Dry-Caribbean Bacillus spp. Strains Ameliorate Drought Stress in Maize by a Strain-Specific Antioxidant Response Modulation
Abstract
1. Introduction
2. Materials and Methods
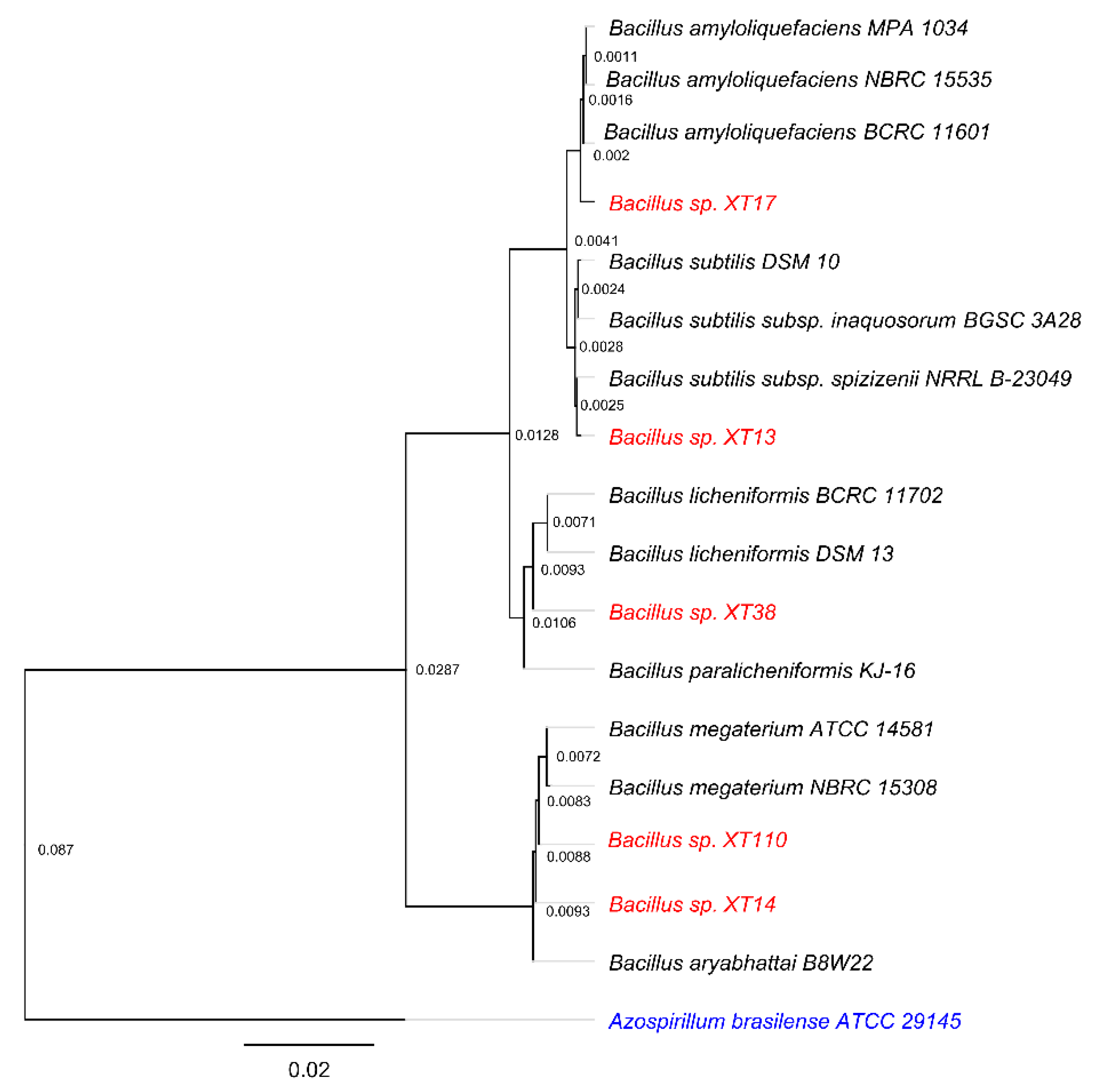
2.1. Strains, Phylogenetic Analysis and Culture Conditions
2.2. Plant Growth-Promoting (PGP) Traits
2.3. Effect of Drought and Bacillus spp. Inoculation on Maize Plant Growth
2.3.1. Antioxidant Enzymatic Activities and Proline Content in Plant Tissue
2.3.2. Determination of P+, K+, and Ca2+ in Plant Tissue
2.4. Statistical Analysis
3. Results
3.1. Strains Characterization and Plant Growth-Promoting (PGP) Traits
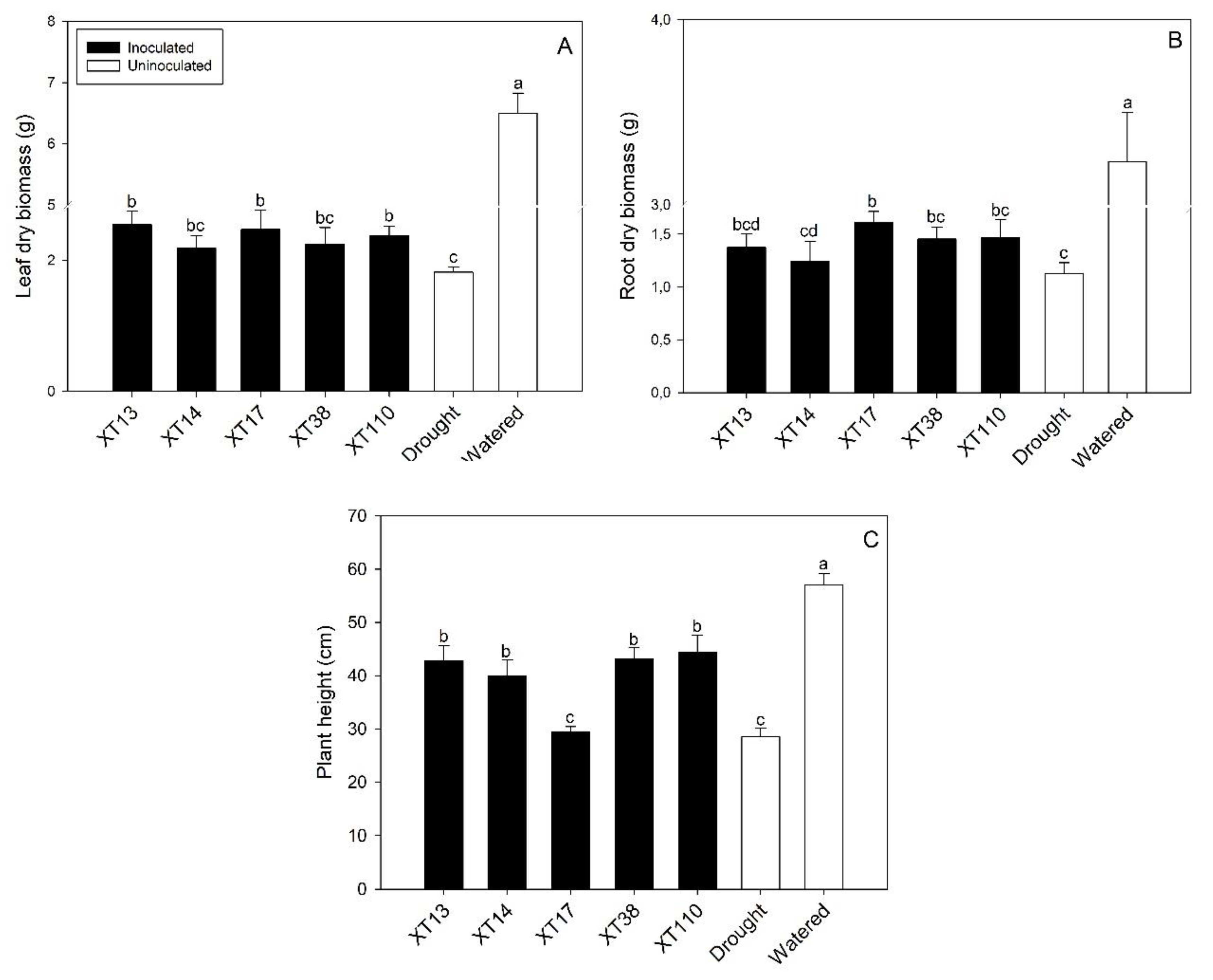
3.2. Bacillus spp. Inoculation Promotes Maize Growth and Nutrient Uptake under Drought Stress
3.3. Bacillus spp. Differentially Modulates Antioxidant Response in Maize Inducing Drought Stress Mitigation
3.4. Correlation between Plant Growth and Antioxidant Response with Bacillus Inoculation under Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P.; Dey, P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2013, 29, 789–803. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Santos, S.N.; da Silva, J.L.; Parma, M.M.; Ávila, L.A.; Visconti, A.; Zucchi, T.D.; Taketani, R.; Andreote, F.D.; de Melo, I.S. Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol. Res. 2013, 168, 183–191. [Google Scholar] [CrossRef]
- Su, A.-Y.; Niu, S.-Q.; Liu, Y.-Z.; He, A.-L.; Zhao, Q.; Paré, P.; Li, M.-F.; Han, Q.-Q.; Ali Khan, S.; Zhang, J.-L. Synergistic effects of Bacillus amyloliquefaciens (GB03) and water retaining agent on drought tolerance of perennial ryegrass. Int. J. Mol. Sci. 2017, 18, 2651. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Gepstein, S.; Glick, B.R. Strategies to ameliorate abiotic stress-induced plant senescence. Plant Mol. Biol. 2013, 82, 623–633. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Claeys, H.; Van Landeghem, S.; Dubois, M.; Maleux, K.; Inzé, D. What is stress? Dose-response effects in commonly used in vitro stress assays. Plant Physiol. 2014, 165, 519–527. [Google Scholar] [CrossRef]
- Daffonchio, D.; Hirt, H.; Berg, G. Plant-microbe interactions and water management in arid and saline soils. In Principles of Plant-Microbe Interactions; Springer International Publishing: Cham, Switzerland, 2015; pp. 265–276. [Google Scholar] [CrossRef]
- Szymanska, R.; Latowski, D.; Nowicka, B.; Strzałka, K. Chapter 10—Lipophilic molecules as a part of antioxidant system in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 321–344. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil 2019. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.; Pandey, P.; Jha, C.K.; Aeron, A. Bacilli and Agrobiotechnology; Springer: Cham, Switzerland, 2016; pp. 1–416. [Google Scholar] [CrossRef]
- Reddy, C.A.; Saravanan, R.S. Chapter Three—Polymicrobial Multi-functional Approach for Enhancement of Crop Productivity. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 82, pp. 53–113. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2012, 35, 1381–1396. [Google Scholar] [CrossRef]
- Moreno-Galván, A.E.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl. Soil Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, H.H.P. Influence of diazotrophic bacteria on antioxidant enzymes and some biochemical characteristics of soybean subjected to water stress. Biotechnol. Lett. 2001, 23, 405–409. [Google Scholar] [CrossRef]
- Ali, L.; Khalid, M.; Asghar, H.N.; Asgher, M. Scrutinizing of rhizobacterial isolates for improving drought resilience in maize (Zea mays). Int. J. Agric. Biol. 2017, 19, 1054–1064. [Google Scholar] [CrossRef]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016, 192, 1–12. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.P.H. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Lyngwi, N.A.; Nongkhlaw, M.; Kalita, D.; Joshi, S.R. Bioprospecting of Plant Growth Promoting Bacilli and Related Genera Prevalent in Soils of Pristine Sacred Groves: Biochemical and Molecular Approach. PLoS ONE 2016, 11, e0152951. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Estrada, G.A.; Baldani, V.L.D.; de Oliveira, D.M.; Urquiaga, S.; Baldani, J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 2013, 369, 115–129. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 29, 677. [Google Scholar] [CrossRef]
- Shulse, C.N.; Chovatia, M.; Agosto, C.; Yoshikuni, G.Y.; Hamilton, M.; Deutsch, S.; Yoshikuni, Y.; Blow, M.J. Engineered root bacteria release plant-available phosphate from phytate. Appl. Environ. Microbiol. 2019, 85, e01210-19. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]
- Meneses, C.H.S.G.; Rouws, L.F.M.; Simões-Araújo, J.L.; Vidal, M.S.; Baldani, J.I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol. Plant-Microbe Interact. 2011, 24, 1448–1458. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Hassen, A.I.; Labuschagne, N. Rhizobacteria-induced systemic tolerance against drought stress in Sorghum bicolor (L.) Moench. Microbiol. Res. 2020, 232, 126388. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance. Methods in Molecular Biology; Sunkar, R., Ed.; Human Press: Totowa, NJ, USA, 2010; pp. 273–280. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shirinbayan, S.; Khosravi, H.; Malakouti, M.J. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl. Soil Ecol. 2019, 133, 138–145. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V.; Sandhya, V.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Armada, E.; Roldán, A.; Azcon, R. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2014, 67, 410–420. [Google Scholar] [CrossRef]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2010, 125, 173–184. [Google Scholar] [CrossRef]
- Jemo, M.; Sulieman, S.; Bekkaoui, F.; Olomide, O.A.K.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Tran, L.-S.P. Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 2017, 8, 2111. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ying, Y.Q.; Zhang, Y.B.; Bi, Y.F.; Wang, A.K.; Du, X.H. Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci. Rep. 2018, 8, 228. [Google Scholar] [CrossRef]
- Meneses, C.; Gonçalves, T.; Alquéres, S.; Rouws, L.; Serrato, R.; Vidal, M.; Baldani, J.I. Gluconacetobacter diazotrophicus exopolysaccharide protects bacterial cells against oxidative stress in vitro and during rice plant colonization. Plant Soil 2017, 416, 133–147. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Vidal, M.; Baldani, J.I.; Meneses, C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020, 21, 333. [Google Scholar] [CrossRef]
- Sukumar, P.; Legué, V.; Vayssières, A.; Martin, F.; Tuskan, G.; Kalluri, U.C. Involvement of auxin pathways in modulating root architecture during beneficial plant-microorganism interactions. Plant Cell Environ. 2013, 36, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Zhou, M.L.; Zhang, Q.; Sun, Z.M.; Chen, L.H.; Liu, B.X.; Zhang, K.X.; Zhu, X.M.; Shao, J.R.; Tang, Y.X.; Wu, Y.M. Trehalose metabolism-related genes in maize. J. Plant Growth Regul. 2014, 33, 256–271. [Google Scholar] [CrossRef]
- Azarmi, F.; Mozafari, V.; Abbaszadeh Dahaji, P.; Hamidpour, M. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol. Plant. 2016, 38, 1–16. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Pakniyat, H.; Pirasteh-Anosheh, H.; Azooz, M.M. Role of ROS as Signaling Molecules in Plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 585–620. [Google Scholar] [CrossRef]
- Surender Reddy, P.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Hameed, A.; Sharma, I.; Kumar, A.; Azooz, M.; Ahmad Lone, H.; Ahmad, P. Chapter 6—Glutathione Metabolism in Plants under Environmental Stress. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 183–200. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gładysz, O.; Szentner, K.; Goliński, P. Chapter 5—Role of Glutathione in Abiotic Stress Tolerance. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 149–181. [Google Scholar] [CrossRef]


| Treatment | Description | Drought (Days) 1 |
|---|---|---|
| Watered | Uninoculated control | None |
| Drought | Uninoculated control | 33 |
| XT13 | Seeds inoculated with the XT13 strain | 33 |
| XT14 | Seeds inoculated with the XT14 strain | 33 |
| XT17 | Seeds inoculated with the XT17 strain | 33 |
| XT38 | Seeds inoculated with the XT38 strain | 33 |
| XT110 | Seeds inoculated with the XT110 strain | 33 |
| pH | O.M | Ca | Mg | K | Na | C.E.C | Fe | Mn | Zn | Cu | P | S | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | cmol(+) kg−1 | mg kg−1 | |||||||||||
| 6.97 | 2.50 | 7.04 | 0.86 | 1.42 | 0.02 | 9.34 | 51.60 | 3.80 | 2.50 | 2.20 | 347.15 | 4.10 | 0.34 |
| Characteristics | XT13 | XT14 | XT17 | XT38 | XT110 |
|---|---|---|---|---|---|
| PGP-features | |||||
| ILM | 0.65 ± 0.00 | 0.64 ± 0.00 | 0.65 ± 0.00 | 0.64 ± 0.01 | 0.66 ± 0.00 |
| Ca3PO4 | 0.19 ± 0.01 | 0.23 ± 0.03 | 0.20 ± 0.02 | 0.19 ± 0.00 | 0.32 ± 0.02 |
| Phytate | 2.83 ± 0.44 | 4.51 ± 0.78 | 2.82 ± 0.54 | 1.13 ± 0.17 | 6.31 ± 0.24 |
| EPS | 0.06 ± 0.01 | 0.14 ± 0.00 | 0.07 ± 0.00 | 0.18 ± 0.00 | 0.07 ± 0.00 |
| ACCd | N.D. | N.D. | N.D. | N.D. | N.D. |
| Activities of hydrolytic enzymes | |||||
| Protease | + | + | + | − | + |
| Cellulase | − | − | + | + | − |
| Pectinase | + | − | + | − | − |
| α-amylase | − | − | + | + | - |
| Treatment | P+ | Ca2+ | K+ |
|---|---|---|---|
| mg g−1 Dry Tissue | |||
| XT13 | 3.27 ± 0.38 | 6.50 ± 0.17 * | 46.03 ± 1.33 * |
| XT14 | 3.73 ± 0.06 * | 8.30 ± 0.27 | 50.23 ± 0.81 * |
| XT17 | 3.20 ± 0.27 | 6.80 ± 0.20 | 46.97 ± 0.21 * |
| XT38 | 3.23 ± 0.21 | 8.30 ± 0.61 | 48.00 ± 2.01 * |
| XT110 | 3.95 ± 0.25 * | 8.40 ± 0.60 | 45.83 ± 0.96 * |
| Drought | 2.77 ± 0.12 | 7.70 ± 0.46 | 41.00 ± 2.10 |
| Watered | 2.75 ± 0.05 | 7.73 ± 0.21 | 37.10 ± 0.66 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Galván, A.; Romero-Perdomo, F.A.; Estrada-Bonilla, G.; Meneses, C.H.S.G.; Bonilla, R.R. Dry-Caribbean Bacillus spp. Strains Ameliorate Drought Stress in Maize by a Strain-Specific Antioxidant Response Modulation. Microorganisms 2020, 8, 823. https://doi.org/10.3390/microorganisms8060823
Moreno-Galván A, Romero-Perdomo FA, Estrada-Bonilla G, Meneses CHSG, Bonilla RR. Dry-Caribbean Bacillus spp. Strains Ameliorate Drought Stress in Maize by a Strain-Specific Antioxidant Response Modulation. Microorganisms. 2020; 8(6):823. https://doi.org/10.3390/microorganisms8060823
Chicago/Turabian StyleMoreno-Galván, Andres, Felipe A. Romero-Perdomo, German Estrada-Bonilla, Carlos Henrique Salvino Gadelha Meneses, and Ruth R. Bonilla. 2020. "Dry-Caribbean Bacillus spp. Strains Ameliorate Drought Stress in Maize by a Strain-Specific Antioxidant Response Modulation" Microorganisms 8, no. 6: 823. https://doi.org/10.3390/microorganisms8060823
APA StyleMoreno-Galván, A., Romero-Perdomo, F. A., Estrada-Bonilla, G., Meneses, C. H. S. G., & Bonilla, R. R. (2020). Dry-Caribbean Bacillus spp. Strains Ameliorate Drought Stress in Maize by a Strain-Specific Antioxidant Response Modulation. Microorganisms, 8(6), 823. https://doi.org/10.3390/microorganisms8060823







