidence for Mycobacterium avium subspecies paratuberculosis as a cause of Crohn’s disease. Epidemiol. Infect. 2007, 135, 1057–1068. [Google Scholar] [CrossRef]
- Chaubey, K.K.; Singh, S.V.; Gupta, S.; Singh, M.; Sohal, J.S.; Kumar, N.; Singh, M.K.; Bhatia, A.K.; Dhama, K. Mycobacterium avium subspecies paratuberculosis—An important food borne pathogen of high public health significance with special reference to India: An update. Vet. Q. 2017, 37, 282–299. [Google Scholar] [CrossRef]
- Zarei-Kordshouli, F.; Geramizadeh, B.; Khodakaram-Tafti, A. Prevalence of Mycobacterium avium subspecies paratuberculosis IS 900 DNA in biopsy tissues from patients with Crohn’s disease: Histopathological and molecular comparison with Johne’s disease in Fars province of Iran. BMC Infect. Dis. 2019, 19, 23. [Google Scholar] [CrossRef]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Rocca, S.; Cubeddu, T.; Nieddu, A.M.; Pirino, S.; Appino, S.; Antuofermo, E.; Tanda, F.; Verin, R.; Sechi, L.A.; Taccini, E.; et al. Detection of Mycobacterium avium spp. paratuberculosis (Map) in samples of sheep paratuberculosis (Johne’s disease or JD) and human Crohn’s disease (CD) using liquid phase RT-PCR, in situ RT-PCR and immunohistochemistry. Small Rumin. Res. 2010, 88, 126–134. [Google Scholar] [CrossRef]
- Yoshimura, H.H.; Graham, D.Y.; Estes, M.K.; Merkal, R.S. Investigation of association of mycobacteria with inflammatory bowel disease by nucleic acid hybridization. J. Clin. Microbiol. 1987, 25, 45–51. [Google Scholar] [CrossRef]
- Naser, S.A.; Schwartz, D.; Shafran, I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am. J. Gastroenterol. 2000, 95, 1094–1095. [Google Scholar] [CrossRef]
- Chiodini, R.J.; Van Kruiningen, H.J.; Merkal, R.S.; Thayer, W.R., Jr.; Coutu, J.A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J. Clin. Microbiol. 1984, 20, 966–971. [Google Scholar] [CrossRef]
- Naser, S.A.; Collins, M.T.; Crawford, J.T.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis (MAP) from the Blood of Patients with Crohn’s disease: A Follow-Up Blind Multi Center Investigation. Open Inflamm. J. 2009, 2, 22–23. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, CDC. Inflammatory Bowel Disease. Available online: https://www.cdc.gov/ibd/index.htm (accessed on 15 June 2020).
- Chamberlin, W.; Borody, T.J.; Campbell, J. Primary treatment of Crohn’s disease: Combined antibiotics taking center stage. Expert Rev. Clin. Immunol. 2011, 7, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Kuenstner, J.T.; Davis, W.C.; Agarwal, P.; Kumar, N.; Singh, D.; Gupta, S.; Chaubey, K.K.; Kumar, A.; Misri, J.; et al. Concurrent Resolution of Chronic Diarrhea Likely Due to Crohn’s Disease and Infection with Mycobacterium avium paratuberculosis. Front. Med. (Lausanne) 2016, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Espeschit, I.F.; Souza, M.C.C.; Lima, M.C.; Moreira, M.A.S. First molecular typing of Mycobacterium avium subspecies paratuberculosis identified in animal and human drinking water from dairy goat farms in Brazil. Braz. J. Microbiol. 2018, 49, 358–361. [Google Scholar] [CrossRef]
- Pierce, E.S. Where are all the Mycobacterium avium subspecies paratuberculosis in patients with Crohn’s disease? PLoS Pathog. 2009, 5, e1000234. [Google Scholar] [CrossRef]
- Paccagnini, D.; Sieswerda, L.; Rosu, V.; Masala, S.; Pacifico, A.; Gazouli, M.; Ikonomopoulos, J.; Ahmed, N.; Zanetti, S.; Sechi, L.A. Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PLoS ONE 2009, 4, e7109. [Google Scholar] [CrossRef]
- Sechi, L.A.; Gazouli, M.; Sieswerda, L.E.; Molicotti, P.; Ahmed, N.; Ikonomopoulos, J.; Scanu, A.M.; Paccagnini, D.; Zanetti, S. Relationship between Crohn’s disease, infection with Mycobacterium avium subspecies paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World J. Gastroenterol. 2006, 12, 7161–7164. [Google Scholar] [CrossRef]
- Steury, E.M.; Templeton, A.C. Crohn’s disease in Africa. A case report and review. Trop. Geogr. Med. 1980, 32, 172–173. [Google Scholar]
- Khalifa, S.E.; Mudawi, H.M.; Fedail, S.S. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop. Gastroenterol. 2005, 26, 194–196. [Google Scholar]
- Elbatea, H.; Enaba, M.; Elkassas, G.; El-Kalla, F.; Elfert, A.A. Indications and outcome of colonoscopy in the middle of Nile delta of Egypt. Dig. Dis. Sci. 2011, 56, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Prince, A.; Hassan, A.A.; Fayed, A.; Zschöck, M.; Naga, M.; Omar, M.; Salem, M.; El-Sayed, A. Epidemiological studies on Johne’s disease in ruminants and Crohn’s disease in humans in Egypt. Int. J. Vet. Sci. Med. 2013, 1, 79–86. [Google Scholar] [CrossRef][Green Version]
- Buchel, O.C.; Bosch, F.J.; Janse van Rensburg, J.; Bezuidenhout, E.; de Vries, C.S.; van Zyl, J.H.; Middlecote, B.D.; de, K.G.H.; Fevery, J. Primary sclerosing cholangitis, Crohn’s disease and HLA-B27 in black South African women. Acta Gastroenterol. Belg. 2012, 75, 454–457. [Google Scholar] [PubMed]
- Segal, I.; Tim, L.O.; Rubin, A.; Solomon, A.; Simon, G.; Lawson, H.H.; Jacobson, M. Rare and unusual manifestations of Crohn’s disease with pyoderma gangrenosum and sclerosing cholangitis. S. Afr. Med. J. 1979, 55, 596–599. [Google Scholar] [PubMed]
- Peghini, M.; Barabe, P.; Morcillo, R.; Diallo, A.; Gueye, P.M.; Desrentes, M.; Mbaye, P.S.; Wade, B. [Crohn’s disease. Apropos of 2 recent cases collected at the Dakar Central Hospital]. Dakar Med. 1990, 35, 52–54. [Google Scholar]
- Casanelli, J.M.; Keli, E.; N’Dri, J.; Bohoussou, P.E.; Blegole, C.; Moussa, B.; N’Guessan, H.A. [Crohn’s disease: First report in Cote-d’Ivoire]. Med. Trop. (Mars) 2004, 64, 384–386. [Google Scholar]
- Singh, A.V.; Chauhan, D.S.; Singh, S.V.; Kumar, V.; Singh, A.; Yadav, A.; Yadav, V.S. Current status of Mycobacterium avium subspecies paratuberculosis infection in animals & humans in India: What needs to be done? Indian J. Med. Res. 2016, 144, 661–671. [Google Scholar] [CrossRef]
- Waddell, L.A.; Rajic, A.; Stark, K.D.; McEwen, S.A. The potential Public Health Impact of Mycobacterium avium ssp. paratuberculosis: Global Opinion Survey of Topic Specialists. Zoonoses Public Health 2016, 63, 212–222. [Google Scholar] [CrossRef]
- Omega, J.A. Epidemiology and Socioeconomic Impact of Bovine and Ovine Paratuberculosis in Kericho County and Konoin Sub-County, Kenya. Master’s Thesis, Chuka University Kenya, Chuka, Kenya, 2019. [Google Scholar]
- OIE. Old Classification of Diseases Notifiable to the OIE. Available online: https://www.oie.int/animal-health-in-the-world/the-world-animal-health-information-system/old-classification-of-diseases-notifiable-to-the-oie-list-b/ (accessed on 15 June 2020).
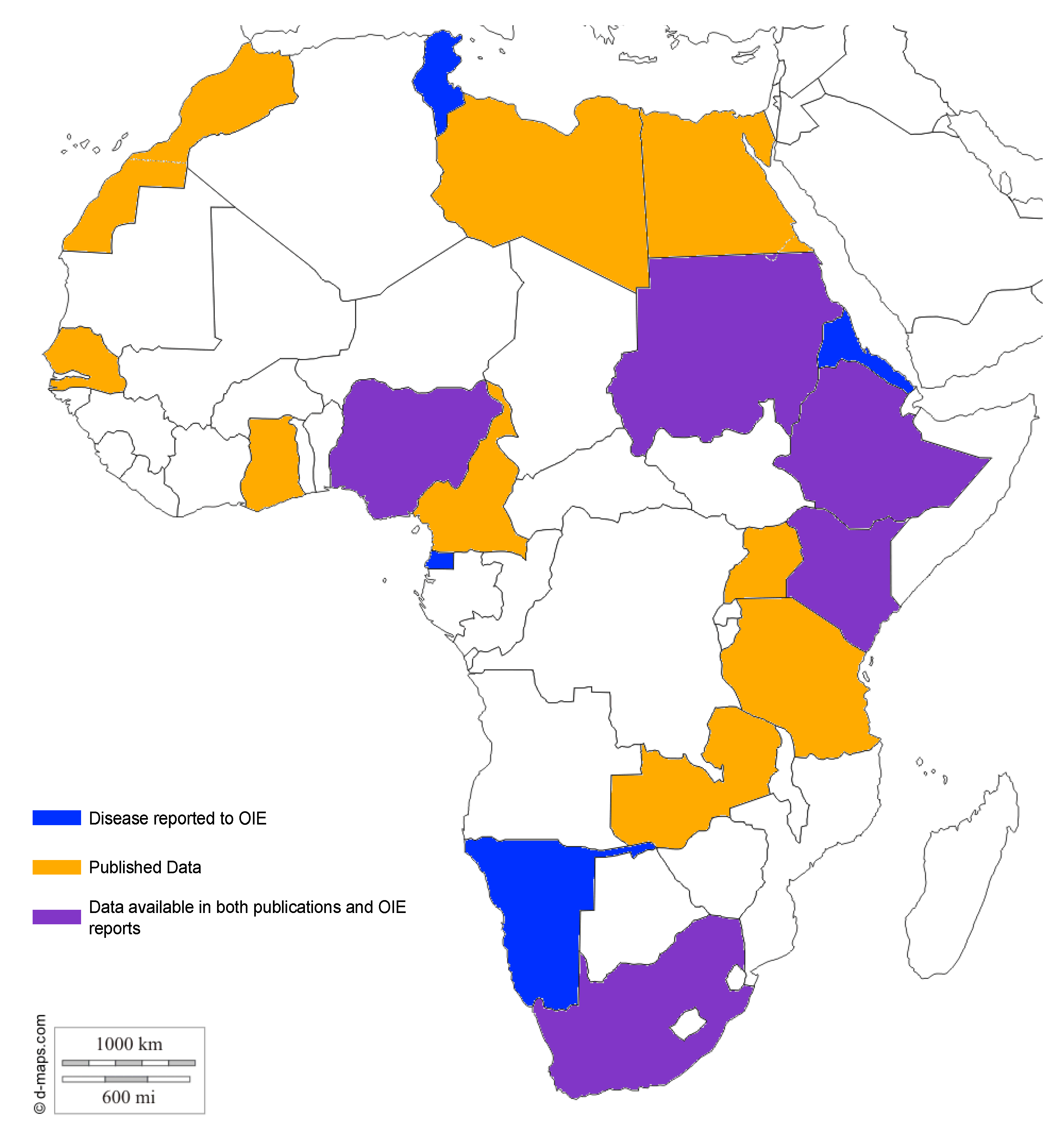
| Country | Species | No. of Reported Cases | Method | Reference | Reported to OIE | ||
|---|---|---|---|---|---|---|---|
| PCR | Culture | Ab Detection | |||||
| Algeria | n.d. | ||||||
| Angola | n.d. | ||||||
| Benin | n.d. | ||||||
| Botswana | n.d. | ||||||
| Burkina Faso | n.d. | ||||||
| Burundi | n.d. | ||||||
| Cabo Verde | n.d. | ||||||
| Cameroon | Cattle | 132 | x | x | [14] | n.d | |
| Central African Republic | n.d. | r.n. | |||||
| Chad | n.d. | r.n. | |||||
| Comoros | n.d. | ||||||
| Democratic Republic of the Congo | n.d. | ||||||
| Republic of the Congo | n.d. | ||||||
| Cote d’Ivoire | n.d. | ||||||
| Djibouti | n.d. | r.n. | |||||
| Egypt | Cattle | 17 | x | [15] | r.n. | ||
| 75 | x | [16] | |||||
| Equatorial Guinea | n.d. | s (2013) | |||||
| Eritrea | n.d. | + (2008) | |||||
| Ethiopia | Cattle | 5 | x | [17] | + (1996) | ||
| x | [18] | ||||||
| Gabon | n.d. | ||||||
| Gambia | n.d. | ||||||
| Ghana | human | 13 | x | [19] | r.n. | ||
| Guinea | n.d. | ||||||
| Guinea Bissau | n.d. | ||||||
| Lesotho | n.d. | ||||||
| Liberia | n.d. | ||||||
| Libya | n.d. | ||||||
| Kenya | Cattle | x | x | x | [20,21] | + (2015) | |
| Camel/Cattle | 102/69 | x | [22] | ||||
| Sheep | x | x | x | [21] | n.d. | ||
| Madagascar | n.d. | r.n. | |||||
| Malawi | n.d. | ||||||
| Mali | n.d. | ||||||
| Mauritania | n.d. | ||||||
| Mauritius | rusa deer | 351 | x | [23] | + (1995) | ||
| 2 | x | ||||||
| Morocco | sheep | 180 | x | x | [24] | n.d. | |
| 56 | x | x | [25] | ||||
| 2 | x | [26] | |||||
| Mozambique | n.d. | ||||||
| Namibia | n.d. | + (1988/2004) | |||||
| Niger | n.d. | ||||||
| Nigeria | Cattle | n.d. | [11] | + (2017) | |||
| Rwanda | n.d. | ||||||
| Sao Tome and Principe | n.d. | ||||||
| Senegal | Cattle | 1 | [27] | n.d. | |||
| Seychelles | n.d. | ||||||
| Sierra Leone | n.d. | r.n. | |||||
| Somalia | n.d. | ||||||
| South Africa | Rock Hyraxes | 2 | x | x | [28] | + (2017) | |
| Dog | 1 | x | [29] | ||||
| Sheep | 197 | x | [30] | ||||
| South Sudan | n.d. | ||||||
| Sudan | Goat | 2 | [31] | + (2004) | |||
| 3 | x | [32] | |||||
| 1 | x | x | [33] | ||||
| Cattle | [34,35] | ||||||
| 23 | x | [36] | |||||
| 13 | x | ||||||
| 11 | x | ||||||
| 25 | x | [37] | |||||
| 4 | x | [38] | |||||
| Swaziland | n.d. | ||||||
| Tanzania | Goat | 21 | x | [39] | n.d. | ||
| Cattle | 11 | x | |||||
| Togo | n.d. | ||||||
| Tunisia | n.d. | + (2002) | |||||
| Uganda | Cattle | 48 | x | x | [40] | n.d. | |
| Cattle | 11 | x | x | [41] | |||
| Cattle | 35 | x | [42] | ||||
| Zambia | Sheep | 16 | x | x | [43] | r.n. | |
| Zimbabwe | n.d. | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuni, J.B.; Hansen, S.; Eltom, K.H.; Eltayeb, E.; Amanzada, A.; Omega, J.A.; Czerny, C.P.; Abd El Wahed, A.; Ojok, L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms 2020, 8, 1007. https://doi.org/10.3390/microorganisms8071007
Okuni JB, Hansen S, Eltom KH, Eltayeb E, Amanzada A, Omega JA, Czerny CP, Abd El Wahed A, Ojok L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms. 2020; 8(7):1007. https://doi.org/10.3390/microorganisms8071007
Chicago/Turabian StyleOkuni, Julius Boniface, Sören Hansen, Kamal H. Eltom, ElSagad Eltayeb, Ahmad Amanzada, Joseph Amesa Omega, Claus Peter Czerny, Ahmed Abd El Wahed, and Lonzy Ojok. 2020. "Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa" Microorganisms 8, no. 7: 1007. https://doi.org/10.3390/microorganisms8071007
APA StyleOkuni, J. B., Hansen, S., Eltom, K. H., Eltayeb, E., Amanzada, A., Omega, J. A., Czerny, C. P., Abd El Wahed, A., & Ojok, L. (2020). Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms, 8(7), 1007. https://doi.org/10.3390/microorganisms8071007






