Complete Genome of Isoprene Degrading Nocardioides sp. WS12
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Growth
2.2. Genome Sequencing
2.3. Genome Analysis and Comparison
3. Results and Discussion
3.1. Genome Sequencing and Identification
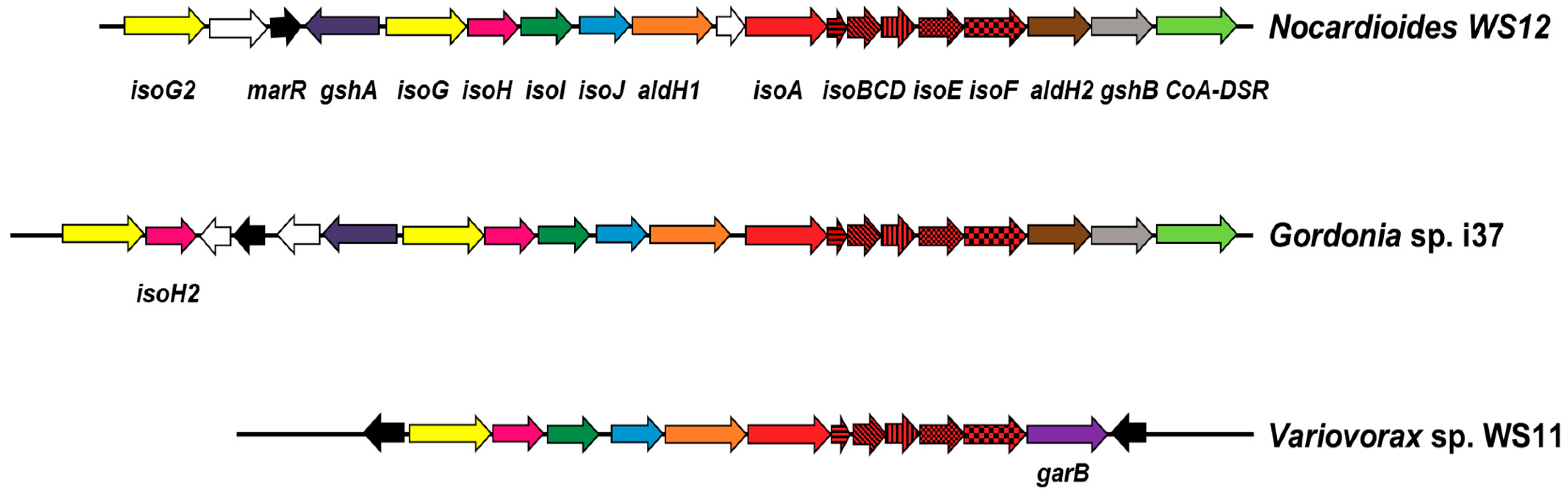
3.2. Isoprene Degradation Gene Cluster
3.3. Rubber Degradation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atkinson, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Steinfeld, J.I. Atmospheric chemistry and physics: From air pollution to climate change. Environ. Sci. Policy Sustain Dev. 1998, 40, 26. [Google Scholar] [CrossRef]
- Pacifico, F.; Harrison, S.P.; Jones, C.D.; Sitch, S. Isoprene emissions and climate. Atmos. Environ. 2009, 43, 6121–6135. [Google Scholar] [CrossRef]
- Henze, D.K.; Seinfeld, J.H. Global secondary organic aerosol from isoprene oxidation. Geophys. Res. Lett. 2006, 33, L09812. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprene photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef]
- Hantson, S.; Knorr, W.; Schurgers, G.; Pugh, T.A.M.; Arneth, A. Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use. Atmos. Environ. 2017, 155, 35–45. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2007, 101, 5–18. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Brancaleoni, E.; Valentini, R.; De Lillis, M.; Csiky, O.; Seufert, G. A hypothesis on the evolution of isoprenoid emission by oaks based on the correlation between emission type and Quercus taxonomy. Oecologia 1998, 115, 302–305. [Google Scholar] [CrossRef]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why only some plants emit isoprene. Plant Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef]
- Sharkey, T.D. Is it useful to ask why plants emit isoprene? Plant Cell Environ. 2013, 36, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Copolovici, L.; Hüve, K. High within-canopy variation in isoprene emission potentials in temperate trees: Implications for predicting canopy-scale isoprene fluxes. J. Geophys. Res. Biogeosci. 2010, 115, G04029. [Google Scholar] [CrossRef]
- Larke-Mejia, N.L.; Crombie, A.; Pratscher, J.; McGenity, T.J.; Murrell, C. Novel isoprene-degrading proteobacteria from soil and leaves identified by cultivation and metagenomics analysis of stable isotope probing experiments. Front. Microbiol. 2019, 10, 2700. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Yavitt, J.B. Consumption of atmospheric isoprene in soil. Geophys. Res. Lett. 1997, 24, 2379–2382. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Yavitt, J.B. Microbial consumption of atmospheric isoprene in a temperate forest soil. Appl. Environ. Microbiol. 1998, 64, 172–177. [Google Scholar] [CrossRef]
- Gray, C.M.; Helmig, D.; Fierer, N. Bacteria and fungi associated with isoprene consumption in soil. Elem. Sci. Anthr. 2015, 3, 53. [Google Scholar] [CrossRef]
- McGenity, T.J.; Crombie, A.T.; Murrell, J.C. Microbial cycling of isoprene, the most abundantly produced biological volatile organic compound on Earth. ISME J. 2018, 12, 931–941. [Google Scholar] [CrossRef]
- Crombie, A.T.; Khawand, M.E.; Rhodius, V.A.; Fengler, K.A.; Miller, M.C.; Whited, G.M.; McGenity, T.J.; Murrell, J.C. Regulation of plasmid-encoded isoprene metabolism in Rhodococcus, a representative of an important link in the global isoprene cycle. Environ. Microbiol. 2015, 17, 3314–3329. [Google Scholar] [CrossRef]
- Van Hylckama Vlieg, J.E.; Kingma, J.; van den Wijngaard, A.J.; Janssen, D.B. A glutathione s-transferase with activity towards cis-1, 2-dichloroepoxyethane is involved in isoprene utilization by Rhodococcus sp. strain AD45. Appl. Environ. Microbiol. 1998, 64, 2800–2805. [Google Scholar] [CrossRef]
- Van Hylckama Vlieg, J.E.; Kingma, J.; Kruizinga, W.; Janssen, D.B. Purification of a glutathione S-transferase and a glutathione conjugate-specific dehydrogenase involved in isoprene metabolism in Rhodococcus sp. strain AD45. J. Bacteriol. 1999, 181, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.G.; Batchelor, P.J.; Morcomb, S.M. Evolution of the soluble diiron monooxygenases. FEMS Microbiol. Rev. 2003, 27, 449–479. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Lata Rana, K. Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int. J. Environ. Sci. Nat. Resour. 2017. [Google Scholar] [CrossRef]
- Franco, C.; Michelsen, P.; Percy, N.; Conn, V.; Listiana, E.; Moll, S.; Loria, R.; Coombs, J. Actinobacterial endophytes for improved crop performance. Int. J. Environ. Sci. Nat. Resour. 2007, 36, 524–531. [Google Scholar] [CrossRef]
- Bao, L.; Gu, L.; Sun, B.; Cai, W.; Zhang, S.; Zhuang, G.; Bai, Z.; Zhuang, X. Seasonal variation of epiphytic bacteria in the phyllosphere of Gingko biloba, Pinus bungeana and Sabina chinensis. FEMS Microbiol. Ecol. 2020, 96, fiaa017. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Exton, D.A.; Timmis, K.N.; Suggett, D.J.; McGenity, T.J. Characterization of marine isoprene-degrading communities. Environ. Microbiol. 2009, 11, 3280–3291. [Google Scholar] [CrossRef]
- Johnston, A.; Crombie, A.T.; El Khawand, M.; Sims, L.; Whited, G.M.; McGenity, T.J.; Murrell, J.C. Identification and characterisation of isoprene-degrading bacteria in an estuarine environment. Environ. Microbiol. 2017, 19, 3526–3537. [Google Scholar] [CrossRef]
- Brenner, D.J. Family Enterobacteriaceae. In Bergey’s Manual of Systemic Bacteriology, 1st ed.; Wiliams and Wilkins: Baltimore, MD, USA, 1984; pp. 408–420. [Google Scholar]
- Bolger Anthony, M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Vallenet, D.; Calteau, A.; Dubois, M.; Amours, P.; Bazin, A.; Beuvin, M.; Burlot, L.; Bussell, X.; Fouteau, S.; Gautreau, G.; et al. MicroScope: An integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res. 2020, 48, D579–D589. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Gunturu, S.; Harvey, W.T.; Rosselló-Mora, R.; Tiedje, J.M.; Cole, J.R.; Konstantinidis, K.T. The Microbial Genomes Atlas (MIGA) webserver: Taxonomic and gene diversity analysis of archaea and bacteria at the whole genome level. Nucleic Acids Res. 2018, 46, W282–W288. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Cho, Y.G.; Lee Taik, S.; Suzuki, K.I.; Nakase, T.; Park, Y.H. Nocardioides nitrophenolicus sp. nov., a p-nitrophenol-degrading bacterium. Int. J. Syst. Bacteriol. 1999, 49, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Kawahara, K.; Sekiya, K.; Uchida, T.; Hattori, Y.; Futamata, H.; Hiraishi, A. Nocardioides aromaticivorans sp. nov., a dibenzofuran-degrading bacterium isolated from dioxin-polluted environments. Syst. Appl. Microbiol. 2005, 28, 165–174. [Google Scholar] [CrossRef]
- Suzuki, K.I.; Komagata, K. Pimelobacter gen. nov., a new genus of coryneform bacteria with LL-diaminopimelic acid in the cell wall. J. Gen. Appl. Microbiol. 1983, 29, 59–71. [Google Scholar] [CrossRef]
- Kim, M.K.; Srinivasan, S.; Park, M.J.; Sathiyaraj, G.; Kim, Y.J.; Yang, D.C. Nocardioides humi sp. nov., a β-glucosidase-producing bacterium isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 2009, 59, 2724–2728. [Google Scholar] [CrossRef][Green Version]
- Alvarez, H.M.; Mayer, F.; Fabritius, D.; Steinbüchel, A. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch. Microbiol. 1996, 165, 377–386. [Google Scholar] [CrossRef]
- Ali Shah, A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeterior. Biodegrad. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Jendrossek, D.; Reinhardt, S. Sequence analysis of a gene product synthesized by Xanthomonas sp. during growth on natural rubber latex. FEMS Microbiol. Lett. 2003, 224, 61–65. [Google Scholar] [CrossRef]
- Jendrossek, D.; Birke, J. Rubber oxygenases. Appl. Microbiol. Biotechnol. 2019, 103, 125–142. [Google Scholar] [CrossRef]
- Oetermann, S.; Jongsma, R.; Coenen, A.; Keller, J.; Steinbüchel, A. LcpR vh2-regulating the expression of latex-clearing proteins in Gordonia polyisoprenivorans vh2. Microbiology 2019, 165, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Tenberge, K.B.; Steinbüchel, A. Identification and characterization of genes from Streptomyces sp. strain k30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 2005, 6, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Linos, A.; Steinbüchel, A.; Spröer, C.; Kroppenstedt, R.M. Gordonia polyisoprenivorans sp. nov., a rubber-degrading actinomycete isolated from an automobile tyre. Int. J. Syst. Bacteriol. 1999, 49, 1785–1791. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Dworakowska, S.; Reis, A.; Gouveia, L.; Matos, C.T.; Bogdał, D.; Bogel-Łukasik, R. Chemical and biological-based isoprene production: Green metrics. Catal. Today 2015, 239, 38–43. [Google Scholar] [CrossRef]
- Carrión, O.; Larke-Mejía, N.L.; Gibson, L.; Farhan Ul Haque, M.; Ramiro-García, J.; McGenity, T.J.; Murrell, J.C. Gene probing reveals the widespread distribution, diversity and abundance of isoprene-degrading bacteria in the environment. Microbiome 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Length (bp) | 5,171,066 |
| Undetermined bases | 0 |
| GC (%) | 68.66 |
| Contigs | 1 |
| N50 | 5,171,066 |
| Predicted Proteins | 4975 |
| Ave. Length (aa) | 323 |
| Coding Density (%) | 93.3 |
| Completeness | 99.23% |
| Contamination | 0.52% |
| Pseudogenes | 2 |
| tRNA types | 21 |
| Total tRNAs | 52 |
| Polypeptide | Closest bona-fide Isoprene Degrader | References | Coverage % | (aa) ID% |
|---|---|---|---|---|
| IsoA | Gordonia polyisoprenivorans strain i37 IsoA | [26,27] | 99 | 85.19 |
| IsoB | Rhodococcus opacus strain PD630 IsoB | [18,38] | 100 | 57.95 |
| IsoC | Mycobacterium sp. strain AT1 IsoC | [27] | 100 | 65.77 |
| IsoD | Rhodococcus sp. AD45 IsoD | [19] | 100 | 67.3 |
| IsoE | Rhodococcus opacus strain PD630 IsoE | [18,38] | 100 | 62.96 |
| IsoF | Gordonia polyisoprenivorans strain i37 IsoF | [26,27] | 99 | 52.52 |
| IsoG | Rhodococcus opacus strain PD630 IsoG | [18,38] | 100 | 76.56 |
| IsoH | Rhodococcus sp. AD45 IsoH | [19] | 100 | 73.45 |
| IsoI | Rhodococcus sp. strain WS4 IsoI | [13] | 100 | 67.23 |
| IsoJ | Rhodococcus sp. strain WS4 IsoJ | [13] | 100 | 69 |
| AldH1 | Gordonia sp. strain OPL2 AldH1 | (in prep) | 98 | 65.3 |
| IsoG2 | Rhodococcus sp. strain WS4 IsoG | [13] | 96 | 59.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, L.; Larke-Mejía, N.L.; Murrell, J.C. Complete Genome of Isoprene Degrading Nocardioides sp. WS12. Microorganisms 2020, 8, 889. https://doi.org/10.3390/microorganisms8060889
Gibson L, Larke-Mejía NL, Murrell JC. Complete Genome of Isoprene Degrading Nocardioides sp. WS12. Microorganisms. 2020; 8(6):889. https://doi.org/10.3390/microorganisms8060889
Chicago/Turabian StyleGibson, Lisa, Nasmille L. Larke-Mejía, and J. Colin Murrell. 2020. "Complete Genome of Isoprene Degrading Nocardioides sp. WS12" Microorganisms 8, no. 6: 889. https://doi.org/10.3390/microorganisms8060889
APA StyleGibson, L., Larke-Mejía, N. L., & Murrell, J. C. (2020). Complete Genome of Isoprene Degrading Nocardioides sp. WS12. Microorganisms, 8(6), 889. https://doi.org/10.3390/microorganisms8060889





