In vivo Fitness of Acinetobacter baumannii Strains in Murine Infection Is Associated with International Lineage II-rep-2 and International Lineage III Clones Showing High Case Fatality Rates in Human Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Growth Curve Assay
2.3. Murine Infection Models
2.4. Thigh Muscle Model
2.5. Sepsis Model
2.6. Statistical Analysis
2.7. Ethics
3. Results
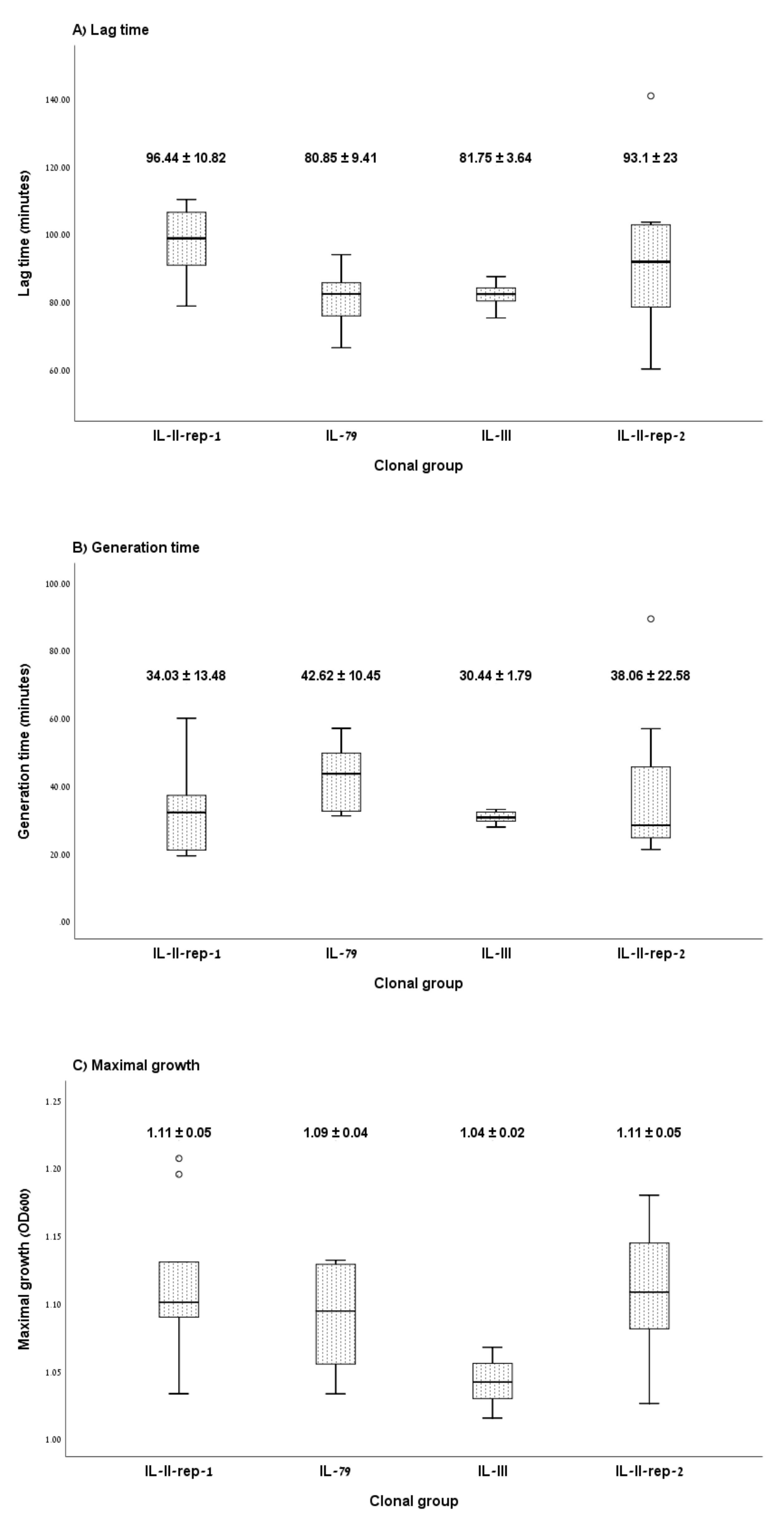
3.1. Growth Curve Assay
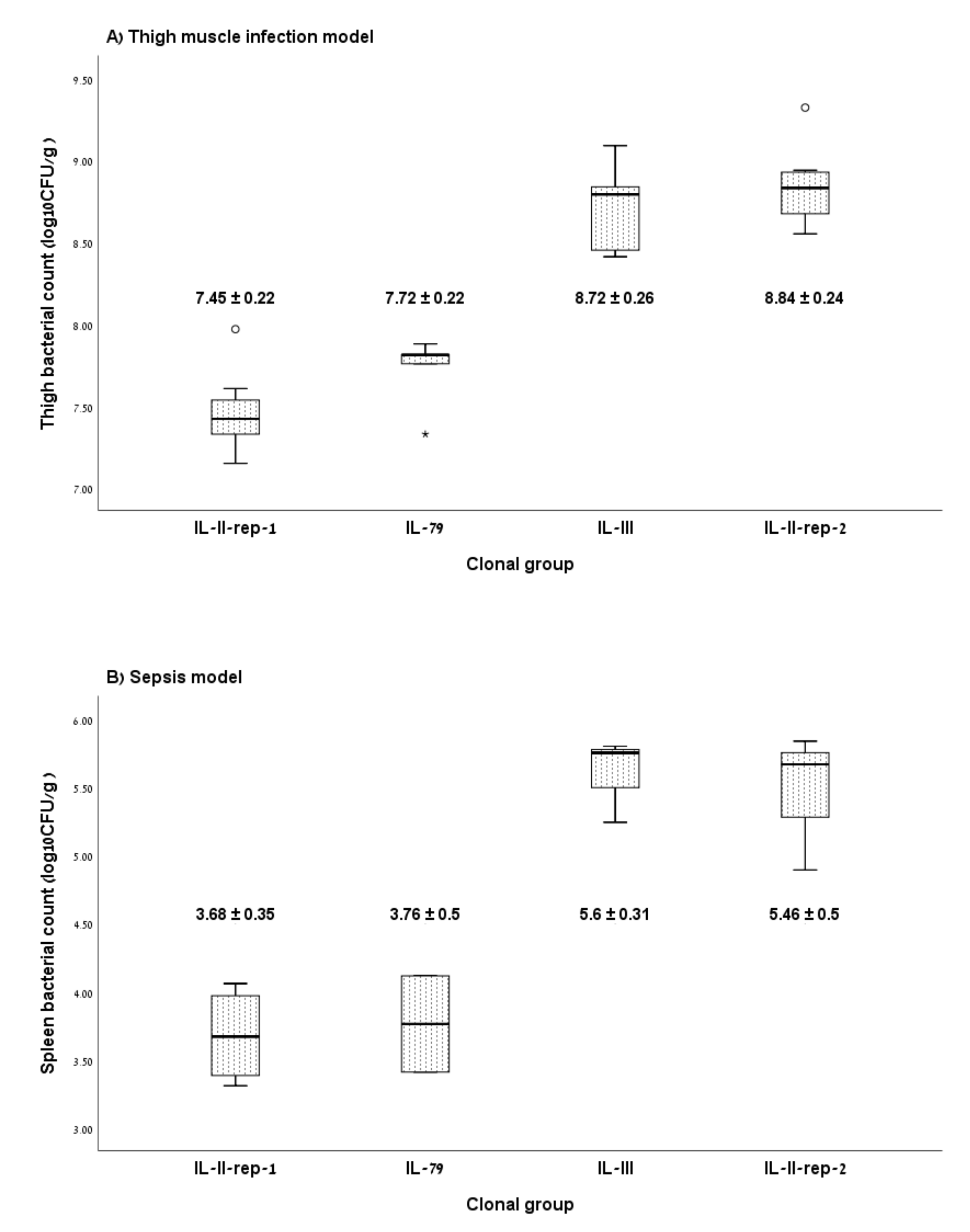
3.2. Murine Infection Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [Green Version]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options Against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Lemos, E.V.; de la Hoz, F.P.; Alvis, N.; Einarson, T.R.; Quevedo, E.; Castañeda, C.; Leon, Y.; Amado, C.; Cañon, O.; Kawai, K. Impact of carbapenem resistance on clinical and economic outcomes among patients with Acinetobacter baumannii infection in Colombia. Clin. Microbiol. Infect. 2014, 20, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Beceiro, A.; Moreno, A.; Fernández, N.; Vallejo, J.A.; Aranda, J.; Adler, B.; Harper, M.; Boyce, J.D.; Bou, G. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.F.; McHugh, T.D.; Gillespie, S.H. Methods to determine fitness in bacteria. Methods Mol. Biol. 2010, 642, 113–121. [Google Scholar] [CrossRef]
- Yoon, E.J.; Balloy, V.; Fiette, L.; Chignard, M.; Courvalin, P.; Grillot-Courvalin, C. Contribution of the ade resistance-nodulation-cell division-type efflux pumps to fitness and pathogenesis of Acinetobacter baumannii. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, A.C.; Hood, I.; Boyd, K.L.; Olson, P.D.; Morrison, J.M.; Carson, S.; Sayood, K.; Iwen, P.C.; Skaar, E.P.; Dunman, P.M. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect. Immun. 2010, 78, 1952–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eveillard, M.; Soltner, C.; Kempf, M.; Saint-André, J.P.; Lemarié, C.; Randrianarivelo, C.; Seifert, H.; Wolff, M.; Joly-Guillou, M.L. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J. Infect. 2010, 60, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkshoorn, L.; Brouwer, C.P.J.M.; Bogaards, S.J.P.; Nemec, A.; Van Den Broek, P.J.; Nibbering, P.H. The synthetic n-terminal peptide of human lactoferrin, hLF(1-11), is highly effective against experimental infection caused by multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2004, 48, 4919–4921. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Tegos, G.P.; Lu, Z.; Huang, L.; Zhiyentayev, T.; Franklin, M.J.; Baer, D.G.; Hamblin, M.R. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 2009, 53, 3929–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddy, J.A.; Actis, L.A.; Arivett, B.A.; Mcconnell, M.J.; Rafael, L.R.; Pachón, J. Role of Acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 2012, 80, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, W.C.; Lee, H.C.; Chiang, S.R.; Yan, J.J.; Wu, J.J.; Lu, C.L.; Chuang, Y.C. In vitro and in vivo activity of meropenem and sulbactam against a multidrug-resistant Acinetobacter baumannii strain. J. Antimicrob. Chemother. 2004, 53, 393–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neou, E.; Michail, G.; Tsakris, A.; Pournaras, S. Virulence of Acinetobacter baumannii Exhibiting Phenotypic Heterogeneous Growth against Meropenem in a Murine Thigh Infection Model. Antibiotics 2013, 2, 73–82. [Google Scholar] [CrossRef]
- Fan, B.; Guan, J.; Wang, X.; Cong, Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Tsuji, M.; Yamano, Y.; Echols, R.; Nicolaua, D.P. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Gaiarsa, S.; Batisti Biffignandi, G.; Esposito, E.P.; Castelli, M.; Jolley, K.A.; Brisse, S.; Sassera, D.; Zarrilli, R. Comparative analysis of the two Acinetobacter baumannii multilocus sequence typing (MLST) schemes. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pournaras, S.; Gogou, V.; Giannouli, M.; Dimitroulia, E.; Dafopoulou, K.; Tsakris, A.; Zarrilli, R. Single-locus-sequence-based typing of blaOXA-51-like genes for rapid assignment of Acinetobacter baumannii clinical isolates to international clonal lineages. J. Clin. Microbiol. 2014, 52, 1653–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutman, A.; Glick, R.; Temkin, E.; Hoshen, M.; Edgar, R.; Braun, T.; Carmeli, Y. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin. Microbiol. Infect. 2014, 20, O1028–O1034. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, F.R.; Wikler, M.A.; Bush, K.; Dudley, M.N.; Eliopoulos, G.M.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; Powell, M.; et al. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational SupplementClinical and Laboratory Standards Institute: New York, NY, USA, 2011; Volume 31, ISBN 1562387421.
- Vila, J.; Marcos, M.A.; Jimenez De Anta, M.T. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus-A. baumannii complex. J. Med. Microbiol. 1996, 44, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Bou, G.; Cerveró, G.; Domínguez, M.A.; Quereda, C.; Martínez-Beltrán, J. PCR-based DNA fingerprinting (REP-PCR, AP-PCR) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2000, 6, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.M.; Bratchell, N.; Roberts, T.A. Predicting microbial growth: Growth responses of Salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int. J. Food Microbiol. 1988, 6, 155–178. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [Green Version]
- Giannouli, M.; Antunes, L.C.S.; Marchetti, V.; Triassi, M.; Visca, P.; Zarrilli, R. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect. Dis. 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Luna, B.M.; Yan, J.; Reyna, Z.; Moon, E.; Nielsen, T.B.; Reza, H.; Lu, P.; Bonomo, R.; Louie, A.; Drusano, G.; et al. Natural history of Acinetobacter baumannii infection in mice. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Esterly, J.S.; McLaughlin, M.M.; Malczynski, M.; Qi, C.; Zembower, T.R.; Scheetz, M.H. Pathogenicity of clinical Acinetobacter baumannii isolates in a Galleria mellonella host model according to blaOXA-40 gene and epidemiological outbreak status. Antimicrob. Agents Chemother. 2014, 58, 1240–1242. [Google Scholar] [CrossRef] [Green Version]
- Peleg, A.Y.; Jara, S.; Monga, D.; Eliopoulos, G.M.; Moellering, R.C.; Mylonakis, E. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 2009, 53, 2605–2609. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.A.; Beceiro, A.; Rumbo-Feal, S.; Rodríguez-Palero, M.J.; Russo, T.A.; Bou, G. Optimisation of the Caenorhabditis elegans model for studying the pathogenesis of opportunistic Acinetobacter baumannii. Int. J. Antimicrob. Agents 2015. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutman, A.; Lellouche, J.; Lifshitz, Z.; Glick, R.; Carmeli, Y. In vivo Fitness of Acinetobacter baumannii Strains in Murine Infection Is Associated with International Lineage II-rep-2 and International Lineage III Clones Showing High Case Fatality Rates in Human Infections. Microorganisms 2020, 8, 847. https://doi.org/10.3390/microorganisms8060847
Nutman A, Lellouche J, Lifshitz Z, Glick R, Carmeli Y. In vivo Fitness of Acinetobacter baumannii Strains in Murine Infection Is Associated with International Lineage II-rep-2 and International Lineage III Clones Showing High Case Fatality Rates in Human Infections. Microorganisms. 2020; 8(6):847. https://doi.org/10.3390/microorganisms8060847
Chicago/Turabian StyleNutman, Amir, Jonathan Lellouche, Ziv Lifshitz, Rivka Glick, and Yehuda Carmeli. 2020. "In vivo Fitness of Acinetobacter baumannii Strains in Murine Infection Is Associated with International Lineage II-rep-2 and International Lineage III Clones Showing High Case Fatality Rates in Human Infections" Microorganisms 8, no. 6: 847. https://doi.org/10.3390/microorganisms8060847
APA StyleNutman, A., Lellouche, J., Lifshitz, Z., Glick, R., & Carmeli, Y. (2020). In vivo Fitness of Acinetobacter baumannii Strains in Murine Infection Is Associated with International Lineage II-rep-2 and International Lineage III Clones Showing High Case Fatality Rates in Human Infections. Microorganisms, 8(6), 847. https://doi.org/10.3390/microorganisms8060847




