A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Processing Water
2.3. Bioremediation through Bacillus pumilus and Candida boidinii
2.4. Bioremediation through Trichoderma harzianum
2.5. Analyses
2.6. Design of Experiment Analysis
2.7. Prediction Profiles
2.8. Multivariate ANOVA
3. Results
3.1. Bacllus pumilus/Candida boidinii
3.2. Trichoderma harzianum
3.3. Effect on the Colour and on the Biomass
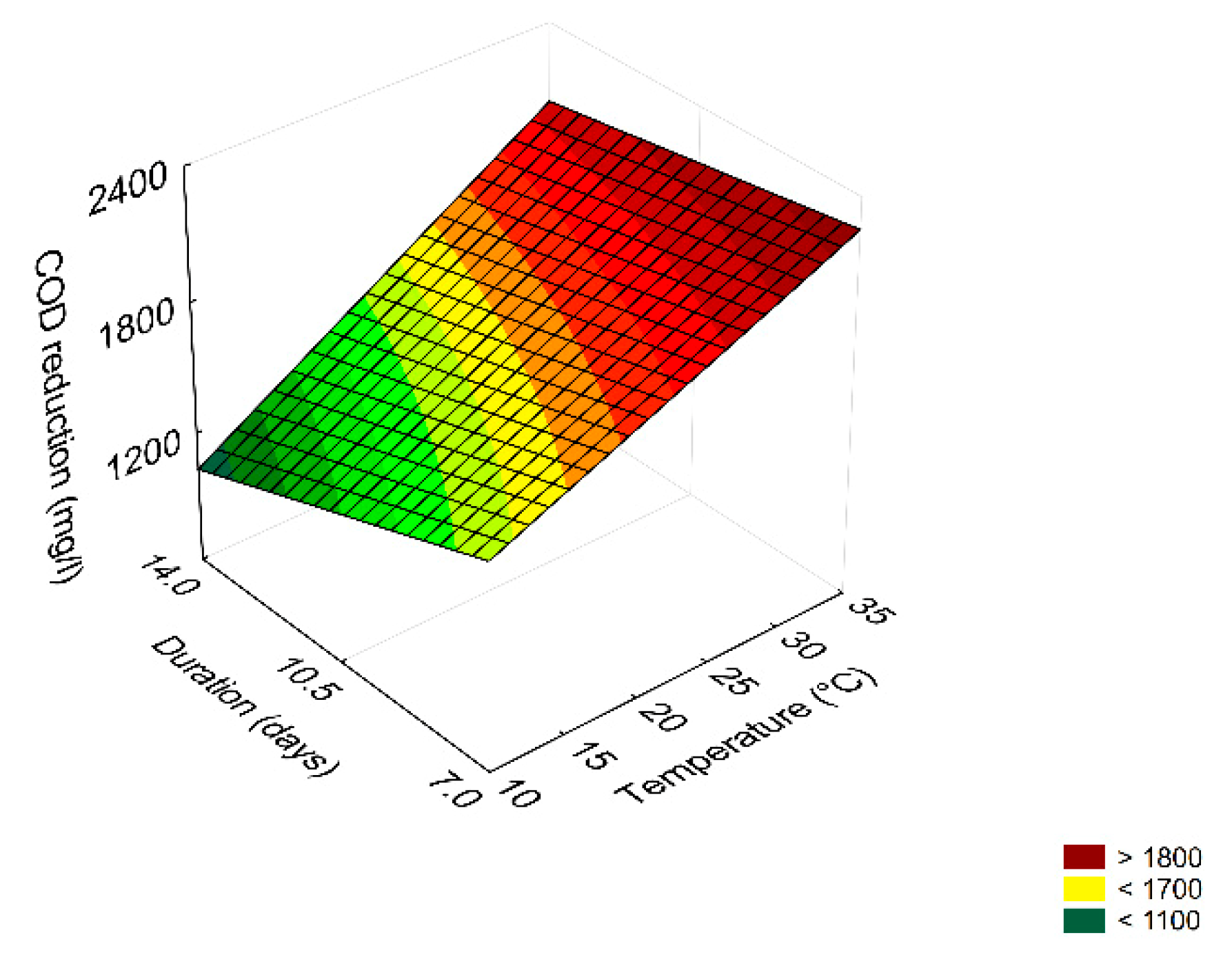
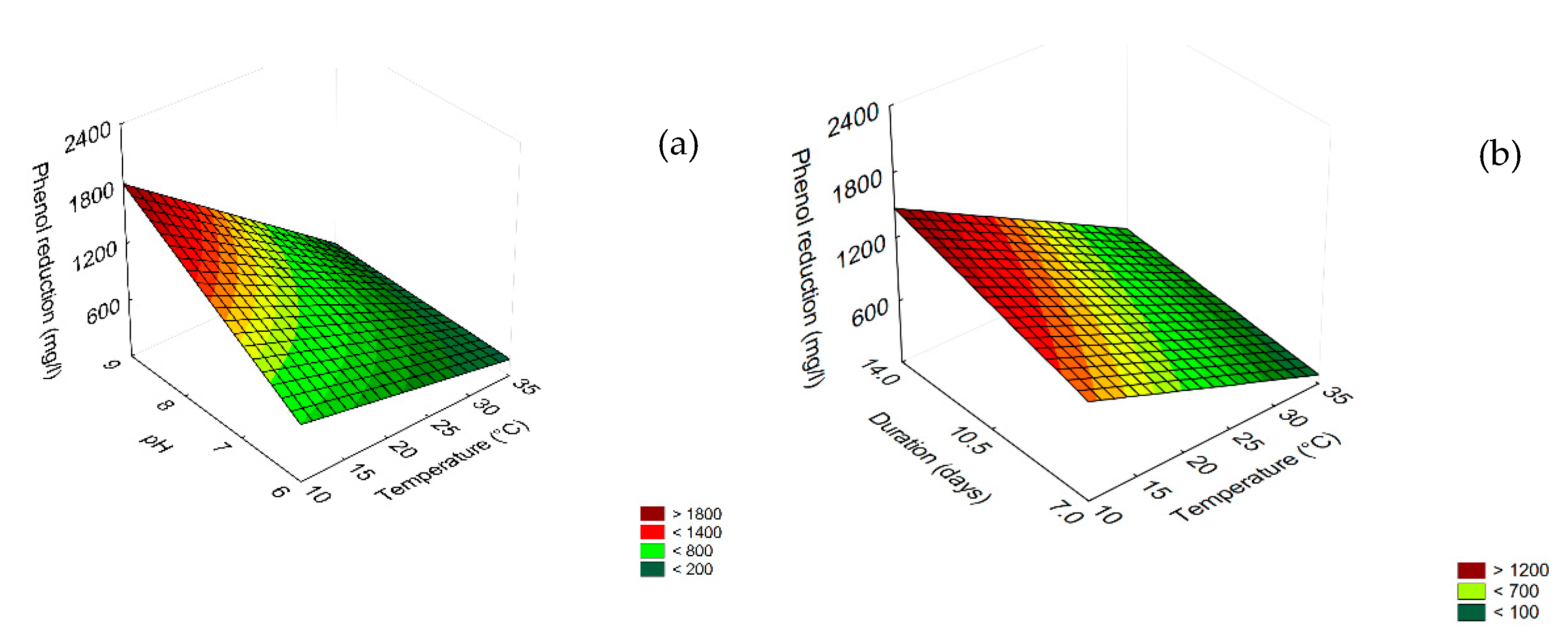
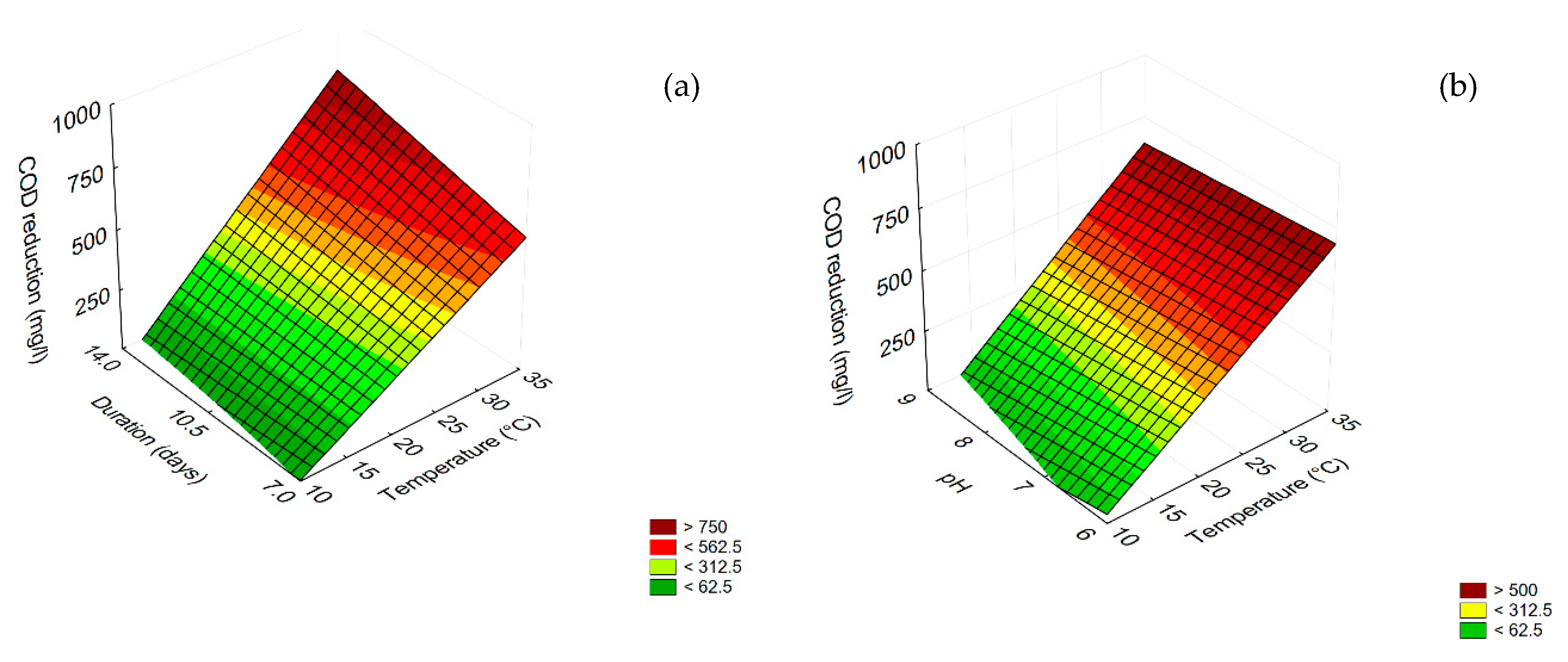
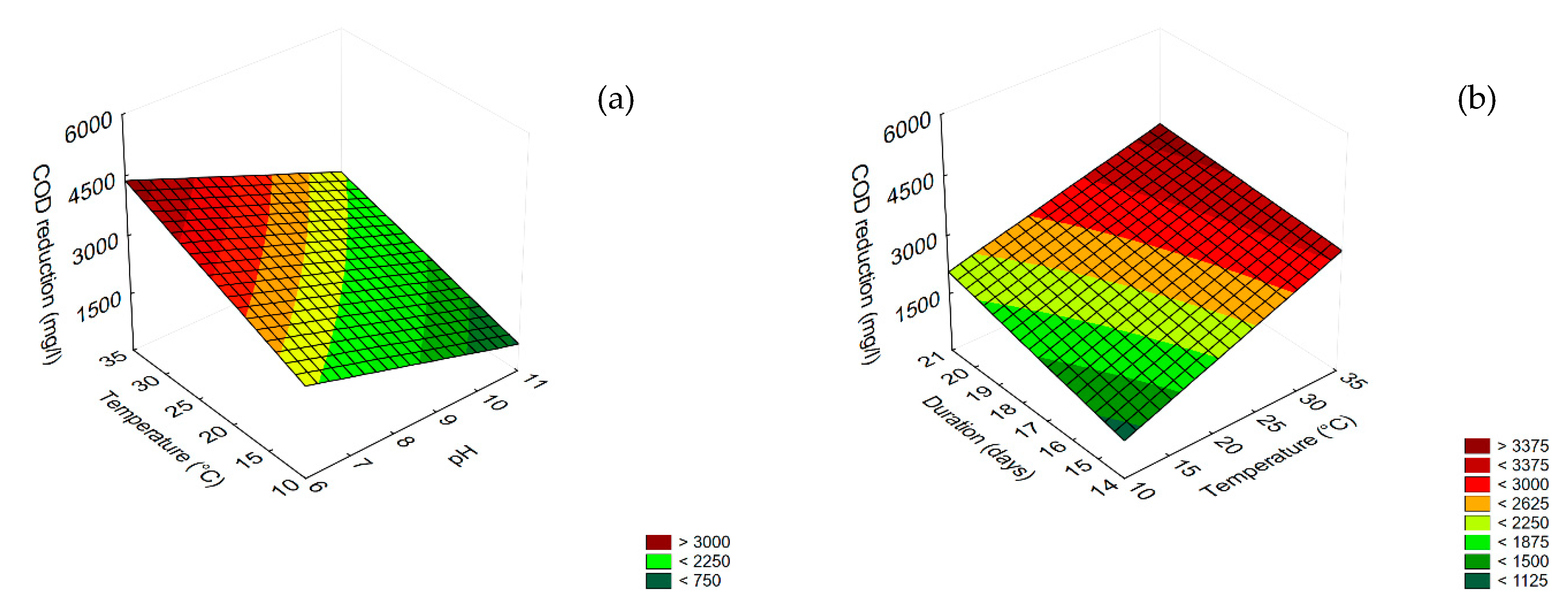
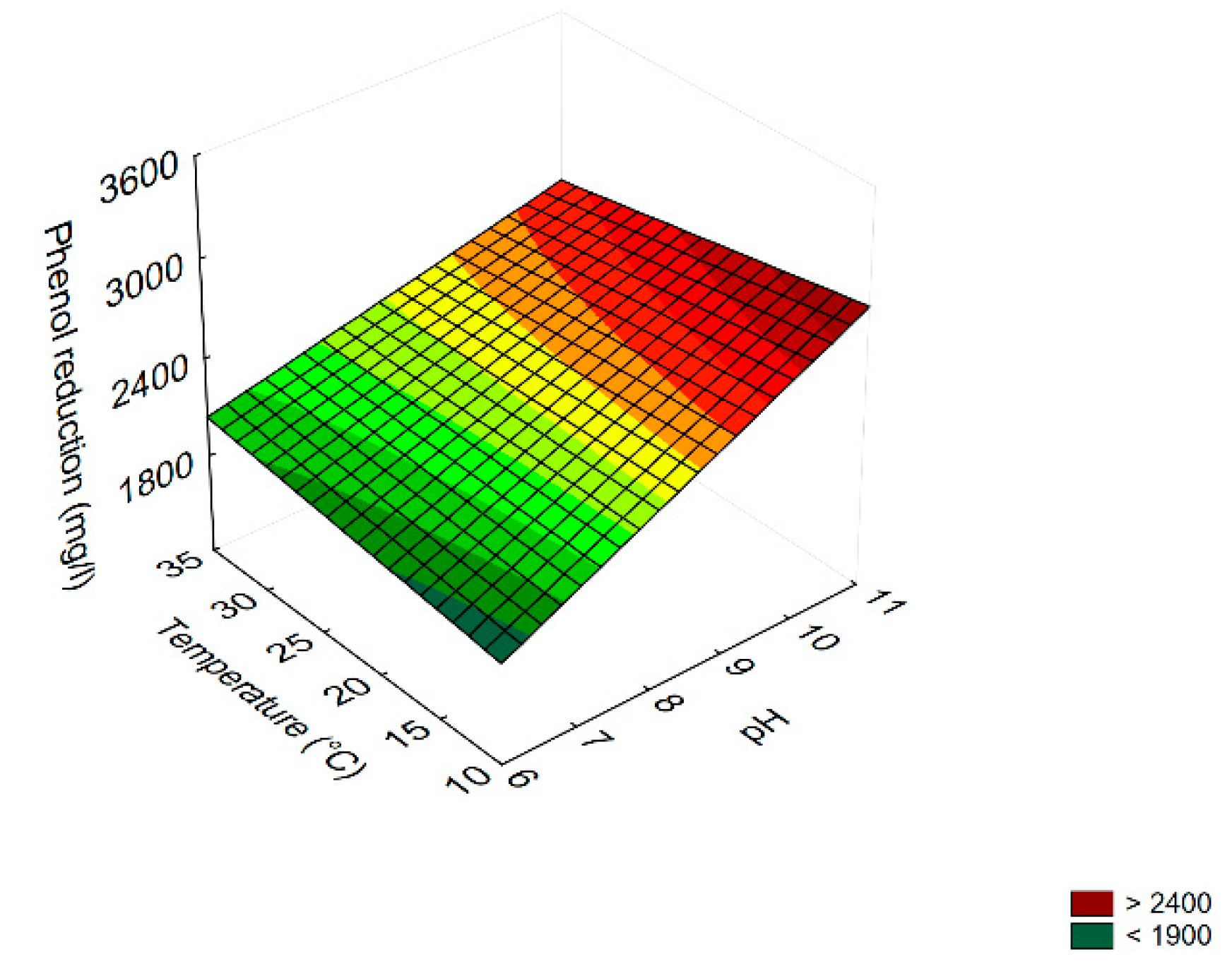
3.4. Prediction
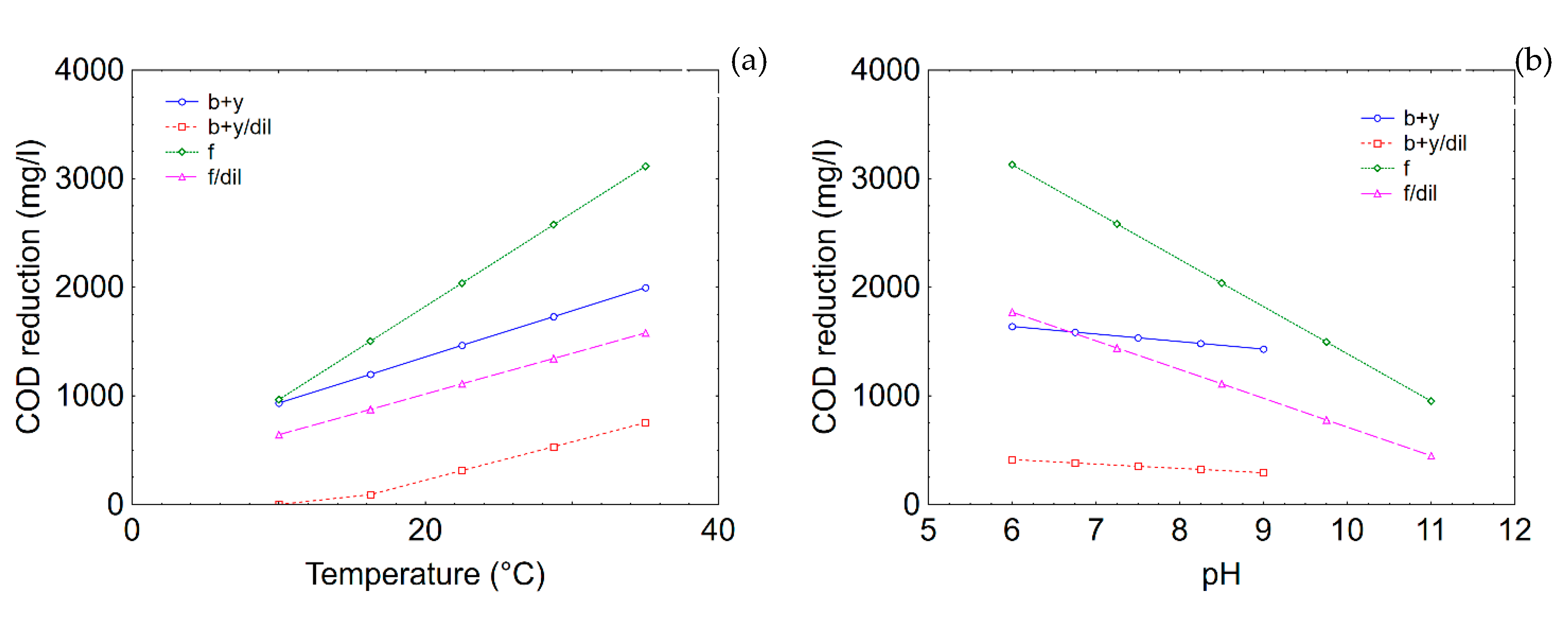
3.5. Effect of Dilution
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Rincón-Llorente, B.; De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table olive wastewater: Problem, treatments and future strategy. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [PubMed]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial waste-water resuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Papadaki, E.; Tsimidou, M.Z.; Mantzouridou, F.T. Changes in phenolic compounds and phytotoxicity of the Spanish-style green olive processing wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 2018, 66, 4891–4901. [Google Scholar] [CrossRef] [PubMed]
- Ben Othman, N.; Ayed, L.; Assas, N.; Kachouri, F.; Hammami, M.; Hamdi, M. Ecological removal of recalcitrant phenolic compounds of treated olive mill wastewater by Pediococcus pentosauceus. Bioresour. Biotechnol. 2008, 99, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Augello, S.; De Stefano, F.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Bioremediation of wastes of table olives and olive oil: Environment-friendly approaches. Ind. Aliment. 2014, 547, 5–11. [Google Scholar]
- Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Selection of promising bacterial strains as potential tools for the bioremediation of olive mill wastewater. In Wastewater Treatment Engineering; Samer, M., Ed.; InTech Publisher: Rijeka, Croatia, 2013; pp. 85–100. [Google Scholar] [CrossRef][Green Version]
- Sinigaglia, M.; Di Benedetto, N.; Bevilacqua, A.; Corbo, M.R.; Capece, A.; Romano, P. Yeasts isolated from olive mill wastewaters from Southern Italy: Technological characterization and potential use for phenol removal. Appl. Microbiol. Biotechnol. 2010, 87, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Cibelli, F.; Raimondo, M.L.; Carlucci, A.; Lops, F.; Sinigaglia, M.; Corbo, M.R. Fungal bioremediation of olive mill wastewater: Using a multi-step approach to model inhibition or stimulation. J. Sci. Food Agric. 2017, 97, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, F.; Bevilacqua, A.; Raimondo, M.L.; Campaniello, D.; Carlucci, A.; Ciccarone, C.; Sinigaglia, M.; Corbo, M.R. Evaluation of fungal growth on olive-mill wastewaters treated at high temperature and by high pressure homogenization. Front. Microbiol. 2017, 8, 2515. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cormenzana, A.; Juarez-Jimenez, B.; Garcia-Pareja, M.P. Antimicrobial activity of olive mill wastewaters (Alpechin) and biotransformed olive oil mill wastewater. Int. Biodeterior. Biodegrad. 1996, 38, 283–290. [Google Scholar] [CrossRef]
- Ayed, L.; Asses, N.; Chammen, N.; Othman, N.B.; Hamdi, M. Advanced oxidation process and biological treatments for table olive processing wastewaters: Constraints and a novel approach to integrated recycling process: A review. Biodegradation 2017, 28, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lasaridi, K.E.; Chroni, C.; Fortatos, S.; Chatzipavlidis, I.; Kyriacou, A. Estimating the bioremediation of green table olive processing wastewater using a selected strain of Aspergillus niger. Desalin. Water Treat. 2010, 23, 26–31. [Google Scholar] [CrossRef]
- Kyriacou, A.; Lasaridi, K.E.; Kotsou, M.; Balis, C.; Pilidis, G. Combined bioremediation and advanced oxidation of green table olives processing wastewater. Process Biochem. 2005, 40, 1401–1408. [Google Scholar] [CrossRef]
- Ayed, L.; Chammen, N.; Asses, N.; Hamdi, M. Optimization of biological pretreatment of green table olive processing wastewaters using Aspergillus niger. J. Bioremediat. Biodegrad. 2013, 4, 212. [Google Scholar] [CrossRef]
- D’Urso, A.; Gapes, D.; Bravi, M. Bioremediation of Olive Oil Mill Wastewaters by Fungal Trichoderma Viride, Strain 8/90sSequencing Batch Reactor. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.541.3308&rep=rep1&type=pdf (accessed on 15 May 2020).
- Lawrance, I.; Sivaranjani, V.; Selvakumar, A.M.; Khambhaty, Y.; Saravan, P. Biodegradation of phenolic resin used in leather processing by laccase producing Trichoderma aureoviridae. Int. J. Environ. Sci. Technol. 2019, 16, 6857–6862. [Google Scholar] [CrossRef]
- Saravanan, S.; Kumari, A.; Sriram, A. Comparative study on treatment of cassava wastewater using free, immobilized and biofilm of Trichoderma harzianum-Trichoderma viride consortium. Water Desalin. Treat. 2019, 156, 370–377. [Google Scholar] [CrossRef]
- Mendoça, E.; Pereira, P.; Martins, A.; Anselmo, A.M. Fungal biodegradation and detoxification of boiling cork water. Eng. Life Sci. 2004, 4, 144–149. [Google Scholar] [CrossRef]
- Sharma, S.; Malaviya, P. Decolorization and detoxification of tannery wastewater by Trichoderma viride SPFT1. Environ. Eng. Manag. J. 2018, 17, 545–550. [Google Scholar]
- Aggelis, G.; Ehaliotis, C.; Nerud, F.; Stoychev, I.; Lyberatos, G.; Zervakis, G.I. Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Appl. Microbiol. Biotechnol. 2002, 59, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, M.; Kyriacou, A.; Lasaridi, K.; Pilidis, G. Integrated aerobic biological treatment and chemical oxidation with Fenton’s reagent for the processing of green table olive wastewater. Process Biochem. 2004, 39, 1653–1660. [Google Scholar] [CrossRef]
- Dyan, M.O.; Putra, G.P.; Budiyono, B.; Sumardiono, S.; Kusworo, T.D. The effect of pH and operation mode for COD removal of slaughterhouse wastewater with Anaerobic Batch Reactor (ABR). Waste Technol. 2015, 3, 7–13. [Google Scholar] [CrossRef]
- Kapoor, R.K.; Raian, K.; Carrier, D.J. Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour. Biotechnol. 2015, 189, 99–106. [Google Scholar] [CrossRef] [PubMed]




| Combinations | Temperature (°C) | pH | Duration (days) |
|---|---|---|---|
| A | 10 | 6 | 7 |
| B | 10 | 6 | 14 |
| C | 10 | 9 | 7 |
| D | 10 | 9 | 14 |
| E | 35 | 6 | 7 |
| F | 35 | 6 | 14 |
| G | 35 | 9 | 7 |
| H | 35 | 9 | 14 |
| Combinations | Temperature (°C) | pH | Duration (Days) |
|---|---|---|---|
| A | 10 | 6 | 14 |
| B | 10 | 6 | 21 |
| C | 10 | 11 | 14 |
| D | 10 | 11 | 21 |
| E | 35 | 6 | 14 |
| F | 35 | 6 | 21 |
| G | 35 | 11 | 14 |
| H | 35 | 11 | 21 |
| Bacillus/Candida | ||||
|---|---|---|---|---|
| TOPW | Diluted TOPW | |||
| COD Red. | Phenol Red. | COD Red. | Phenol Red. | |
| Temperature | 857.25 | −1000.25 | 700.75 | - |
| pH | - | 626.38 | −97.75 | - |
| Duration | −371.75 | 384.50 | - | - |
| Temp × pH | - | −582.87 | - | - |
| Temp × Dur | - | - | 152.25 | - |
| pH × dur | - | - | - | - |
| R2ad | 0.997 | 0.998 | 0.999 | |
| SE | 20.25 | 20.38 | 105.13 | |
| T. harzianum | ||||
| TOPW | Diluted TOPW | |||
| COD Red. | Phenol Red. | COD Red. | Phenol Red. | |
| Temperature | 1820.37 | - | 815.25 | 491.00 |
| pH | −1869.13 | 809.06 | −1375.25 | 1645.25 |
| Duration | 779.63 | - | 628 | −956.00 |
| Temp × pH | - | -278.06 | - | −668.13 |
| Temp × Dur | −329.37 | - | - | 381.23 |
| pH × dur | 305.62 | - | - | 923.13 |
| R2ad | 0.976 | 0.825 | 0.926 | 0.935 |
| SE | 109.23 | 98.60 | 121.02 | 154.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campaniello, D.; Carlucci, A.; Speranza, B.; Raimondo, M.L.; Cibelli, F.; Rosaria Corbo, M.; Bevilacqua, A. A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms 2020, 8, 878. https://doi.org/10.3390/microorganisms8060878
Campaniello D, Carlucci A, Speranza B, Raimondo ML, Cibelli F, Rosaria Corbo M, Bevilacqua A. A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms. 2020; 8(6):878. https://doi.org/10.3390/microorganisms8060878
Chicago/Turabian StyleCampaniello, Daniela, Antonia Carlucci, Barbara Speranza, Maria Luisa Raimondo, Francesca Cibelli, Maria Rosaria Corbo, and Antonio Bevilacqua. 2020. "A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water" Microorganisms 8, no. 6: 878. https://doi.org/10.3390/microorganisms8060878
APA StyleCampaniello, D., Carlucci, A., Speranza, B., Raimondo, M. L., Cibelli, F., Rosaria Corbo, M., & Bevilacqua, A. (2020). A Comparative Study on Trichoderma harzianum and a Combination of Candida/Bacillus as Tools for the Bioremediation of Table Olive Processing Water. Microorganisms, 8(6), 878. https://doi.org/10.3390/microorganisms8060878







