A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite
Abstract
1. Introduction
2. Materials and Methods
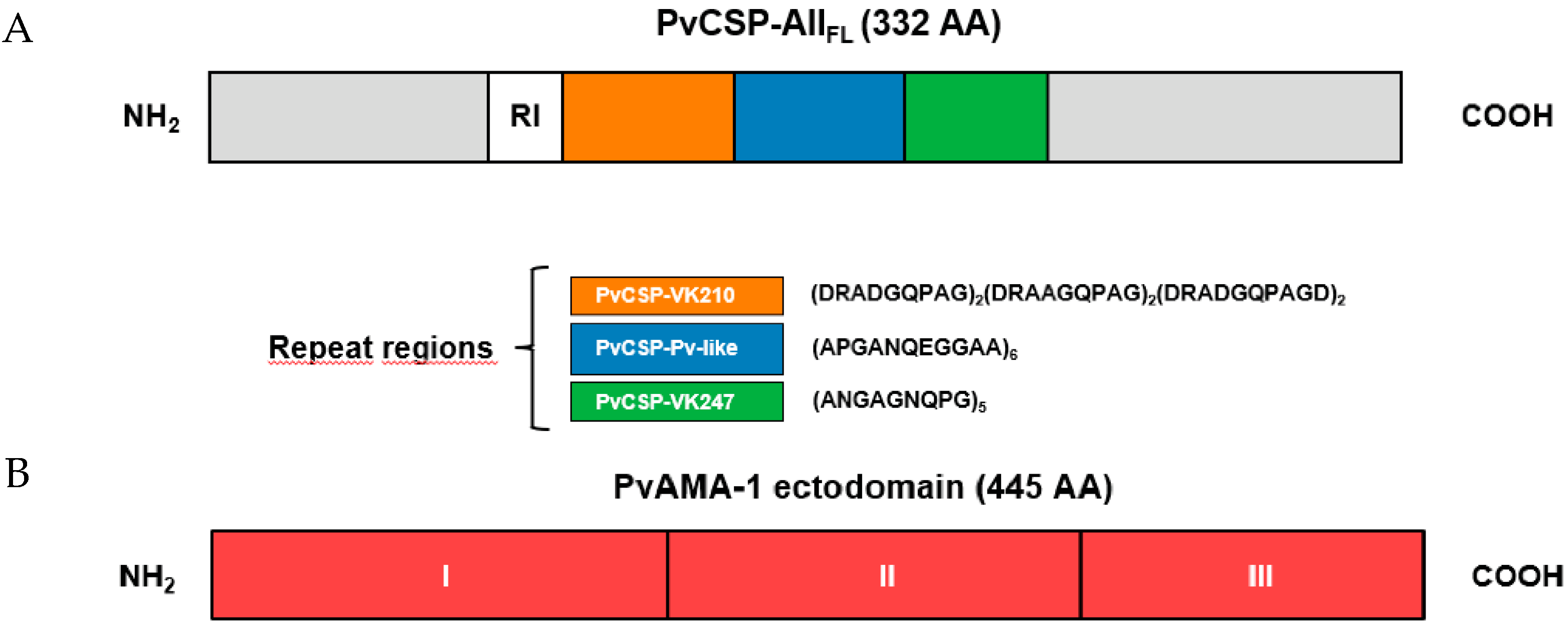
2.1. Recombinant Protein Expressed in Pichia Pastoris
2.2. Recombinant Proteins Expressed in Escherichia coli
2.3. Animals
2.4. Analysis of IgG Antibody by ELISA
2.5. Indirect Immunofluorescence Assay
2.6. Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE)-Based Proliferation Assay
2.7. Parasites, Mice, and Mosquitoes
2.8. Transgenic P. berghei Sporozoite Challenge
2.9. Statistical Analysis
3. Results
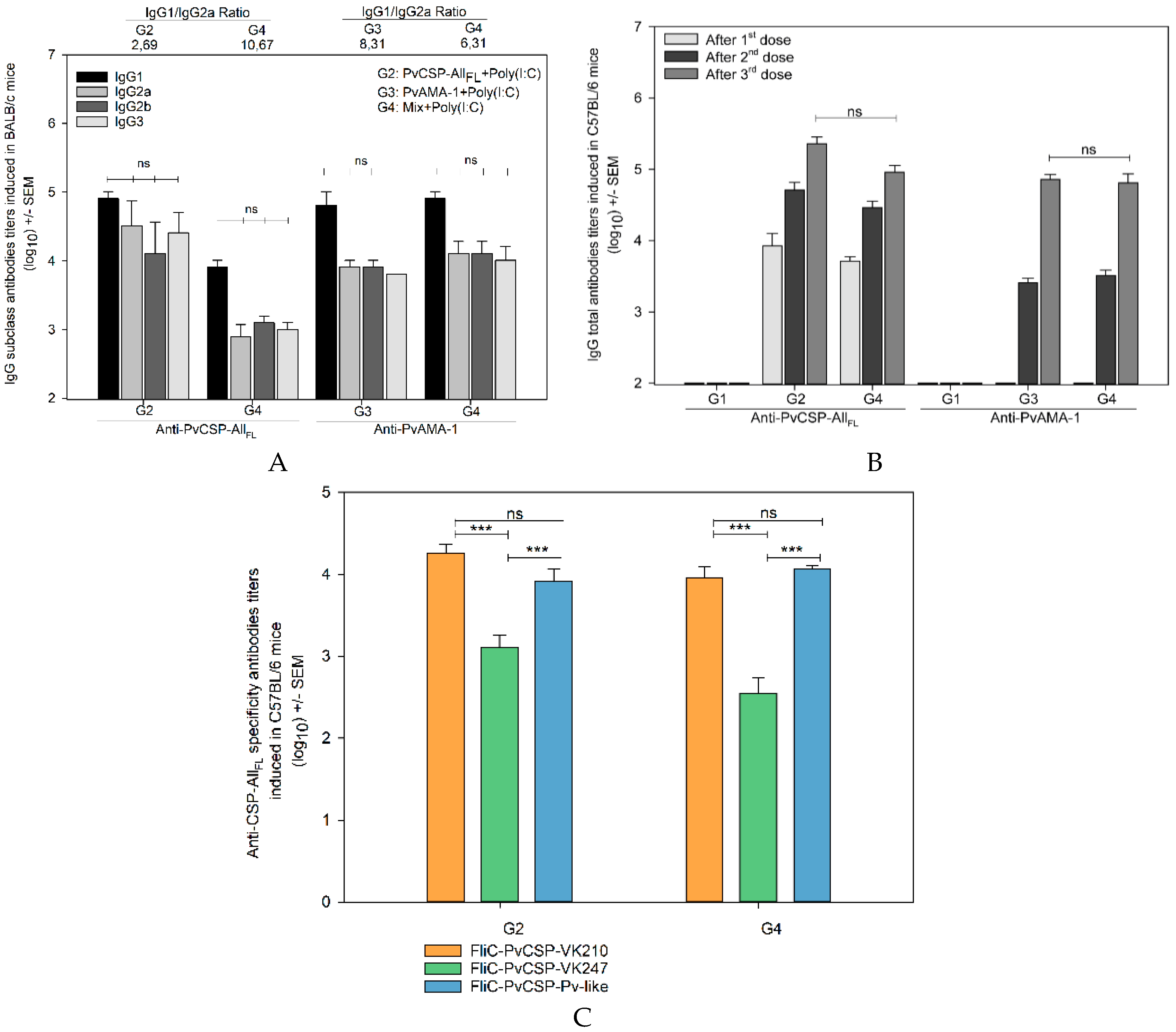
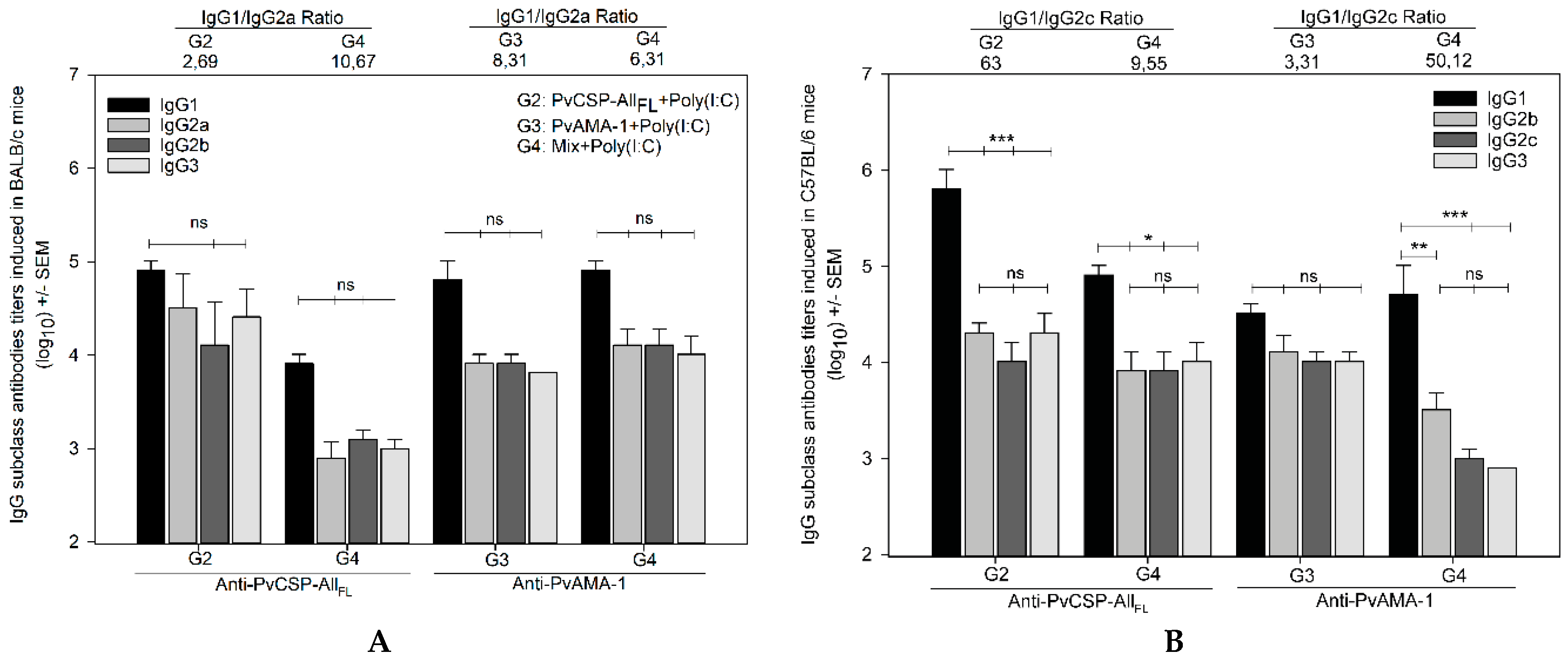
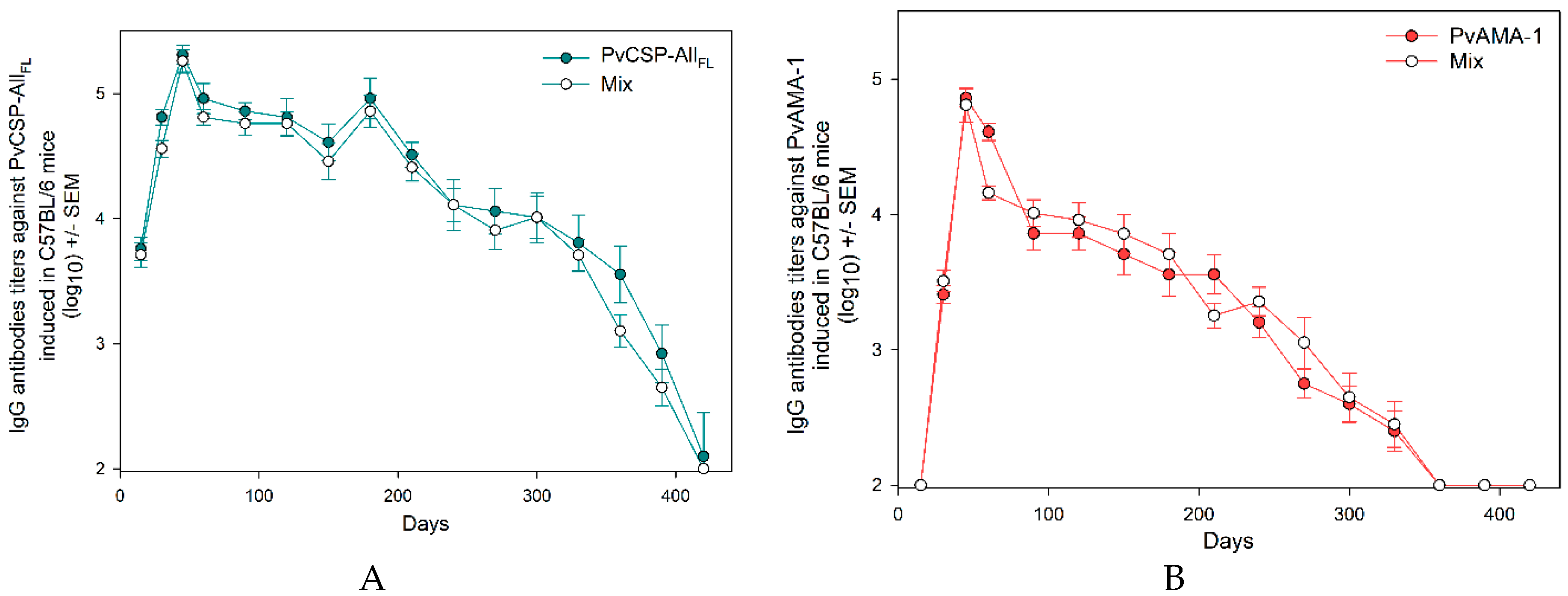
3.1. Antibody Responses Induced by the Vaccine Formulations in BALB/c and C57BL/6 Mice
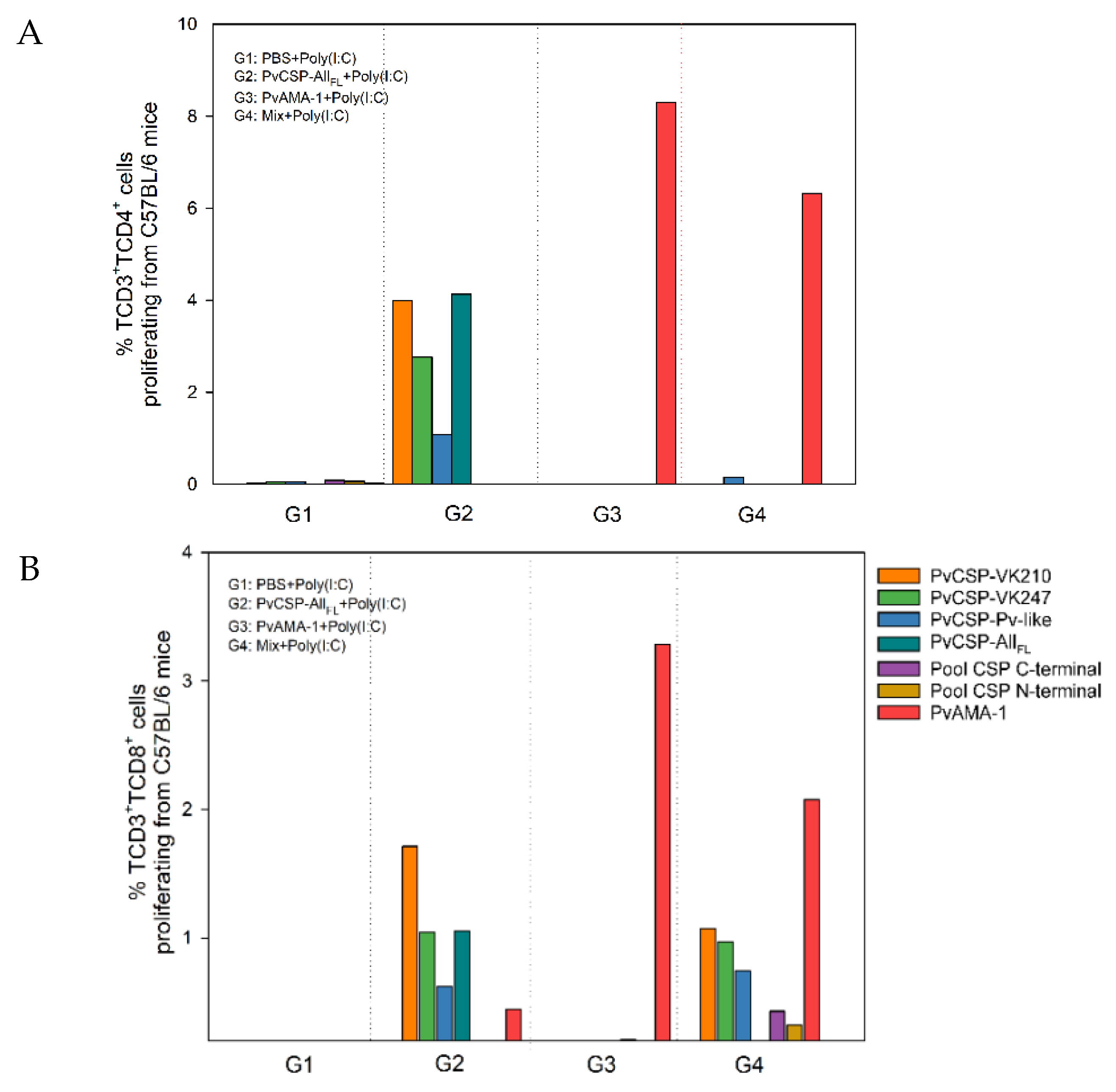
3.2. Cell-Mediated Immune Response
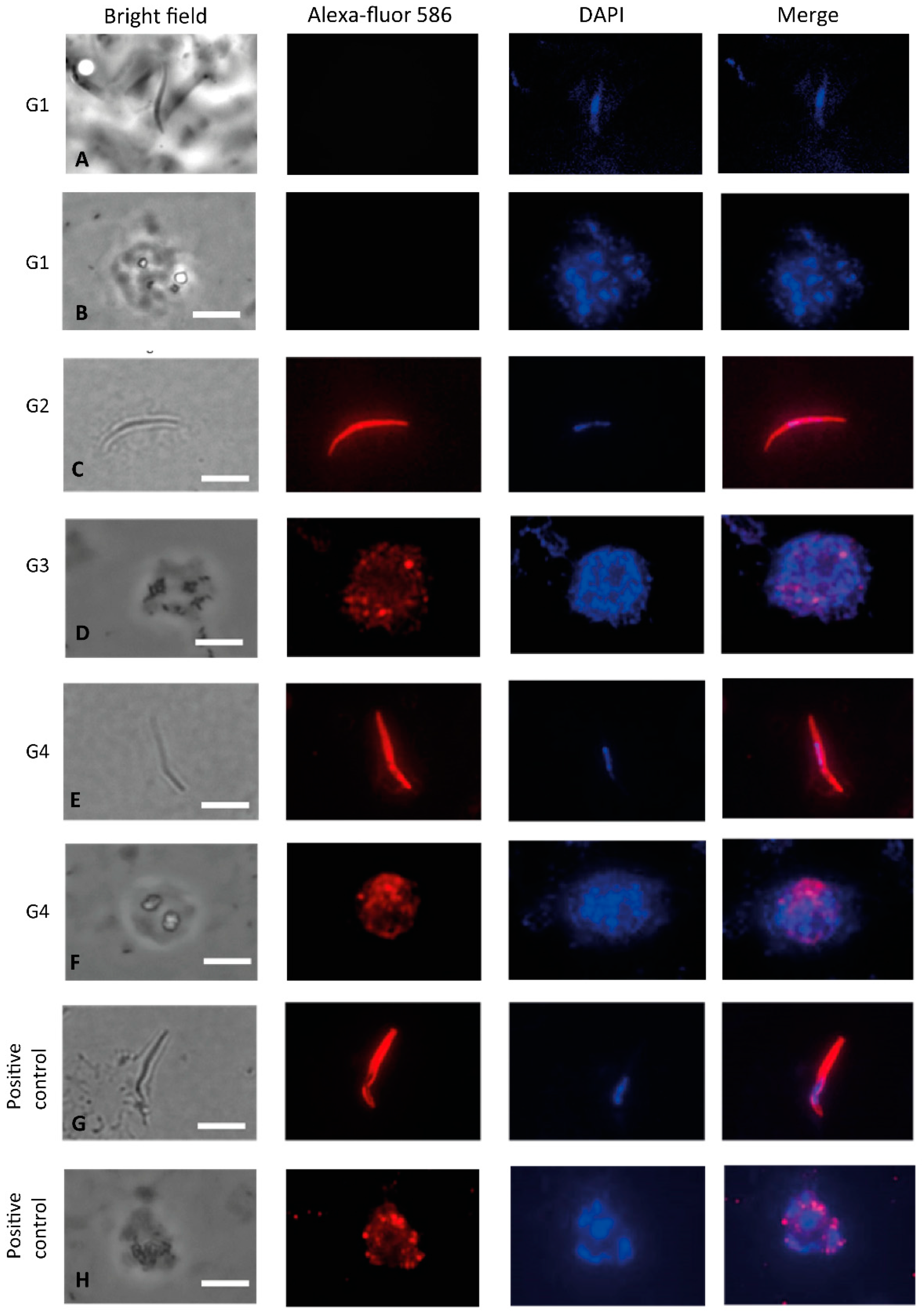
3.3. Recognition of the Native Protein PvCSP
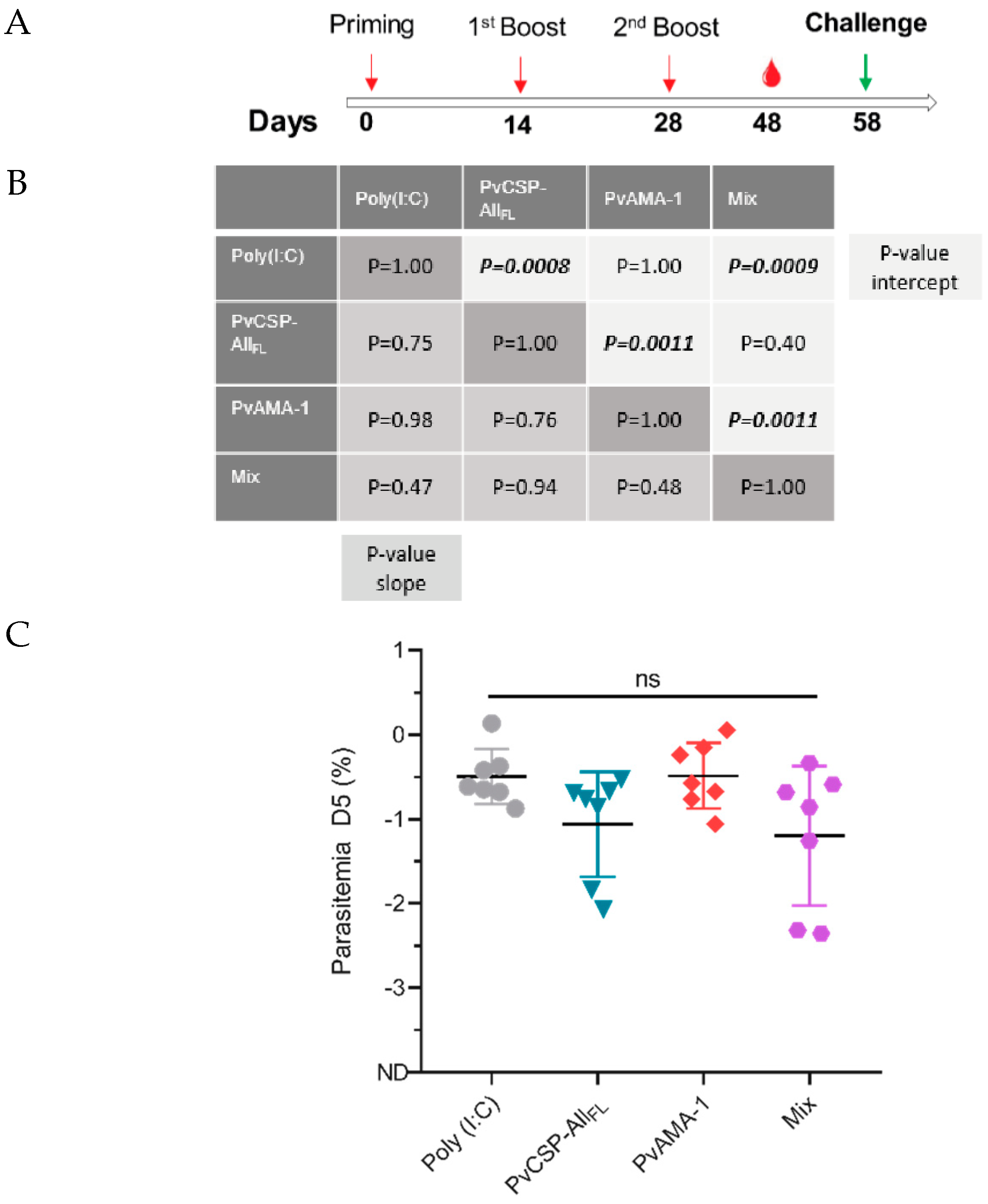
3.4. Pb/PvCSP-VK210 Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Long, C.A.; Zavala, F. Immune Responses in Malaria. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- World Health Organization. Malaria vaccine: WHO position paper, January 2016—Recommendations. Vaccine 2018, 36, 3576–3577. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.H.; Tararam, C.A.; Lasaro, M.O.; Camacho, A.G.; Ersching, J.; Leal, M.T.; Herrera, S.; Bruna-Romero, O.; Soares, I.S.; Nussenzweig, R.S.; et al. Immunogenicity of a prime-boost vaccine containing the circumsporozoite proteins of Plasmodium vivax in rodents. Infect. Immun. 2014, 82, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, A.M.; Lima, L.C.; Francoso, K.S.; Denapoli, P.M.A.; Panatieri, R.; Bargieri, D.Y.; Thiberge, J.M.; Andolina, C.; Nosten, F.; Renia, L.; et al. Vaccine Containing the Three Allelic Variants of the Plasmodium vivax Circumsporozoite Antigen Induces Protection in Mice after Challenge with a Transgenic Rodent Malaria Parasite. Front. Immunol. 2017, 8, 1275. [Google Scholar] [CrossRef]
- De Camargo, T.M.; de Freitas, E.O.; Gimenez, A.M.; Lima, L.C.; de Almeida Caramico, K.; Francoso, K.S.; Bruna-Romero, O.; Andolina, C.; Nosten, F.; Renia, L.; et al. Prime-boost vaccination with recombinant protein and adenovirus-vector expressing Plasmodium vivax circumsporozoite protein (CSP) partially protects mice against Pb/Pv sporozoite challenge. Sci. Rep. 2018, 8, 1118. [Google Scholar] [CrossRef]
- Yadava, A.; Waters, N.C. Rationale for Further Development of a Vaccine Based on the Circumsporozoite Protein of Plasmodium vivax. PLoS Negl. Trop. Dis. 2017, 11, e0005164. [Google Scholar] [CrossRef]
- Laurens, M.B. The Promise of a Malaria Vaccine-Are We Closer? Annu. Rev. Microbiol. 2018, 72, 273–292. [Google Scholar] [CrossRef]
- Sedegah, M.; Tamminga, C.; McGrath, S.; House, B.; Ganeshan, H.; Lejano, J.; Abot, E.; Banania, G.J.; Sayo, R.; Farooq, F.; et al. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: Safety and immunogenicity in seronegative adults. PLoS ONE 2011, 6, e24586. [Google Scholar] [CrossRef]
- Tamminga, C.; Sedegah, M.; Regis, D.; Chuang, I.; Epstein, J.E.; Spring, M.; Mendoza-Silveiras, J.; McGrath, S.; Maiolatesi, S.; Reyes, S.; et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: Safety, immunogenicity and protective efficacy of the CSP component. PloS ONE 2011, 6, e25868. [Google Scholar] [CrossRef] [PubMed]
- Chuang, I.; Sedegah, M.; Cicatelli, S.; Spring, M.; Polhemus, M.; Tamminga, C.; Patterson, N.; Guerrero, M.; Bennett, J.W.; McGrath, S.; et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS ONE 2013, 8, e55571. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.; Sedegah, M.; Maiolatesi, S.; Fedders, C.; Reyes, S.; Reyes, A.; Vasquez, C.; Alcorta, Y.; Chuang, I.; Spring, M.; et al. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum. Vaccines Immunother. 2013, 9, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Herrera, M.; Vera, O.; Castellanos, A.; Cespedes, N.; Soto, L.; Corradin, G.; Herrera, S. Preclinical vaccine study of Plasmodium vivax circumsporozoite protein derived-synthetic polypeptides formulated in montanide ISA 720 and montanide ISA 51 adjuvants. Am. J. Trop. Med. Hyg. 2011, 84, 21–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrera, S.; Bonelo, A.; Perlaza, B.L.; Valencia, A.Z.; Cifuentes, C.; Hurtado, S.; Quintero, G.; Lopez, J.A.; Corradin, G.; Arevalo-Herrera, M. Use of long synthetic peptides to study the antigenicity and immunogenicity of the Plasmodium vivax circumsporozoite protein. Int. J. Parasitol. 2004, 34, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Bonelo, A.; Perlaza, B.L.; Fernandez, O.L.; Victoria, L.; Lenis, A.M.; Soto, L.; Hurtado, H.; Acuna, L.M.; Velez, J.D.; et al. Safety and elicitation of humoral and cellular responses in colombian malaria-naive volunteers by a Plasmodium vivax circumsporozoite protein-derived synthetic vaccine. Am. J. Trop. Med. Hyg. 2005, 73, 3–9. [Google Scholar] [CrossRef][Green Version]
- Yadava, A.; Sattabongkot, J.; Washington, M.A.; Ware, L.A.; Majam, V.; Zheng, H.; Kumar, S.; Ockenhouse, C.F. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect. Immun. 2007, 75, 1177–1185. [Google Scholar] [CrossRef]
- Bell, B.A.; Wood, J.F.; Bansal, R.; Ragab, H.; Cargo, J., 3rd; Washington, M.A.; Wood, C.L.; Ware, L.A.; Ockenhouse, C.F.; Yadava, A. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine 2009, 27, 1448–1453. [Google Scholar] [CrossRef]
- Lumsden, J.M.; Pichyangkul, S.; Srichairatanakul, U.; Yongvanitchit, K.; Limsalakpetch, A.; Nurmukhambetova, S.; Klein, J.; Bertholet, S.; Vedvick, T.S.; Reed, S.G.; et al. Evaluation of the safety and immunogenicity in rhesus monkeys of a recombinant malaria vaccine for Plasmodium vivax with a synthetic Toll-like receptor 4 agonist formulated in an emulsion. Infect. Immun. 2011, 79, 3492–3500. [Google Scholar] [CrossRef][Green Version]
- Lumsden, J.M.; Nurmukhambetova, S.; Klein, J.H.; Sattabongkot, J.; Bennett, J.W.; Bertholet, S.; Fox, C.B.; Reed, S.G.; Ockenhouse, C.F.; Howard, R.F.; et al. Evaluation of immune responses to a Plasmodium vivax CSP-based recombinant protein vaccine candidate in combination with second-generation adjuvants in mice. Vaccine 2012, 30, 3311–3319. [Google Scholar] [CrossRef]
- Vanloubbeeck, Y.; Pichyangkul, S.; Bayat, B.; Yongvanitchit, K.; Bennett, J.W.; Sattabongkot, J.; Schaecher, K.; Ockenhouse, C.F.; Cohen, J.; Yadava, A.; et al. Comparison of the immune responses induced by soluble and particulate Plasmodium vivax circumsporozoite vaccine candidates formulated in AS01 in rhesus macaques. Vaccine 2013, 31, 6216–6224. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Yadava, A.; Tosh, D.; Sattabongkot, J.; Komisar, J.; Ware, L.A.; McCarthy, W.F.; Cowden, J.J.; Regules, J.; Spring, M.D.; et al. Phase 1/2a Trial of Plasmodium vivax Malaria Vaccine Candidate VMP001/AS01B in Malaria-Naive Adults: Safety, Immunogenicity, and Efficacy. PLoS Negl. Trop. Dis. 2016, 10, e0004423. [Google Scholar] [CrossRef] [PubMed]
- Arnot, D.E.; Barnwell, J.W.; Tam, J.P.; Nussenzweig, V.; Nussenzweig, R.S.; Enea, V. Circumsporozoite protein of Plasmodium vivax: Gene cloning and characterization of the immunodominant epitope. Science 1985, 230, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Wirtz, R.A.; Lanar, D.E.; Sattabongkot, J.; Hall, T.; Waters, A.P.; Prasittisuk, C. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science 1989, 245, 973–976. [Google Scholar] [CrossRef]
- Qari, S.H.; Shi, Y.P.; Povoa, M.M.; Alpers, M.P.; Deloron, P.; Murphy, G.S.; Harjosuwarno, S.; Lal, A.A. Global occurrence of Plasmodium vivax-like human malaria parasite. J. Infect. Dis. 1993, 168, 1485–1489. [Google Scholar] [CrossRef]
- Arevalo-Herrera, M.; Herrera, S. Plasmodium vivax malaria vaccine development. Mol. Immunol. 2001, 38, 443–455. [Google Scholar] [CrossRef]
- Kisalu, N.K.; Idris, A.H.; Weidle, C.; Flores-Garcia, Y.; Flynn, B.J.; Sack, B.K.; Murphy, S.; Schon, A.; Freire, E.; Francica, J.R.; et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018, 24, 408–416. [Google Scholar] [CrossRef]
- Bongfen, S.E.; Ntsama, P.M.; Offner, S.; Smith, T.; Felger, I.; Tanner, M.; Alonso, P.; Nebie, I.; Romero, J.F.; Silvie, O.; et al. The N-terminal domain of Plasmodium falciparum circumsporozoite protein represents a target of protective immunity. Vaccine 2009, 27, 328–335. [Google Scholar] [CrossRef]
- Espinosa, D.A.; Gutierrez, G.M.; Rojas-Lopez, M.; Noe, A.R.; Shi, L.; Tse, S.W.; Sinnis, P.; Zavala, F. Proteolytic Cleavage of the Plasmodium falciparum Circumsporozoite Protein Is a Target of Protective Antibodies. J. Infect. Dis. 2015, 212, 1111–1119. [Google Scholar] [CrossRef]
- Thera, M.A.; Coulibaly, D.; Kone, A.K.; Guindo, A.B.; Traore, K.; Sall, A.H.; Diarra, I.; Daou, M.; Traore, I.M.; Tolo, Y.; et al. Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25-545]) in healthy Malian adults in Bandiagara. Malar. J. 2016, 15, 442. [Google Scholar] [CrossRef]
- Sagara, I.; Dicko, A.; Ellis, R.D.; Fay, M.P.; Diawara, S.I.; Assadou, M.H.; Sissoko, M.S.; Kone, M.; Diallo, A.I.; Saye, R.; et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 2009, 27, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Sagara, I.; Ellis, R.D.; Dicko, A.; Niambele, M.B.; Kamate, B.; Guindo, O.; Sissoko, M.S.; Fay, M.P.; Guindo, M.A.; Kante, O.; et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel + CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine 2009, 27, 7292–7298. [Google Scholar] [CrossRef] [PubMed]
- Spring, M.D.; Cummings, J.F.; Ockenhouse, C.F.; Dutta, S.; Reidler, R.; Angov, E.; Bergmann-Leitner, E.; Stewart, V.A.; Bittner, S.; Juompan, L.; et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PloS ONE 2009, 4, e5254. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.H.; Rodrigues, K.M.; Oliveira, T.R.; Comodo, A.N.; Rodrigues, M.M.; Kocken, C.H.; Thomas, A.W.; Soares, I.S. Antibody response of naturally infected individuals to recombinant Plasmodium vivax apical membrane antigen-1. Int. J. Parasitol. 2005, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.G.; Soares, I.S.; Carvalho, L.H.; Fontes, C.J.; Krettli, A.U.; Braga, E.M. Antibodies to Plasmodium vivax apical membrane antigen 1: Persistence and correlation with malaria transmission intensity. Am. J. Trop. Med. Hyg. 2006, 75, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Barbedo, M.B.; Ricci, R.; Jimenez, M.C.; Cunha, M.G.; Yazdani, S.S.; Chitnis, C.E.; Rodrigues, M.M.; Soares, I.S. Comparative recognition by human IgG antibodies of recombinant proteins representing three asexual erythrocytic stage vaccine candidates of Plasmodium vivax. Mem. Inst. Oswaldo Cruz 2007, 102, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Mufalo, B.C.; Gentil, F.; Bargieri, D.Y.; Costa, F.T.; Rodrigues, M.M.; Soares, I.S. Plasmodium vivax apical membrane antigen-1: Comparative recognition of different domains by antibodies induced during natural human infection. Microbes Infect. 2008, 10, 1266–1273. [Google Scholar] [CrossRef]
- Vicentin, E.C.; Francoso, K.S.; Rocha, M.V.; Iourtov, D.; Dos Santos, F.L.; Kubrusly, F.S.; Sakauchi, M.A.; Raw, I.; Nosten, F.; Renia, L.; et al. Invasion-inhibitory antibodies elicited by immunization with Plasmodium vivax apical membrane antigen-1 expressed in Pichia pastoris yeast. Infect. Immun. 2014, 82, 1296–1307. [Google Scholar] [CrossRef]
- Rocha, M.V.; Francoso, K.S.; Lima, L.C.; Camargo, T.M.; Machado, R.L.D.; Costa, F.T.M.; Renia, L.; Nosten, F.; Russell, B.; Rodrigues, M.M.; et al. Generation, characterization and immunogenicity of a novel chimeric recombinant protein based on Plasmodium vivax AMA-1 and MSP119. Vaccine 2017, 35, 2463–2472. [Google Scholar] [CrossRef]
- Camacho, A.G.; Teixeira, L.H.; Bargieri, D.Y.; Boscardin, S.B.; Soares, I.S.; Nussenzweig, R.S.; Nussenzweig, V.; Rodrigues, M.M. TLR5-dependent immunogenicity of a recombinant fusion protein containing an immunodominant epitope of malarial circumsporozoite protein and the FliC flagellin of Salmonella Typhimurium. Mem. Inst. Oswaldo Cruz 2011, 106 (Suppl. 1), 167–171. [Google Scholar] [CrossRef]
- Leal, M.T.; Camacho, A.G.; Teixeira, L.H.; Bargieri, D.Y.; Soares, I.S.; Tararam, C.A.; Rodrigues, M.M. Immunogenicity of recombinant proteins consisting of Plasmodium vivax circumsporozoite protein allelic variant-derived epitopes fused with Salmonella enterica Serovar Typhimurium flagellin. Clin. Vaccine Immunol. CVI 2013, 20, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Sriprawat, K.; Kaewpongsri, S.; Suwanarusk, R.; Leimanis, M.L.; Lek-Uthai, U.; Phyo, A.P.; Snounou, G.; Russell, B.; Renia, L.; Nosten, F. Effective and cheap removal of leukocytes and platelets from Plasmodium vivax infected blood. Malar. J. 2009, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Suwanarusk, R.; Malleret, B.; Costa, F.T.; Snounou, G.; Kevin Baird, J.; Nosten, F.; Renia, L. Human ex vivo studies on asexual Plasmodium vivax: The best way forward. Int. J. Parasitol. 2012, 42, 1063–1070. [Google Scholar] [CrossRef][Green Version]
- Espinosa, D.A.; Yadava, A.; Angov, E.; Maurizio, P.L.; Ockenhouse, C.F.; Zavala, F. Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect. Immun. 2013, 81, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Aliprandini, E.; Tavares, J.; Panatieri, R.H.; Thiberge, S.; Yamamoto, M.M.; Silvie, O.; Ishino, T.; Yuda, M.; Dartevelle, S.; Traincard, F.; et al. Cytotoxic anti-circumsporozoite antibodies target malaria sporozoites in the host skin. Nat. Microbiol. 2018, 3, 1224–1233. [Google Scholar] [CrossRef]
- Yadava, A.; Nurmukhambetova, S.; Pichugin, A.V.; Lumsden, J.M. Cross-species immunity following immunization with a circumsporozoite protein-based vaccine for malaria. J. Infect. Dis. 2012, 205, 1456–1463. [Google Scholar] [CrossRef]
- Salman, A.M.; Montoya-Diaz, E.; West, H.; Lall, A.; Atcheson, E.; Lopez-Camacho, C.; Ramesar, J.; Bauza, K.; Collins, K.A.; Brod, F.; et al. Rational development of a protective P. vivax vaccine evaluated with transgenic rodent parasite challenge models. Sci. Rep. 2017, 7, 46482. [Google Scholar] [CrossRef]
- Silvie, O.; Franetich, J.F.; Charrin, S.; Mueller, M.S.; Siau, A.; Bodescot, M.; Rubinstein, E.; Hannoun, L.; Charoenvit, Y.; Kocken, C.H.; et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J. Biol. Chem. 2004, 279, 9490–9496. [Google Scholar] [CrossRef]
- Kastenmuller, K.; Espinosa, D.A.; Trager, L.; Stoyanov, C.; Salazar, A.M.; Pokalwar, S.; Singh, S.; Dutta, S.; Ockenhouse, C.F.; Zavala, F.; et al. Full-length Plasmodium falciparum circumsporozoite protein administered with long-chain poly(I.C) or the Toll-like receptor 4 agonist glucopyranosyl lipid adjuvant-stable emulsion elicits potent antibody and CD4+ T cell immunity and protection in mice. Infect. Immun. 2013, 81, 789–800. [Google Scholar] [CrossRef]
- Reece, W.H.; Pinder, M.; Gothard, P.K.; Milligan, P.; Bojang, K.; Doherty, T.; Plebanski, M.; Akinwunmi, P.; Everaere, S.; Watkins, K.R.; et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 2004, 10, 406–410. [Google Scholar] [CrossRef]
- Dobrescu, I.; de Camargo, T.M.; Gimenez, A.M.; Murillo, O.; Amorim, K.; Marinho, C.R.F.; Soares, I.S.; Boscardin, S.B.; Bargieri, D.Y. Protective Immunity in Mice Immunized With P. vivax MSP119-Based Formulations and Challenged With P. berghei Expressing PvMSP119. Front. Immunol. 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.F.; Gimenez, A.M.; Aliprandini, E.; Novais, J.T.; Cury, D.P.; Watanabe, I.S.; Dominguez, M.R.; Silveira, E.L.V.; Amino, R.; Soares, I.S. Protective Malaria Vaccine in Mice Based on the Plasmodium vivax Circumsporozoite Protein Fused with the Mumps Nucleocapsid Protein. Vaccines 2020, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Ockenhouse, C.F.; Regules, J.A.; Dutta, S.; Wallqvist, A.; Jongert, E.; Waters, N.C.; Lemiale, F.; Bergmann-Leitner, E. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar. J. 2016, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Sadegh-Nasseri, S.; Kim, A. Selection of immunodominant epitopes during antigen processing is hierarchical. Mol. Immunol. 2019, 113, 115–119. [Google Scholar] [CrossRef]
- Lopez, C.; Yepes-Perez, Y.; Hincapie-Escobar, N.; Diaz-Arevalo, D.; Patarroyo, M.A. What Is Known about the Immune Response Induced by Plasmodium vivax Malaria Vaccine Candidates? Front. Immunol. 2017, 8, 126. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.C.; Marques, R.F.; Gimenez, A.M.; Françoso, K.S.; Aliprandini, E.; Camargo, T.M.; Aguiar, A.C.C.; Pereira, D.B.; Renia, L.; Amino, R.; et al. A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite. Microorganisms 2020, 8, 916. https://doi.org/10.3390/microorganisms8060916
Lima LC, Marques RF, Gimenez AM, Françoso KS, Aliprandini E, Camargo TM, Aguiar ACC, Pereira DB, Renia L, Amino R, et al. A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite. Microorganisms. 2020; 8(6):916. https://doi.org/10.3390/microorganisms8060916
Chicago/Turabian StyleLima, Luciana C., Rodolfo F. Marques, Alba Marina Gimenez, Katia S. Françoso, Eduardo Aliprandini, Tarsila M. Camargo, Anna Caroline C. Aguiar, Dhelio B. Pereira, Laurent Renia, Rogerio Amino, and et al. 2020. "A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite" Microorganisms 8, no. 6: 916. https://doi.org/10.3390/microorganisms8060916
APA StyleLima, L. C., Marques, R. F., Gimenez, A. M., Françoso, K. S., Aliprandini, E., Camargo, T. M., Aguiar, A. C. C., Pereira, D. B., Renia, L., Amino, R., & Soares, I. S. (2020). A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite. Microorganisms, 8(6), 916. https://doi.org/10.3390/microorganisms8060916




