Impact of Dietary Manganese on Intestinal Barrier and Inflammatory Response in Broilers Challenged with Salmonella Typhimurium
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Animals and Experimental Design
2.2.1. Animals, Housing Conditions, and Diets
2.2.2. Experiment 1 Design
2.2.3. Experiment 2 Design
2.2.4. Experiment 3 Design
2.2.5. Experiment 4 Design
2.3. Intestinal Permeability Assay
2.4. Mn Content Analysis
2.5. Intestinal Histomorphometry
2.6. Quantitation of Serum Cytokines
2.7. Quantitation of mRNA Using Real-Time PCR
2.8. Intestinal Microbial Population
2.9. Enumeration of Salmonella
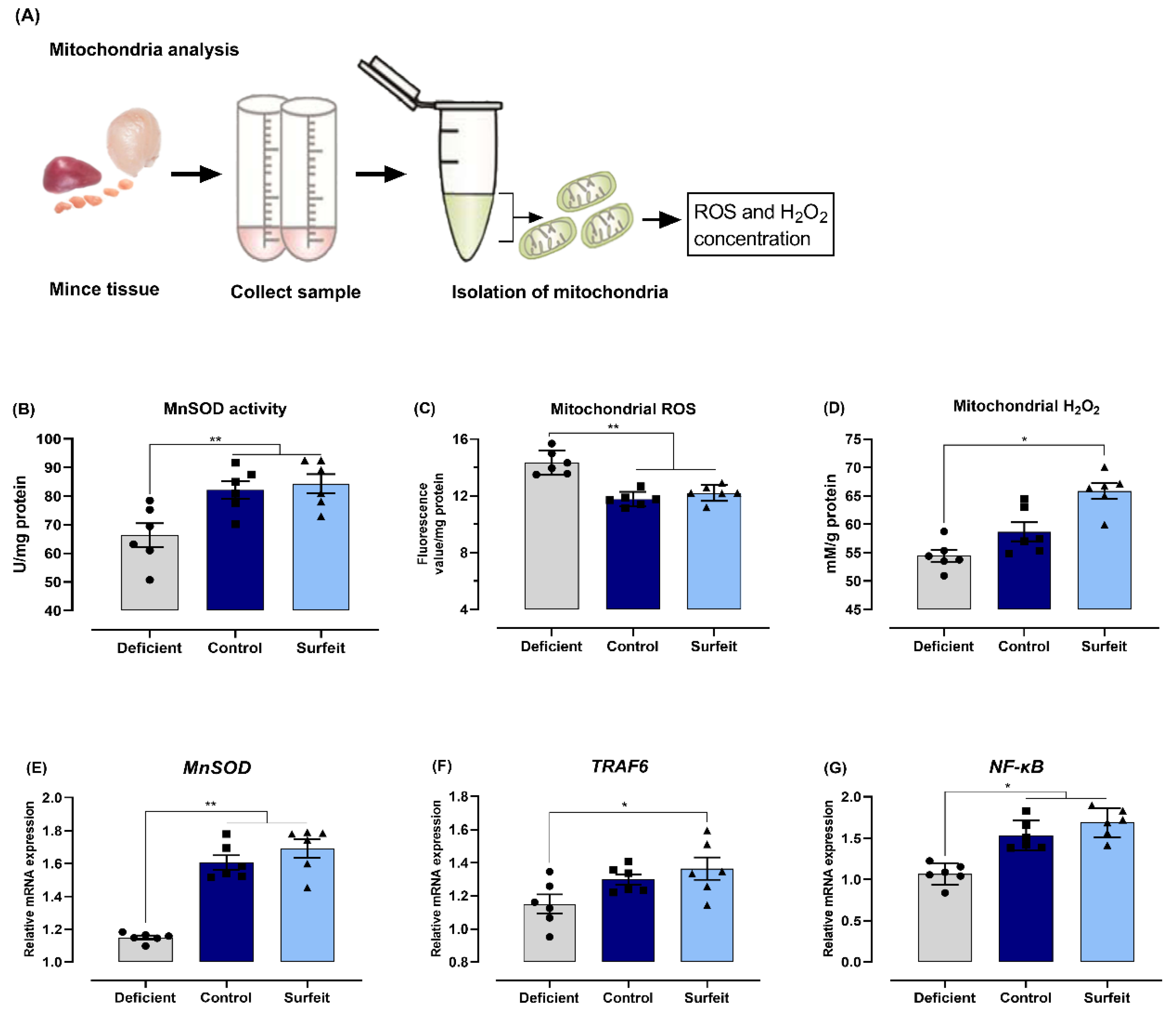
2.10. Mitochondrion Isolation and MnSOD, ROS, and H2O2 Detection
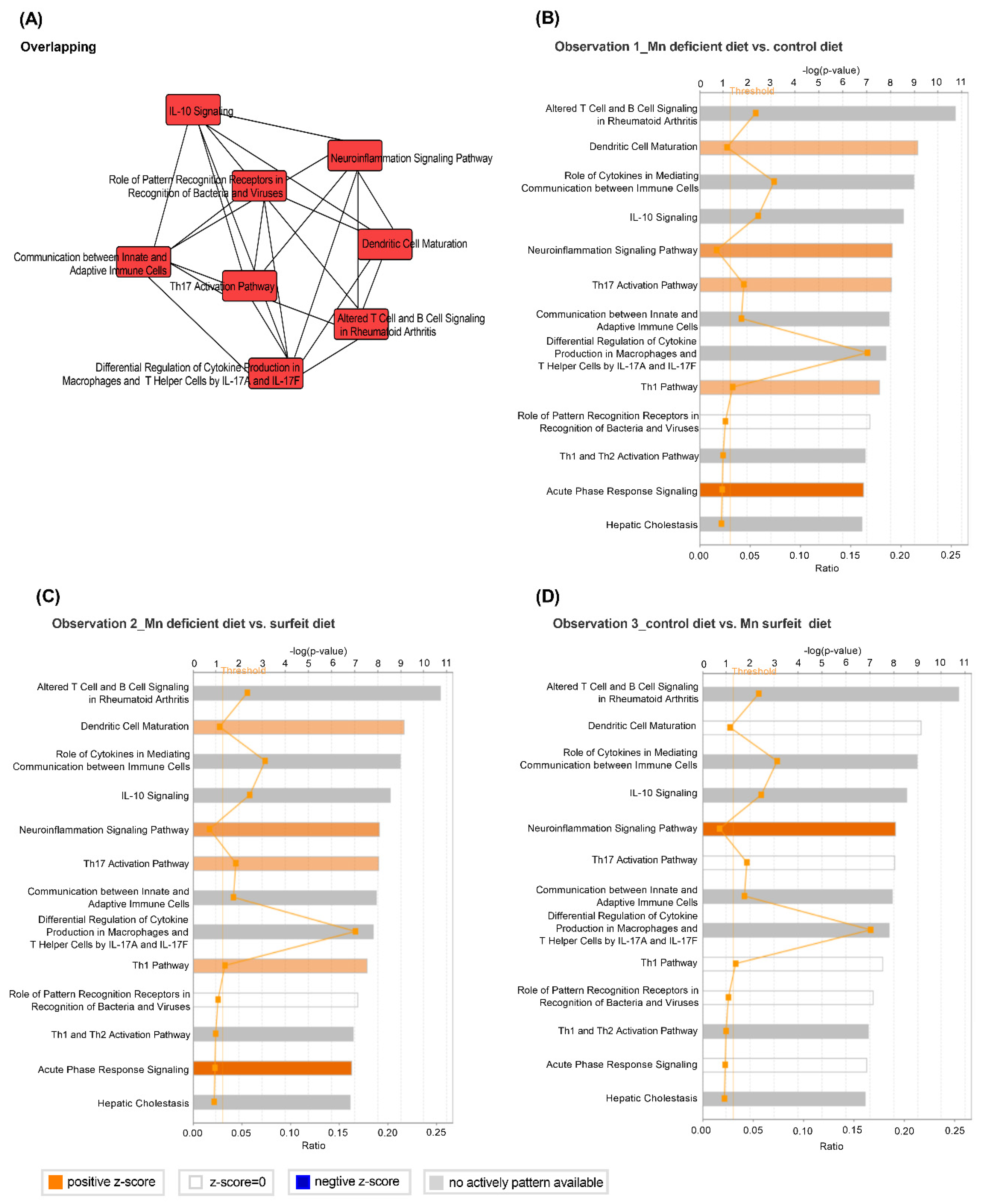
2.11. Ingenuity-Pathway Analysis (IPA)
2.12. Statistical Analyses
3. Results
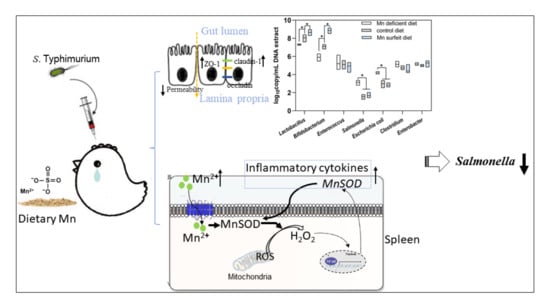
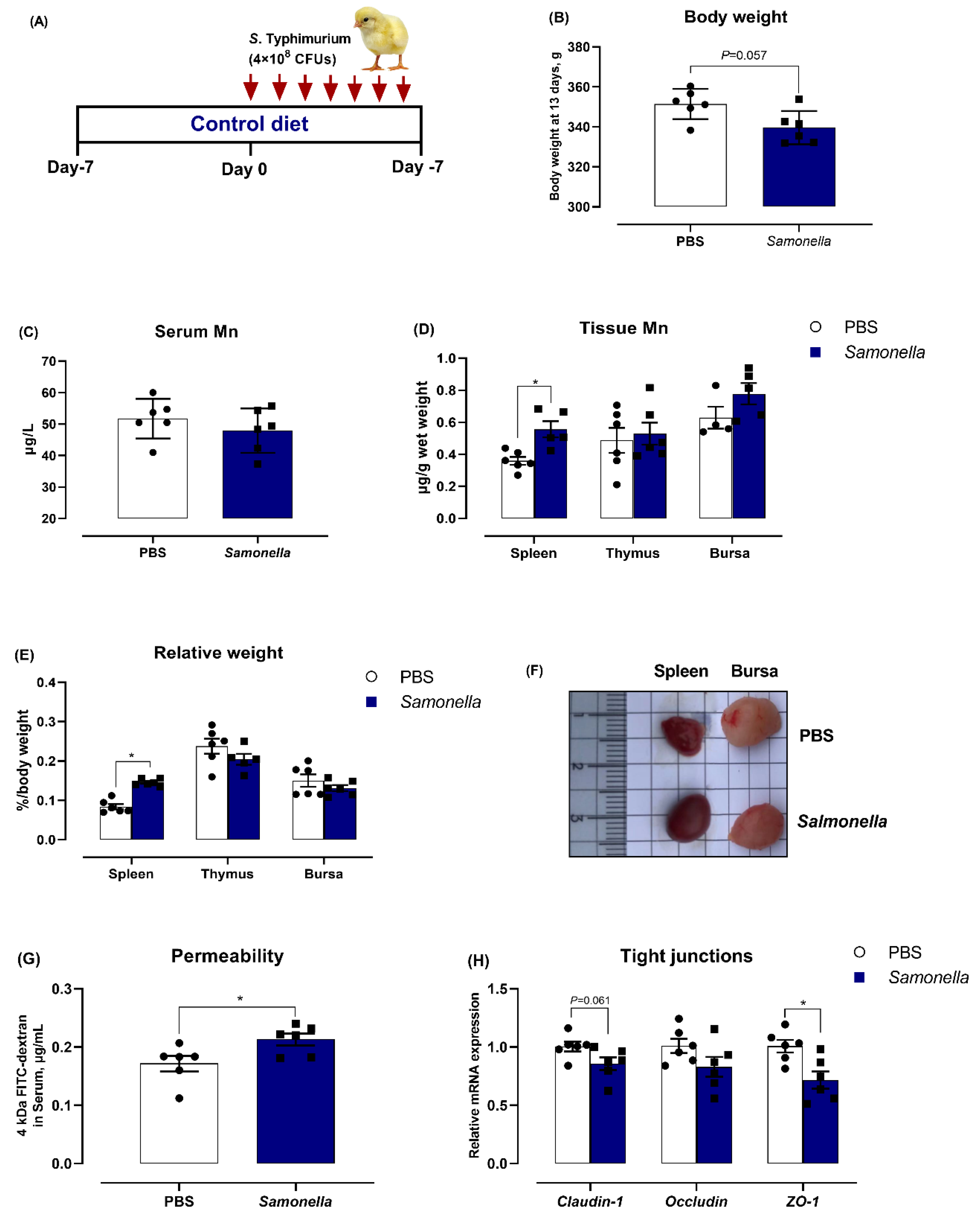
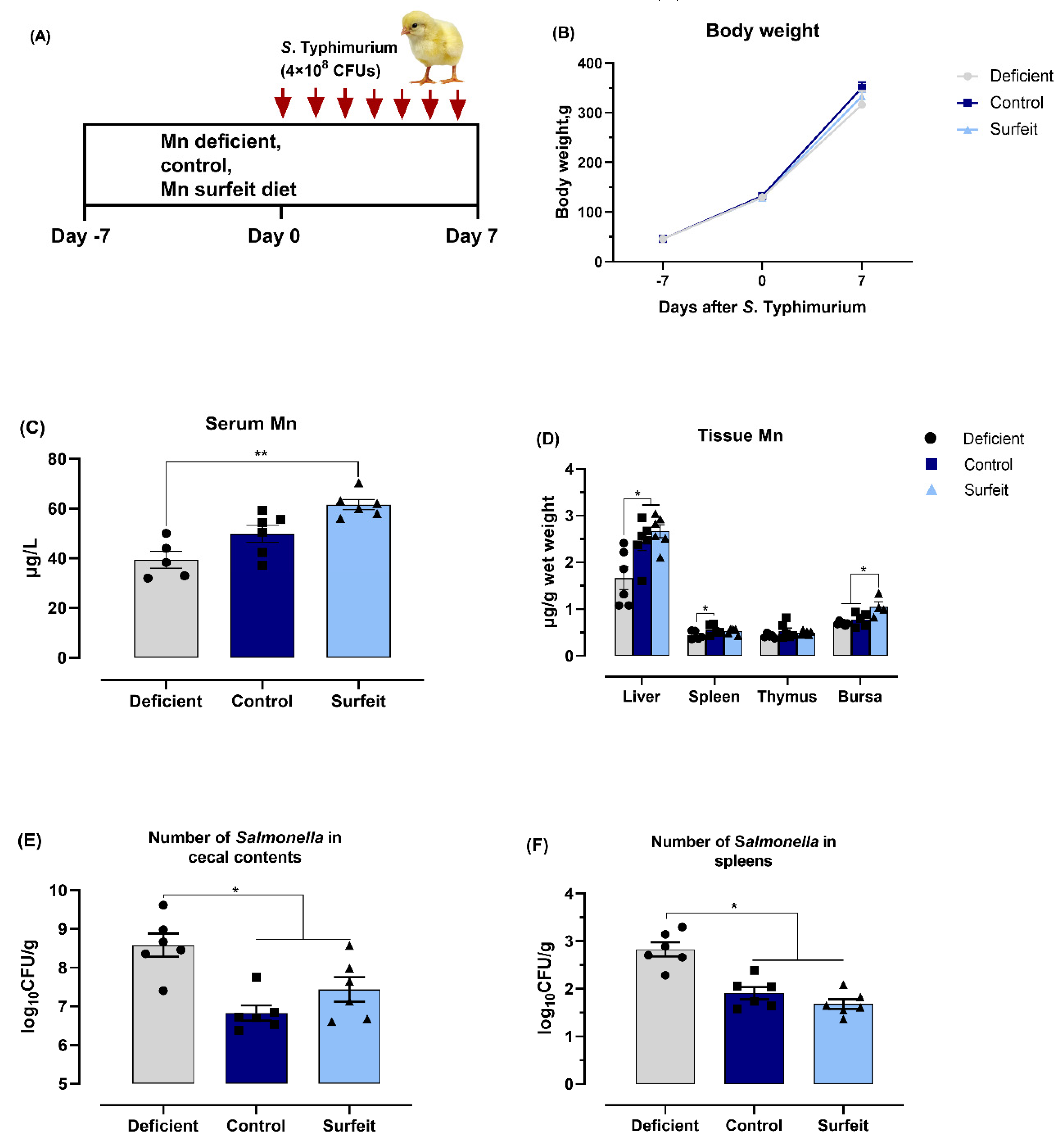
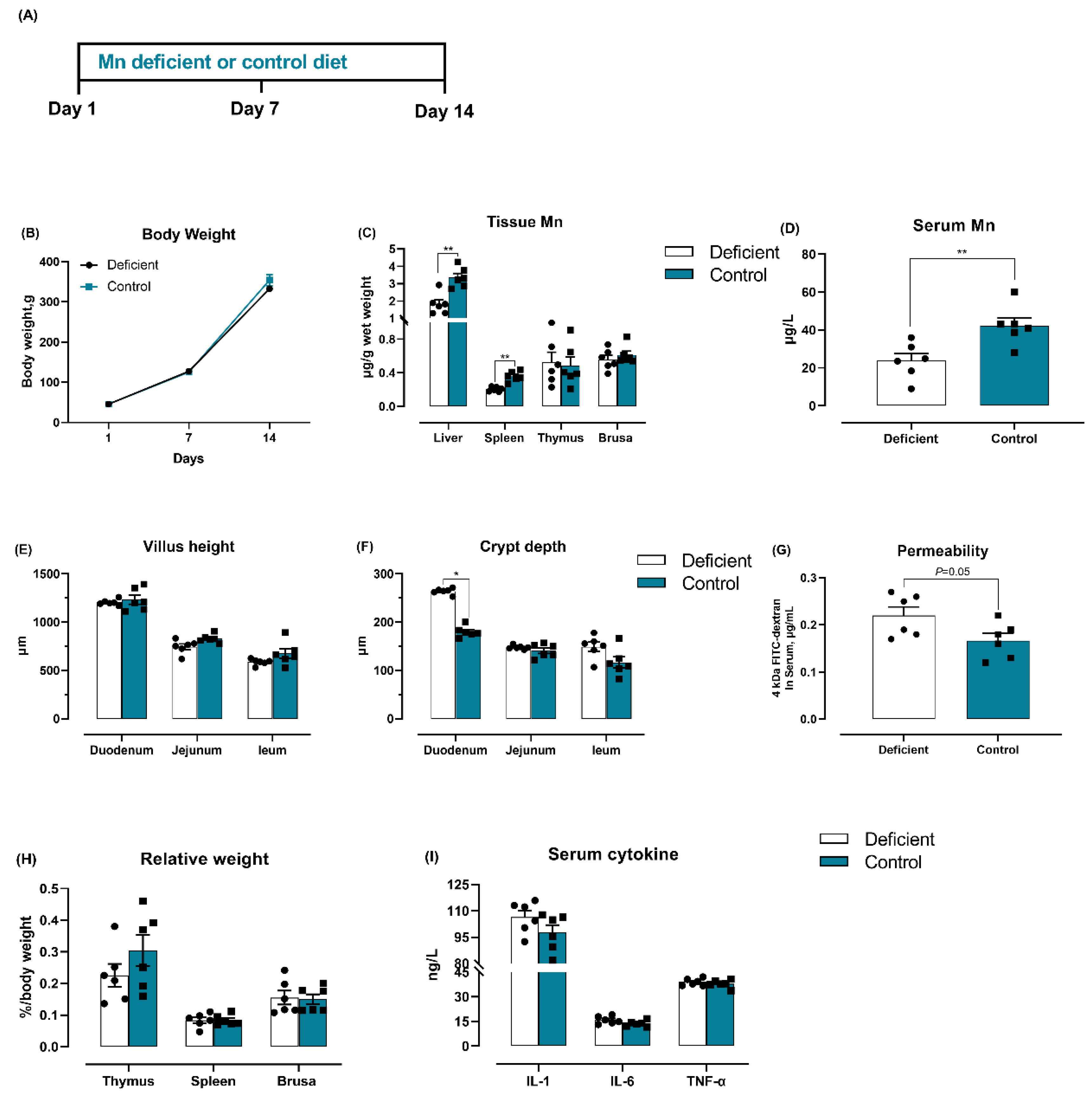
3.1. Salmonella Challenge Induces Splenomegaly and Mn Redistribution
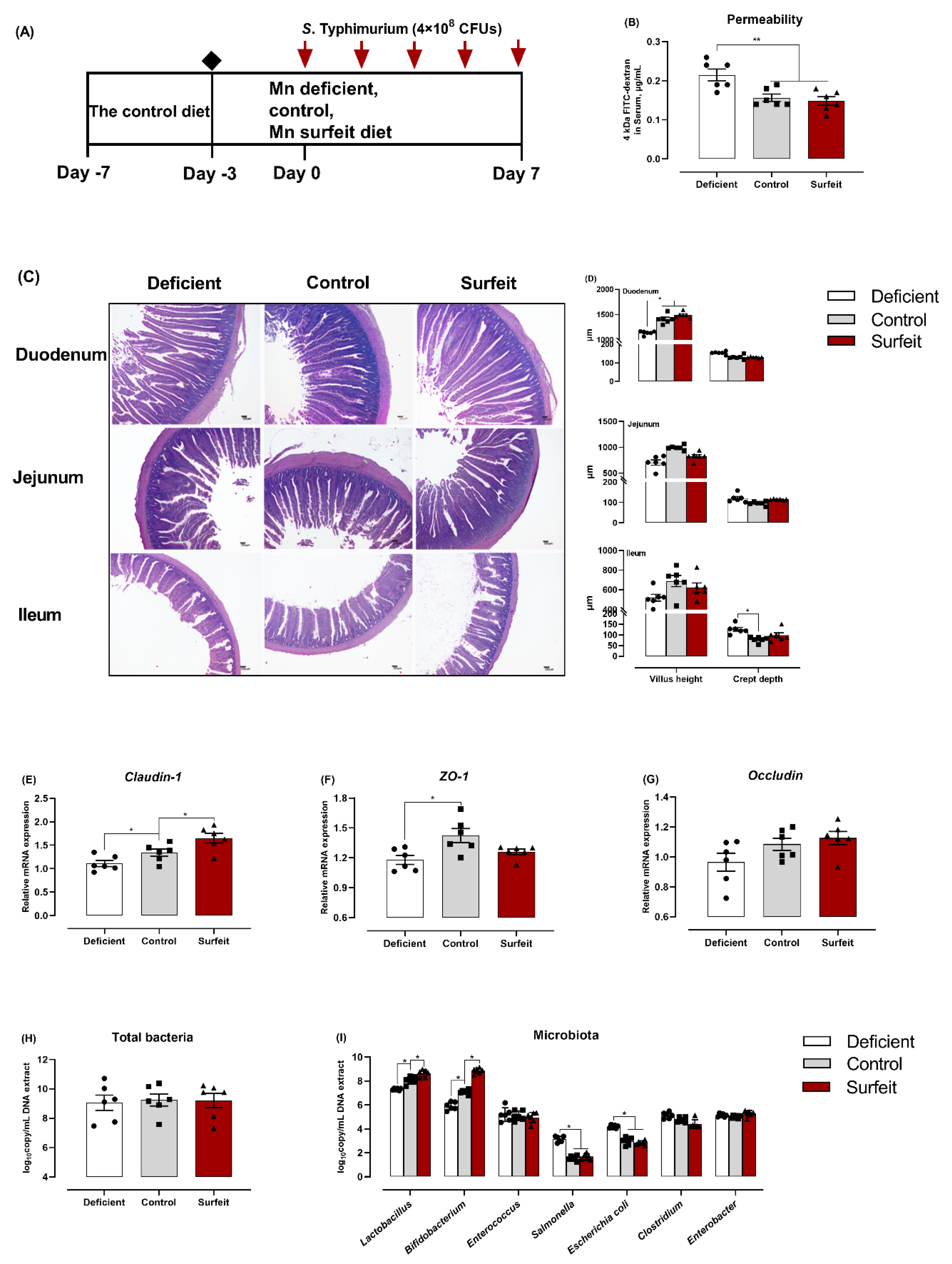
3.2. Dietary Mn Supplementation Improves Intestinal Integrity
3.3. Dietary Mn Supplementation Decreases the Number of Salmonella
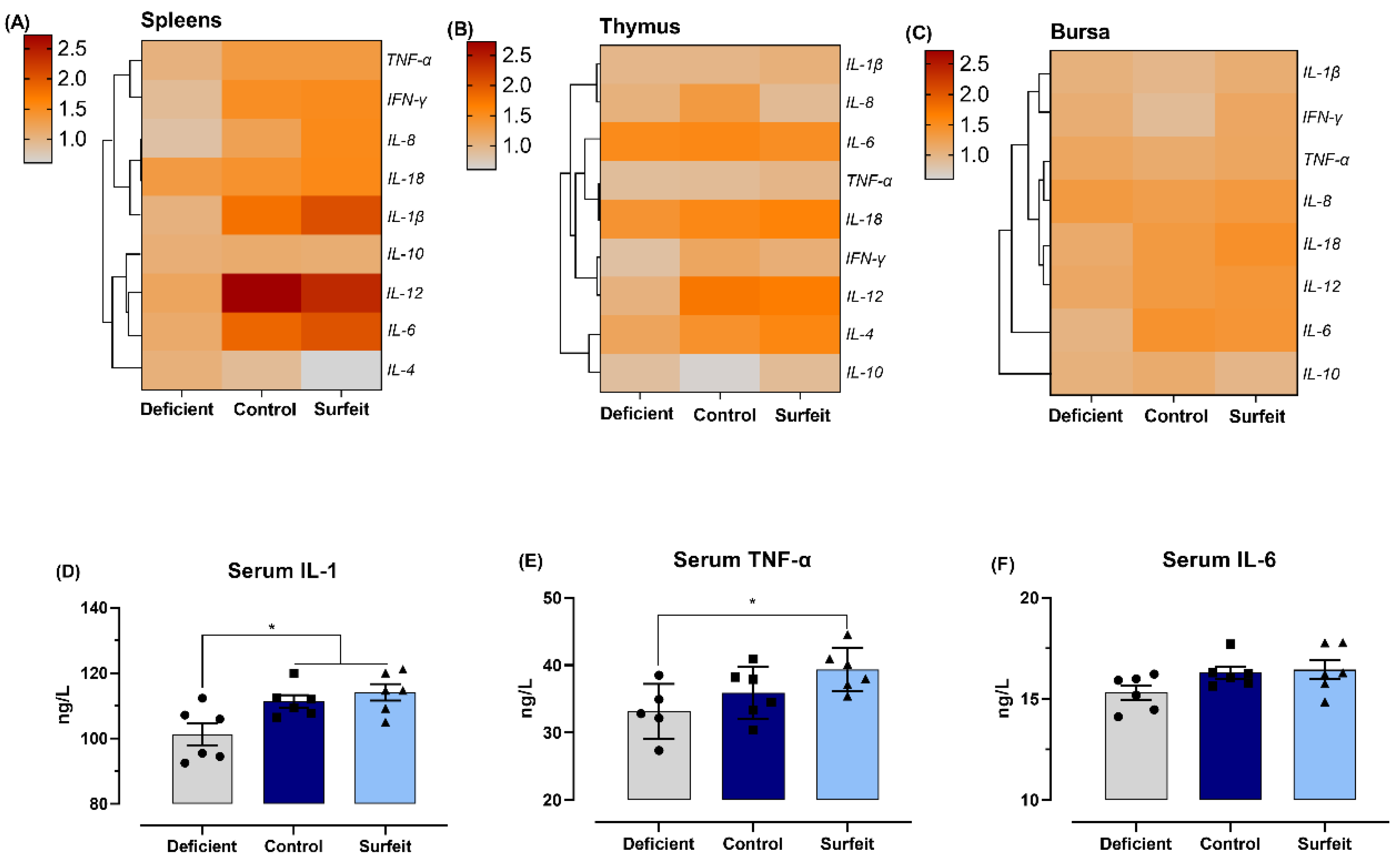
3.4. Dietary Mn Supplementation Promotes Splenic Inflammatory Response
3.5. Dietary Mn Interferes with Acute Inflammatory Reaction in the Spleen against S. Typhimurium Infection
3.6. Dietary Mn Deficiency Itself Induces Intestinal Impairment but Does Not Affect Systemic Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goncalves-Tenorio, A.; Silva, B.N.; Rodrigues, V.; Cadavez, V.; Gonzales-Barron, U. Prevalence of pathogens in poultry meat: A Meta-analysis of European published surveys. Foods 2018, 7, 69. [Google Scholar] [CrossRef]
- Hwang, D.; Rothrock, M.J., Jr.; Pang, H.; Guo, M.; Mishra, A. Predicting Salmonella prevalence associated with meteorological factors in pastured poultry farms in southeastern United States. Sci. Total. Environ. 2020, 713, 136359. [Google Scholar] [CrossRef]
- Matulova, M.; Varmuzova, K.; Sisak, F.; Havlickova, H.; Babak, V.; Stejskal, K.; Zdrahal, Z.; Rychlik, I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet. Res. 2013, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.K.; Wigley, P.; Powers, C.; Hulme, S.D.; Barrow, P.A.; Smith, A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004, 100, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Torres, A.; Jones-Carson, J.; Baumler, A.J.; Falkow, S.; Valdivia, R.; Brown, W.; Le, M.; Berggren, R.; Parks, W.T.; Fang, F.C. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 1999, 401, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shao, Y.; Liu, D.; Yin, P.; Guo, Y.; Yuan, J. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian Pathol. 2012, 41, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.; Sakaguchi, T.; Hurley, B.P.; Kase, B.A.; Reinecker, H.C.; McCormick, B.A. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G178–G187. [Google Scholar] [CrossRef]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella-host interactions—Modulation of the host innate immune system. Front. Immunol. 2014, 5, 481. [Google Scholar] [CrossRef]
- Berndt, A.; Wilhelm, A.; Jugert, C.; Pieper, J.; Sachse, K.; Methner, U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 2007, 75, 5993–6007. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, K.; Ding, X.; Wang, J.; Peng, H.; Zeng, Q.; Xuan, Y.; Su, Z.; Wu, B.; Bai, S. Effect of high dietary manganese on the immune responses of broilers following oral Salmonella Typhimurium inoculation. Biol. Trace Elem. Res. 2018, 181, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.P.; Huang, Y.; Luo, Y.H.; Wang, L.L.; Ding, X.M.; Wang, J.P.; Zeng, Q.F.; Zhang, K.Y. Alteration in lymphocytes responses, cytokine and chemokine profiles in laying hens infected with Salmonella Typhimurium. Vet. Immunol. Immunopathol. 2014, 160, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye. Res. 2007, 85, 462–472. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Son, E.W.; Lee, S.R.; Choi, H.S.; Koo, H.J.; Huh, J.E.; Kim, M.H.; Pyo, S. Effects of supplementation with higher levels of manganese and magnesium on immune function. Arch. Pharm. Res. 2007, 30, 743–749. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Mokgobu, M.I.; Cholo, M.C.; Anderson, R.; Steel, H.C.; Motheo, M.P.; Hlatshwayo, T.N.; Tintinger, G.R.; Theron, A.J. Oxidative induction of pro-inflammatory cytokine formation by human monocyte-derived macrophages following exposure to manganese in vitro. J. Immunotoxicol. 2015, 12, 98–103. [Google Scholar] [CrossRef]
- Du, Y.; Zhu, Y.; Teng, X.; Zhang, K.; Teng, X.; Li, S. Toxicological effect of manganese on NF-kappa B/iNOS-COX-2 signaling pathway in chicken testes. Biol. Trace. Elem. Res. 2015, 168, 227–234. [Google Scholar] [CrossRef]
- Elewaut, D.; DiDonato, J.A.; Kim, J.M.; Truong, F.; Eckmann, L.; Kagnoff, M.F. NF-kappa B is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 1999, 163, 1457–1466. [Google Scholar]
- Sakon, S.; Xue, X.; Takekawa, M.; Sasazuki, T.; Okazaki, T.; Kojima, Y.; Piao, J.H.; Yagita, H.; Okumura, K.; Doi, T.; et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003, 22, 3898–3909. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.J.; Basagoudanavar, S.H.; Nogusa, S.; Irrinki, K.; Mallilankaraman, K.; Slifker, M.J.; Beg, A.A.; Madesh, M.; Balachandran, S. NF-kappa B protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell. Biol. 2011, 31, 2934–2946. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Mokgobu, M.I.; Anderson, R.; Steel, H.C.; Cholo, M.C.; Tintinger, G.R.; Theron, A.J. Manganese promotes increased formation of hydrogen peroxide by activated human macrophages and neutrophils in vitro. Inhal. Toxicol. 2012, 24, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Leto TL, M.S.; Hurt, D.; Ueyama, T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox. Signal. 2009, 11, 2607–2619. [Google Scholar] [CrossRef]
- Bai, S.; Huang, L.; Luo, Y.; Wang, L.; Ding, X.; Wang, J.; Zeng, Q.; Zhang, K. Dietary manganese supplementation influences the expression of transporters involved in iron metabolism in chickens. Biol. Trace. Elem. Res. 2014, 160, 352–360. [Google Scholar] [CrossRef]
- Li, S.; Lu, L.; Hao, S.; Wang, Y.; Zhang, L.; Liu, S.; Liu, B.; Li, K.; Luo, X. Dietary manganese modulates expression of the manganese-containing superoxide dismutase gene in chickens. J. Nutr. 2011, 141, 189–194. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; Natl. Acad. Press: Washington, DC, USA, 1994. [Google Scholar]
- Shao, J.J.; Yao, H.D.; Zhang, Z.W.; Li, S.; Xu, S.W. The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol. Lett. 2012, 214, 99–108. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034.1. [Google Scholar] [CrossRef]
- Rezaei, S.; Faseleh Jahromi, M.; Liang, J.B.; Zulkifli, I.; Farjam, A.S.; Laudadio, V.; Tufarelli, V. Effect of oligosaccharides extract from palm kernel expeller on growth performance, gut microbiota and immune response in broiler chickens. Poult. Sci. 2015, 94, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Yamashita, S.; Hirusaki, K.; Katoh, K.; Ohta, Y. Isolation of mitochondria by gentle cell membrane disruption, and their subsequent characterization. Biochem. Biophys. Res. Commun. 2015, 463, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. 2016, 20, 4742–4749. [Google Scholar]
- Vandeplas, S.; Dauphin, R.D.; Thiry, C.; Beckers, Y.; Welling, G.W.; Thonart, P.; Thewis, A. Efficiency of a Lactobacillus plantarum-xylanase combination on growth performances, microflora populations, and nutrient digestibilities of broilers infected with Salmonella Typhimurium. Poult. Sci. 2009, 88, 1643–1654. [Google Scholar] [CrossRef]
- Marcq, C.; Cox, E.; Szalo, I.M.; Thewis, A.; Beckers, Y. Salmonella Typhimurium oral challenge model in mature broilers: Bacteriological, immunological, and growth performance aspects. Poult. Sci. 2011, 90, 59–67. [Google Scholar] [CrossRef]
- Monack, D.M.; Bouley, D.M.; Falkow, S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 2004, 199, 231–241. [Google Scholar] [CrossRef]
- Koutsos, E.A.; Garcia Lopez, J.C.; Klasing, K.C. Carotenoids from in ovo or dietary sources blunt systemic indices of the inflammatory response in growing chicks (Gallus gallus domesticus). J. Nutr. 2006, 136, 1027–1031. [Google Scholar] [CrossRef][Green Version]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, Y.; Bai, R.; Li, S.; Teng, X. The effect of manganese-induced toxicity on the cytokine mRNA expression of chicken spleen lymphocytes in vitro. Res. Vet. Sci. 2015, 101, 165–167. [Google Scholar] [CrossRef]
- Choi, E.K.; Aring, L.; Das, N.K.; Solanki, S.; Inohara, N.; Iwase, S.; Samuelson, L.C.; Shah, Y.M.; Seo, Y.A. Impact of dietary manganese on experimental colitis in mice. FASEB J. 2020, 34, 2929–2943. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Wolfe, D.; Epperly, M.W.; Huang, S.; Liu, K.; Glorioso, J.C.; Greenberger, J.; Blumberg, D. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. J. Gastrointest. Surg. 2003, 7, 229–235. [Google Scholar] [CrossRef]
- Videnska, P.; Sisak, F.; Havlickova, H.; Faldynova, M.; Rychlik, I. Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Vet. Res. 2013, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Arguello, H.; Estelle, J.; Zaldivar-Lopez, S.; Jimenez-Marin, A.; Carvajal, A.; Lopez-Bascon, M.A.; Crispie, F.; O’Sullivan, O.; Cotter, P.D.; Priego-Capote, F.; et al. Early Salmonella Typhimurium infection in pigs disrupts microbiome composition and functionality principally at the ileum mucosa. Sci. Rep. 2018, 8, 7788. [Google Scholar] [PubMed]
- Chi, L.; Gao, B.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2017, 331, 142–153. [Google Scholar] [CrossRef]
- Matulova, M.; Rajova, J.; Vlasatikova, L.; Volf, J.; Stepanova, H.; Havlickova, H.; Sisak, F.; Rychlik, I. Characterization of chicken spleen transcriptome after infection with Salmonella enterica serovar Enteritidis. PLoS ONE 2012, 7, e48101. [Google Scholar] [CrossRef]
- Liu, J.Z.; Pezeshki, M.; Raffatellu, M. Th17 cytokines and host-pathogen interactions at the mucosa: Dichotomies of help and harm. Cytokine 2009, 48, 156–160. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Saegusa, K.; Noguchi, T.; Sadamitsu, C.; Nishitoh, H.; Nagai, S.; Koyasu, S.; Matsumoto, K.; Takeda, K.; Ichijo, H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 2005, 6, 587–592. [Google Scholar] [CrossRef]
- Lamothe, B.; Besse, A.; Campos, A.D.; Webster, W.K.; Wu, H.; Darnay, B.G. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J. Biol. Chem. 2007, 282, 4102–4112. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Pan, S.; Zhang, K.; Michiels, J.; Zeng, Q.; Ding, X.; Wang, J.; Peng, H.; Bai, J.; Xuan, Y.; et al. Impact of Dietary Manganese on Intestinal Barrier and Inflammatory Response in Broilers Challenged with Salmonella Typhimurium. Microorganisms 2020, 8, 757. https://doi.org/10.3390/microorganisms8050757
Zhang H, Pan S, Zhang K, Michiels J, Zeng Q, Ding X, Wang J, Peng H, Bai J, Xuan Y, et al. Impact of Dietary Manganese on Intestinal Barrier and Inflammatory Response in Broilers Challenged with Salmonella Typhimurium. Microorganisms. 2020; 8(5):757. https://doi.org/10.3390/microorganisms8050757
Chicago/Turabian StyleZhang, Huaiyong, Shuqin Pan, Keying Zhang, Joris Michiels, Qiufeng Zeng, Xuemei Ding, Jianping Wang, Huanwei Peng, Jie Bai, Yue Xuan, and et al. 2020. "Impact of Dietary Manganese on Intestinal Barrier and Inflammatory Response in Broilers Challenged with Salmonella Typhimurium" Microorganisms 8, no. 5: 757. https://doi.org/10.3390/microorganisms8050757
APA StyleZhang, H., Pan, S., Zhang, K., Michiels, J., Zeng, Q., Ding, X., Wang, J., Peng, H., Bai, J., Xuan, Y., Su, Z., & Bai, S. (2020). Impact of Dietary Manganese on Intestinal Barrier and Inflammatory Response in Broilers Challenged with Salmonella Typhimurium. Microorganisms, 8(5), 757. https://doi.org/10.3390/microorganisms8050757