Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Cultures and Incubation
2.2. Experimental Setup and Procedure
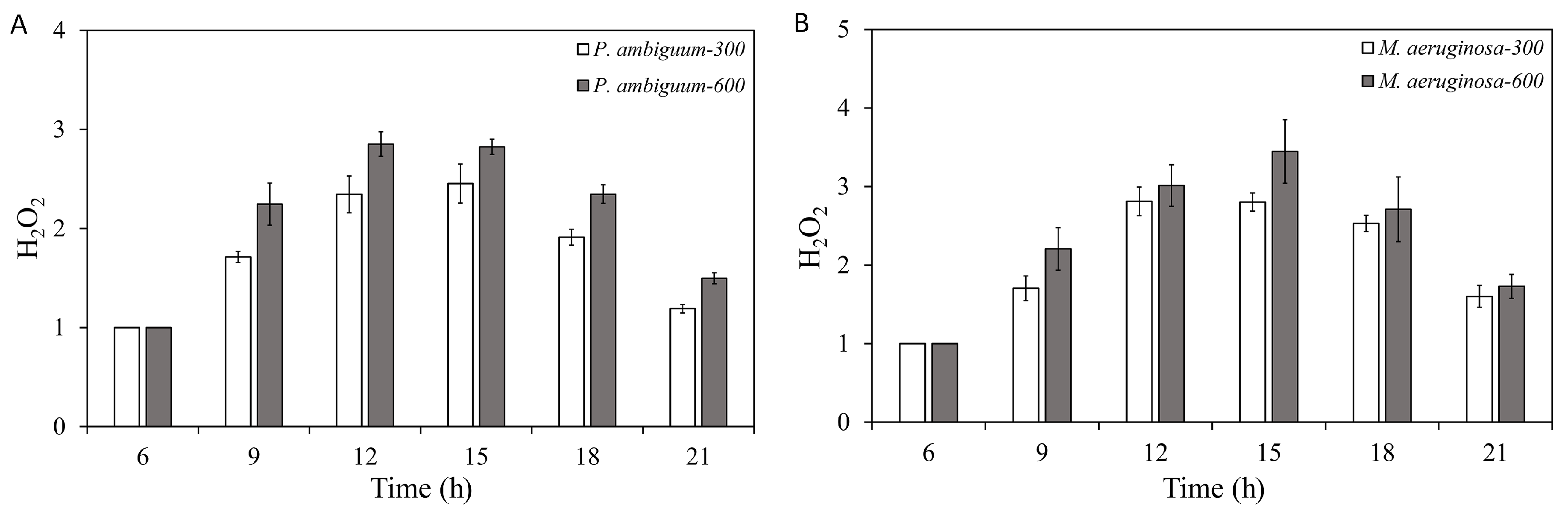
2.3. H2O2 Concentration
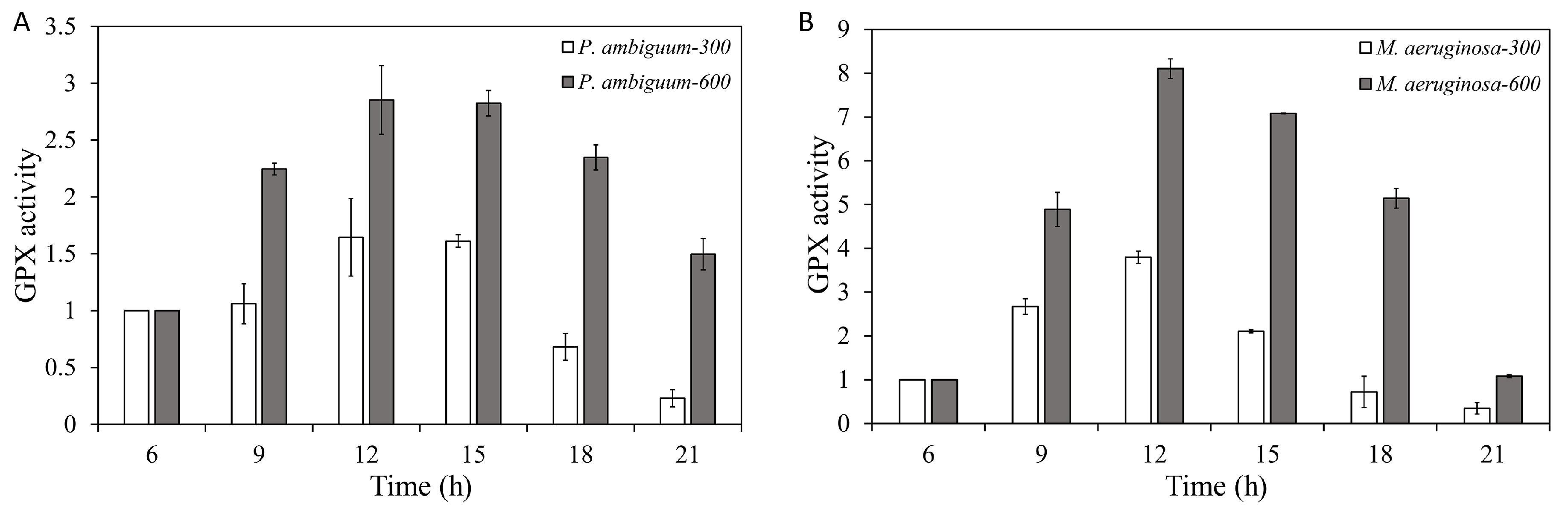
2.4. GPX-Activity Assay
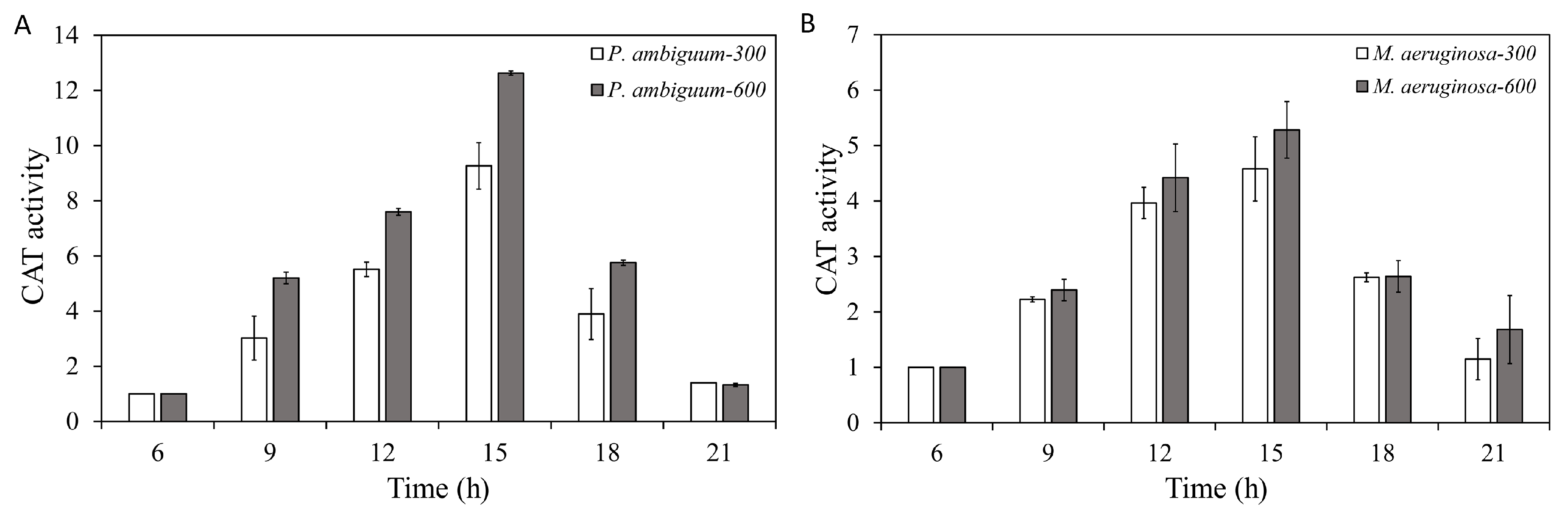
2.5. CAT-Activity Assay
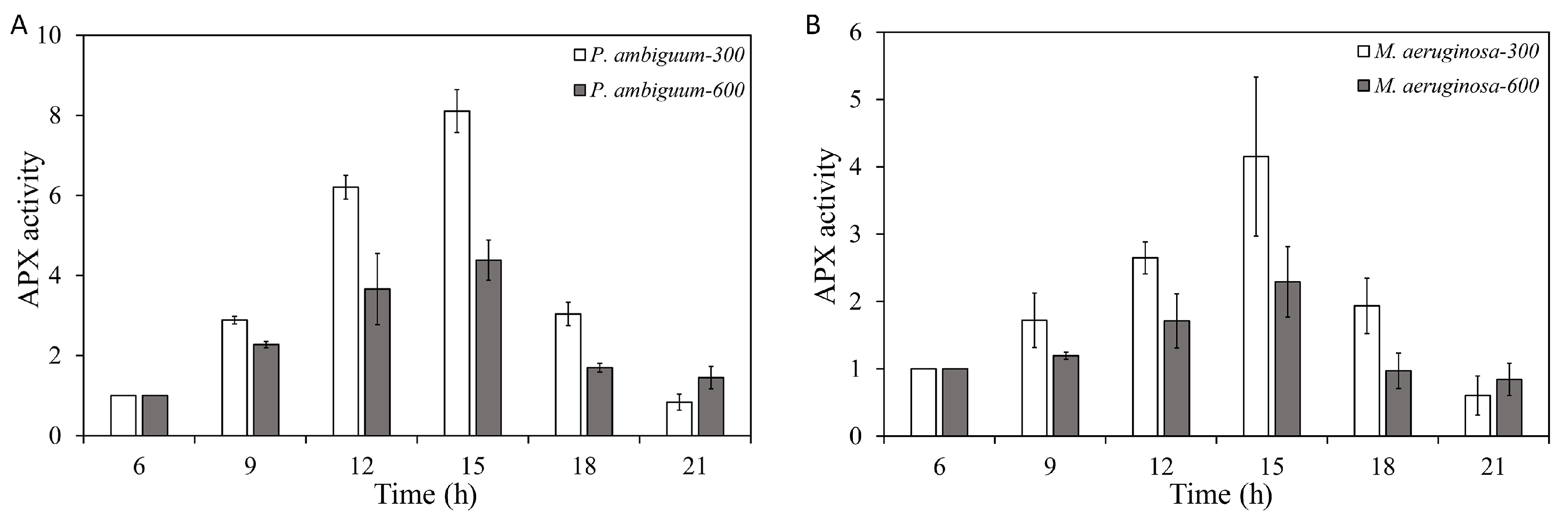
2.6. APX-Activity Assay
2.7. SOD-Activity Assay
2.8. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pham, T.-L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Trolle, D.; Nielsen, A.; Andersen, H.E.; Thodsen, H.; Olesen, J.E.; Børgesen, C.D.; Refsgaard, J.C.; Sonnenborg, T.O.; Karlsson, I.B.; Christensen, J.P.; et al. Effects of changes in land use and climate on aquatic ecosystems: Coupling of models and decomposition of uncertainties. Sci. Total Environ. 2019, 657, 627–633. [Google Scholar] [CrossRef]
- Izaguirre, G.; Hwang, C.J.; Krasner, S.W.; McGuire, M.J. Geosmin and 2-Methylisoborneol from cyanobacteria in three water supply systems. Appl. Environ. Microbiol. 1982, 43, 708–714. [Google Scholar] [CrossRef]
- Butakova, E.A. Specific features of odor-causing compounds (geosmin and 2-methylisoborneol) as secondary metabolites of cyanobacteria. Russ. J. Plant Physiol. 2013, 60, 507–510. [Google Scholar] [CrossRef]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium Pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. 2001, 20, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Ghadouani, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshw. Biol. 2003, 48, 363–381. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Rodríguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2012, 46, 4319–4329. [Google Scholar] [CrossRef]
- Grandgirard, J.; Poinsot, D.; Krespi, L.; Nenon, J.-P.; Cortesero, A.M. Costs of secondary parasitism in the facultative hyperparasitoid Pachycrepoideus dubius: Does host size matter? Èntomol. Exp. Appl. 2002, 103, 239–248. [Google Scholar] [CrossRef]
- Lake, M.; Madsen, M.; Brokaw, T.; Moon, R.; Beardon, C.; Cassell, C.; Collins, D. Mason Lake; Lake Stewardship Consulting: Belfair, WA, USA, 2003. [Google Scholar]
- Jančula, D.; Maršálek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Sidler, W. Phycobilisome and Phycobiliprotein Structures. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; Volume 2, ISBN 978-0-7923-3273-2. [Google Scholar]
- Liu, L.; Chen, H.; Liu, M.; Yang, J.R.; Xiao, P.; Wilkinson, D.M. Response of the eukaryotic plankton community to the cyanobacterial biomass cycle over 6 years in two subtropical reservoirs. ISME J. 2019, 13, 2196–2208. [Google Scholar] [CrossRef]
- Joset, F.; Jeanjean, R.; Hagemann, M. Dynamics of the response of cyanobacteria to salt stress: Deciphering the molecular events. Physiol. Plant. 1996, 96, 738–744. [Google Scholar] [CrossRef]
- Sinetova, M.; Los, D.A. New insights in cyanobacterial cold stress responses: Genes, sensors, and molecular triggers. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic de-regulation in cyanobacteria in response to abiotic stresses. Front. Microbiol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.; Hamilton, D.P.; Brookes, J. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Al Maskari, S.M.S.; Al Sabahi, J.N.; Victor, R. Cyanobacterial mats from hot springs produce antimicrobial compounds and quorum-sensing inhibitors under natural conditions. Environ. Biol. Fishes 2010, 23, 983–993. [Google Scholar] [CrossRef]
- Celeste, A.J.; Beaulieu, K.M.; Bradley, P.M. Environmental factors that influence cyanobacteria and Geosmin occurrence in reservoirs. Curr. Perspect. Contam. Hydrol. Water Resour. Sustain. 2013. [Google Scholar] [CrossRef]
- Watson, S. Cyanobacterial and eukaryotic algal odour compounds: Signals or by-products? A review of their biological activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Briand, J.-F.; Leboulanger, C.; Humbert, J.-F.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, orglobalwarming? J. Phycol. 2004, 40, 231–238. [Google Scholar] [CrossRef]
- Rücker, J.; Tingwey, E.I.; Wiedner, C.; Anu, C.M.; Nixdorf, B. Impact of the inoculum size on the population of Nostocales cyanobacteria in a temperate lake. J. Plankton Res. 2009, 31, 1151–1159. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Visser, P.M.; Ma, R. Diurnal changes of cyanobacteria blooms in Taihu Lake as derived from GOCI observations. Limnol. Oceanogr. 2018, 63, 1711–1726. [Google Scholar] [CrossRef]
- Saha, R.; Liu, D.; Hoynes-O’Connor, A.; Liberton, M.; Yu, J.; Bhattacharyya-Pakrasi, M.; Balássy, A.; Zhang, F.; Moon, T.S.; Maranas, C.D.; et al. Diurnal regulation of cellular processes in the Cyanobacterium. mBio 2016, 7. [Google Scholar] [CrossRef]
- Anthony, J.R.; Warczak, K.L.; Donohue, T.J. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 6502–6507. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2015, 50, 423–441. [Google Scholar] [CrossRef]
- Berrendero, E.; Valiente, E.F.; Perona, E.; Gómez, C.L.; Loza, V.; Martin, M.D.L.; Ángeles, M.; Mateo, P. Nitrogen fixation in a non-heterocystous cyanobacterial mat from a mountain river. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacteriumMicrocystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Princiotta, S.D.; Hendricks, S.P.; White, D.S. Production of Cyanotoxins by Microcystis aeruginosa mediates interactions with the Mixotrophic flagellate Cryptomonas. Toxins 2019, 11, 223. [Google Scholar] [CrossRef]
- Teneva, I.; Dzhambazov, B.; Koleva-Valkova, L.; Mladenov, R.; Schirmer, K. Toxic potential of five freshwater Phormidium species (Cyanoprokaryota). Toxicon 2005, 45, 711–725. [Google Scholar] [CrossRef]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Effects of plant growth regulators on Hill activity of submerged aquatic plants during induced senescence. Aquat. Bot. 1984, 18, 371–376. [Google Scholar] [CrossRef]
- Senousy, H.H.; Ellatif, S.A.; Ali, S. Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ. Sci. Pollut. Res. 2020, 27, 18463–18474. [Google Scholar] [CrossRef] [PubMed]
- Macadam, J.W.; Nelson, C.J.; Sharp, R.E. Peroxidase activity in the leaf elongation zone of tall fescue: I. spatial distribution of Ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992, 99, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by Ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a Nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Arazna, M.; Demkow, U. Biochemistry of oxidative stress. Adv. Exp. Med. Biol. 2015, 878, 9–19. [Google Scholar] [CrossRef]
- Lin, G.G.; Scott, J.G. ROS function in redox signaling. Curr. Biol. 2012, 100, 130–134. [Google Scholar]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef]
- Page†, M.T.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2011, 35, 388–404. [Google Scholar] [CrossRef]
- Virtanen, O.; Valev, D.; Kruse, O.; Wobbe, L.; Tyystjarvi, E. Photoinhibition and continuous growth of the wild-type and a high-light tolerant strain of Chlamydomonas reinhardtii. Photosynthetica 2019, 57, 617–626. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Pedersén, M. Production, scavenging and toxicity of hydrogen peroxide in the green seaweedUlva rigida. Eur. J. Phycol. 1996, 31, 265–271. [Google Scholar] [CrossRef]
- Estervig, D.; Wang, R.J. Sister chromatid exchanges and chromosome aberrations in human cells induced by H2O2 and other photoproducts generated in fluorescent light-exposed medium. Photochem. Photobiol. 1984, 40, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Brutemark, A.; Engstrom-Ost, J.; Vehmaa, A.; Gorokhova, E. Growth, toxicity and oxidative stress of a cultured cyanobacterium (Dolichospermum sp.) under different CO2/pH and temperature conditions. Phycol. Res. 2015, 63, 56–63. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2010, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Muhetaer, G.; Asaeda, T.; Jayasanka, S.M.; Baniya, M.B.; Abeynayaka, H.D.; Rashid, M.H.; Yan, H. Effects of light intensity and exposure period on the growth and stress responses of two cyanobacteria species: Pseudanabaena galeata and Microcystis aeruginosa. Water 2020, 12, 407. [Google Scholar] [CrossRef]
- Zevenboom, W.; Mur, L.R. Growth and photosynthetic response of the cyanobacterium Microcystis aeruginosa in relation to photoperiodicity and irradiance. Arch. Microbiol. 1984, 139, 232–239. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.; Truscott, T. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Paerl, H.W. Cyanobacterial carotenoids: Their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia 1984, 61, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Niu, X.; Song, Q.; Li, Y.; Zhang, R.; Zou, D.; Lai, S.; Yang, Z.; Tang, Z.; Zhou, S.; et al. Physiological and biochemical responses of Microcystis aeruginosa to phosphine. Environ. Pollut. 2019, 247, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Borase, D.; Dhar, D.W.; Singh, N. Diversity indices and growth parameters of cyanobacteria from three lakes of Rajasthan. Vegetos-Int. J. Plant Res. 2013, 26, 377. [Google Scholar] [CrossRef]
- Golden, S.S.; Ishiura, M.; Johnson, C.H.; Kondo, T. Cyanobacterial circadian rhythms. Annu. Rev. Plant Biol. 1997, 48, 327–354. [Google Scholar] [CrossRef] [PubMed]
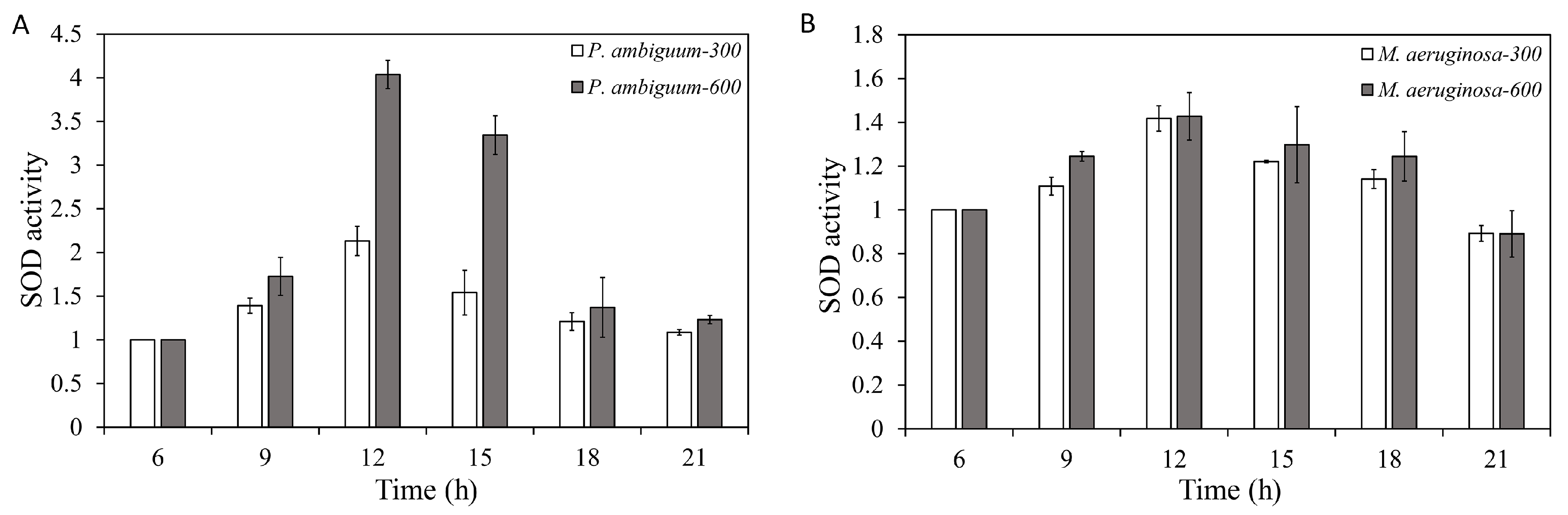
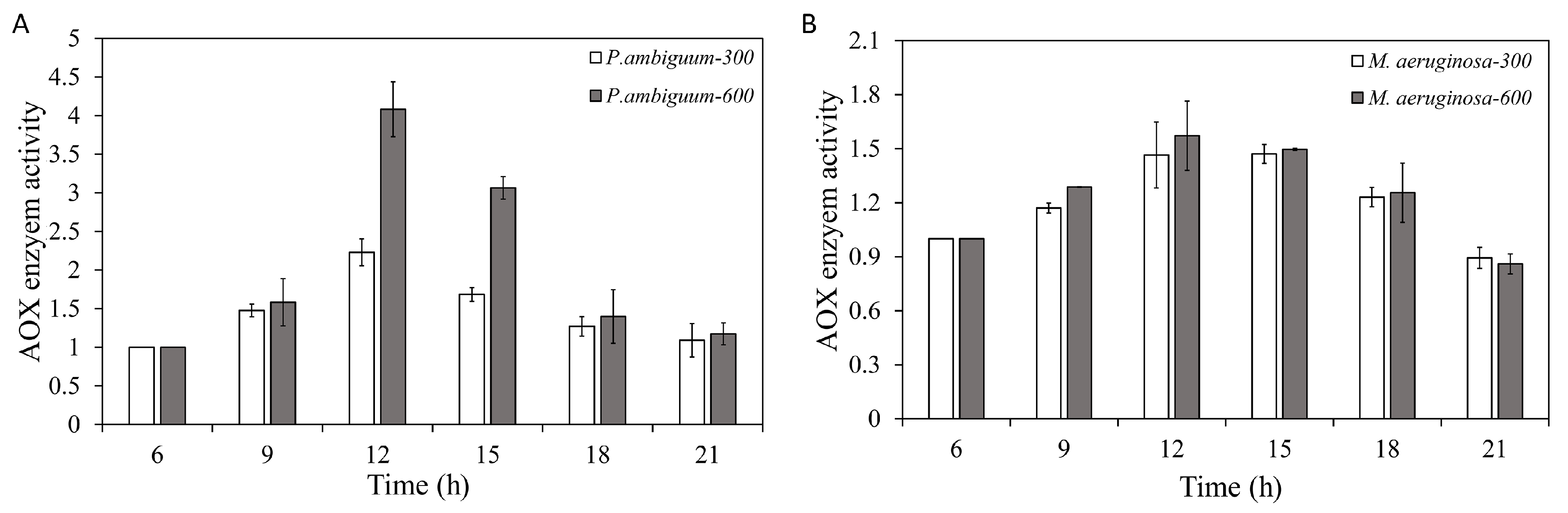
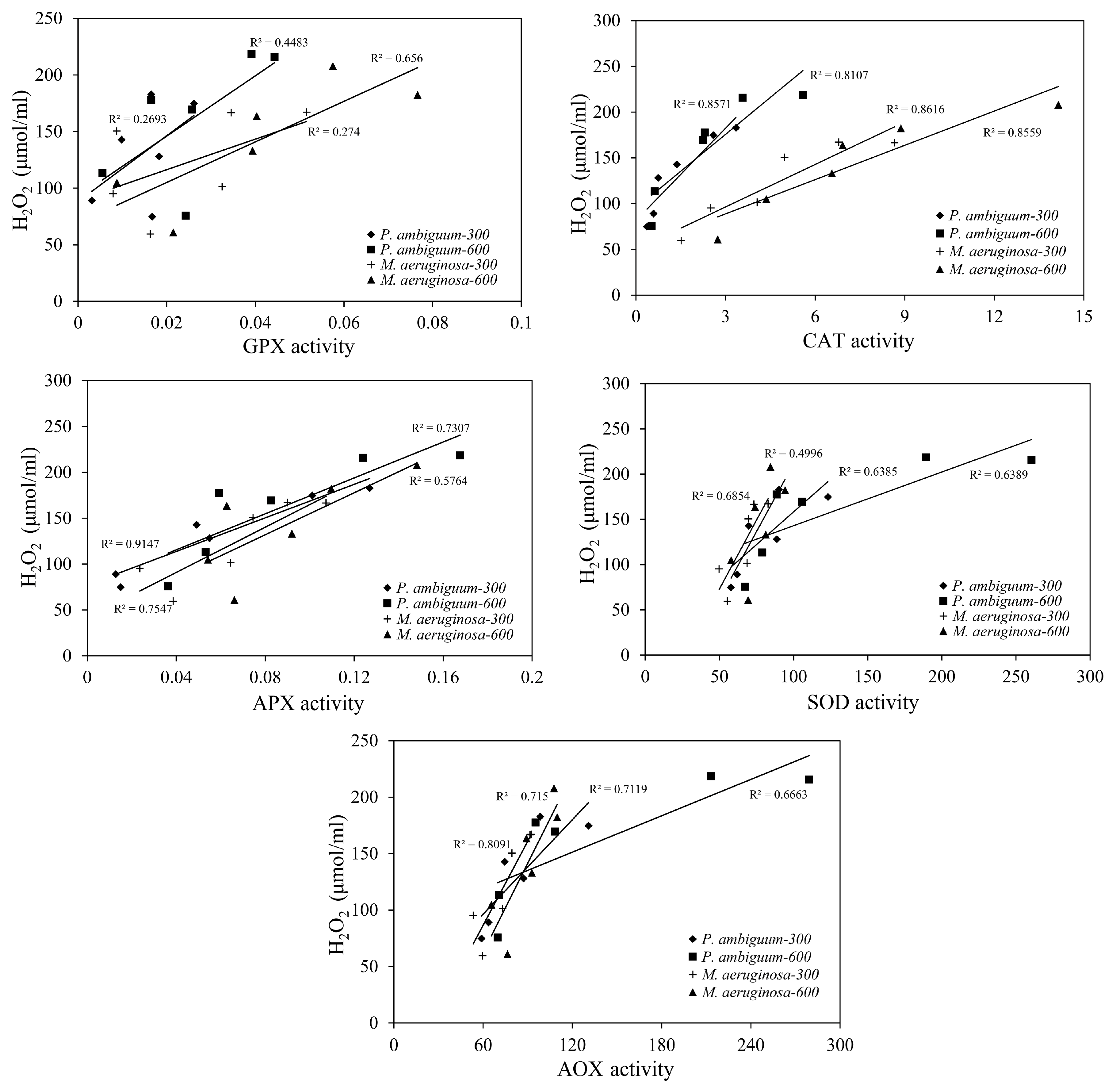
| Condition | Parameter | R2 | p Value |
|---|---|---|---|
| M. aeruginosa–300 1 | SOD | 0.780 | p < 0.01 |
| APX | 0.652 | p < 0.01 | |
| CAT | 0.893 | p < 0.01 | |
| GPX | 0.539 | p < 0.05 | |
| AOX | 0.856 | p < 0.01 | |
| M. aeruginosa-600 | SOD | 0.526 | p < 0.05 |
| APX | 0.683 | p < 0.01 | |
| CAT | 0.924 | p < 0.01 | |
| GPX | 0.692 | p < 0.01 | |
| AOX | 0.720 | p < 0.01 | |
| P. ambiguum-300 | SOD | 0.748 | p < 0.01 |
| APX | 0.962 | p < 0.01 | |
| CAT | 0.824 | p < 0.01 | |
| GPX | 0.383 | p > 0.05 | |
| AOX | 0.803 | p < 0.01 | |
| P. ambiguum-600 | SOD | 0.784 | p < 0.01 |
| APX | 0.738 | p < 0.01 | |
| CAT | 0.830 | p < 0.01 | |
| GPX | 0.624 | p < 0.01 | |
| AOX | 0.796 | p < 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhetaer, G.; Jayasanka, S.M.D.H.; Fujino, T. Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms 2020, 8, 890. https://doi.org/10.3390/microorganisms8060890
Muhetaer G, Jayasanka SMDH, Fujino T. Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms. 2020; 8(6):890. https://doi.org/10.3390/microorganisms8060890
Chicago/Turabian StyleMuhetaer, Guligena, Senavirathna M.D.H. Jayasanka, and Takeshi Fujino. 2020. "Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions" Microorganisms 8, no. 6: 890. https://doi.org/10.3390/microorganisms8060890
APA StyleMuhetaer, G., Jayasanka, S. M. D. H., & Fujino, T. (2020). Oxidative Stress and Antioxidant Responses of Phormidium ambiguum and Microcystis aeruginosa Under Diurnally Varying Light Conditions. Microorganisms, 8(6), 890. https://doi.org/10.3390/microorganisms8060890






