Organoids and Bioengineered Intestinal Models: Potential Solutions to the Cryptosporidium Culturing Dilemma
Abstract
1. Introduction
1.1. Cryptosporidiosis
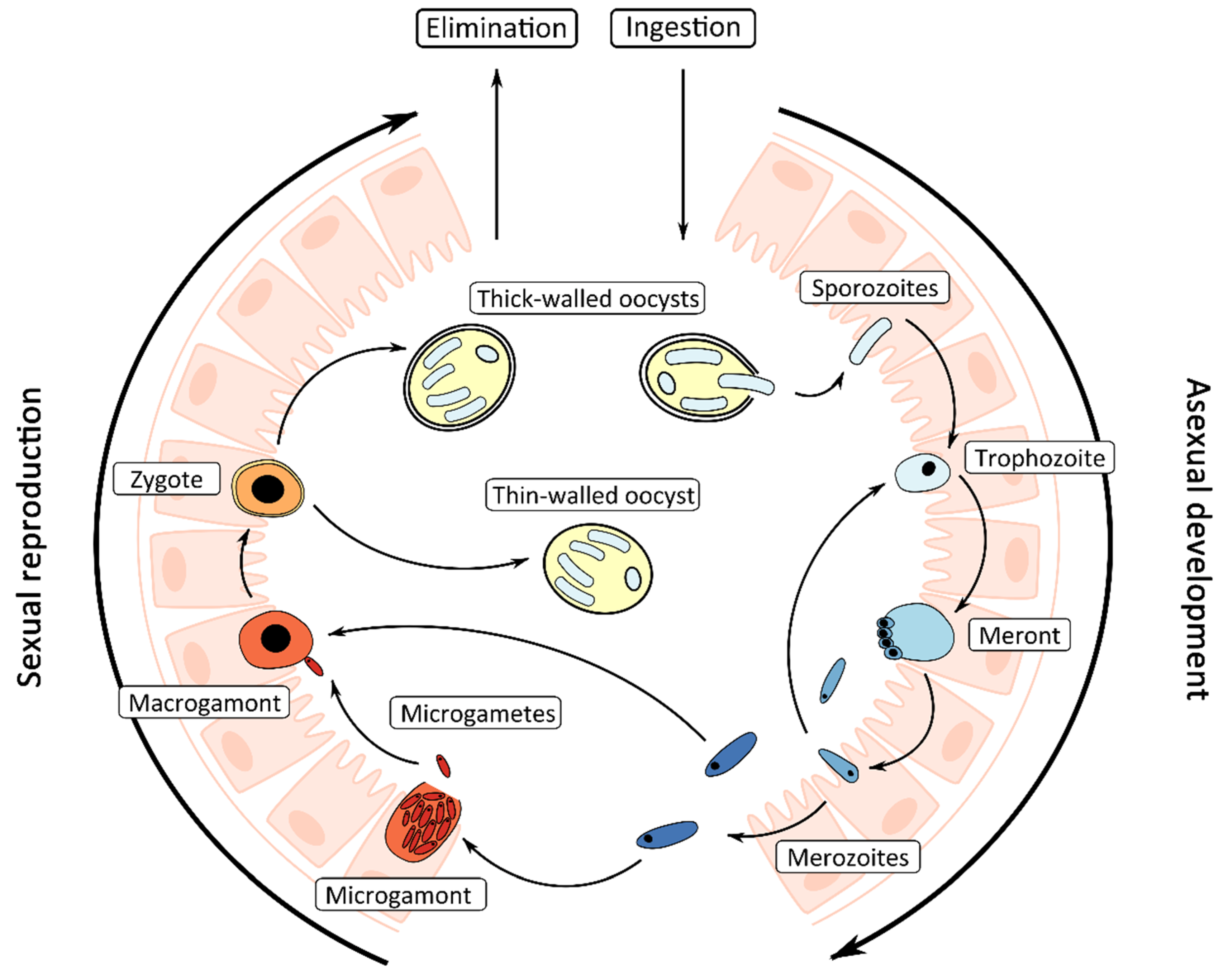
1.2. Life Cycle
1.3. In Vitro and In Vivo Models of Cryptosporidium Infection
2. Cell-Free Culture of Cryptosporidium
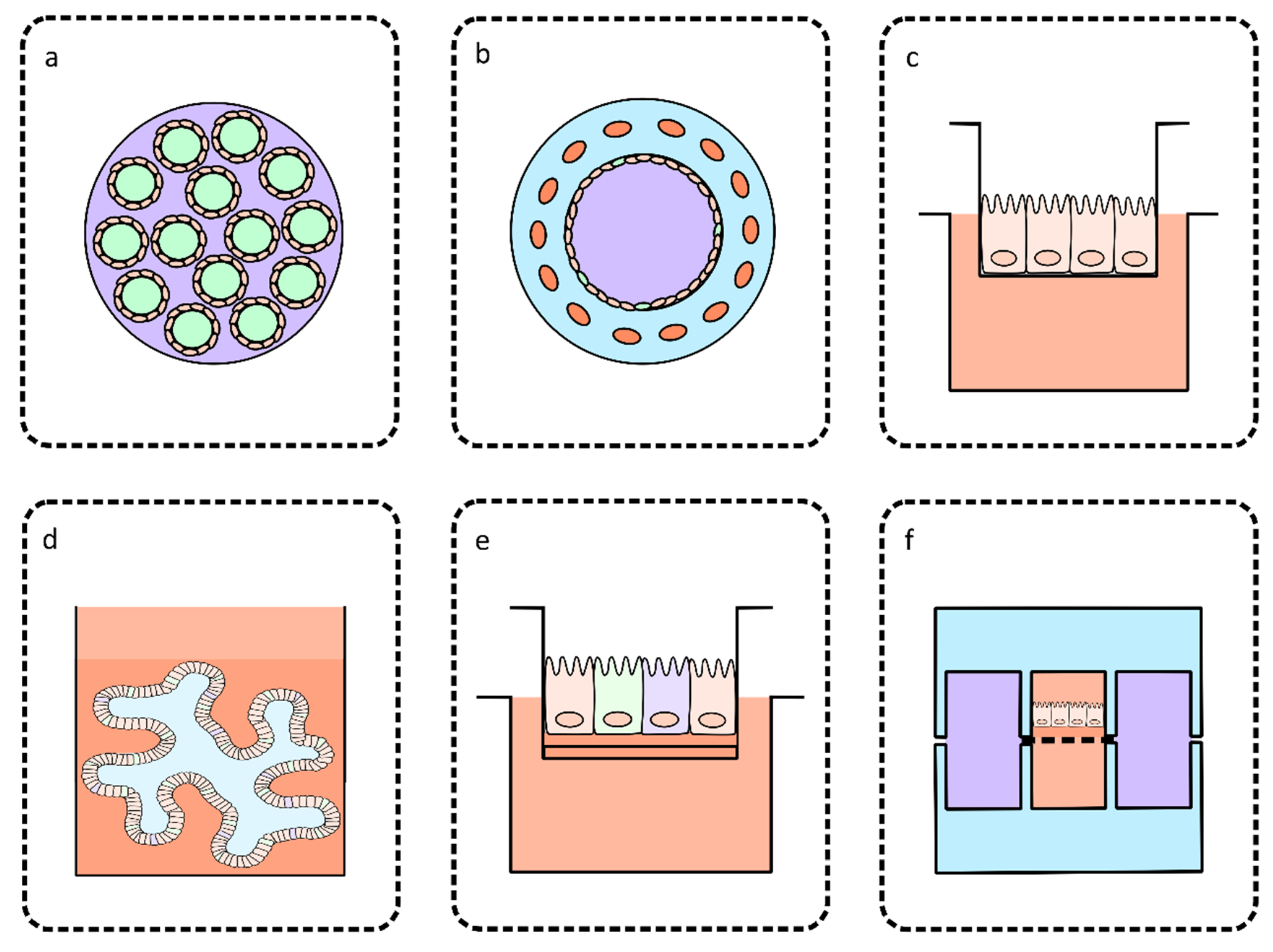
3. Bioengineered Intestinal Models for Culturing Cryptosporidium
3.1. Perfusion Intestinal Models
3.2. Models Based on Co-Culture
3.3. Models Based on Primary or Stem Cell-Derived Cultures
4. Organ-on-a-Chip Technology
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Gharpure, R.; Perez, A.; Miller, A.D.; Wikswo, M.E.; Silver, R.; Hlavsa, M.C. Cryptosporidiosis outbreaks—United States, 2009–2017. Morb. Mortal. Wkly. Rep. 2019, 68, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Rao, P.C.; Blacker, B.F.; Brown, A.; Brewer, T.G.; Colombara, D.V.; De Hostos, E.L.; Engmann, C.; Guerrant, R.L.; et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health 2018, 6, e758–e768. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Hanson, D.; Dworkin, M.S.; Frederick, T.; Bertolli, J.; Lindegren, M.L.; Holmberg, S.; Jones, J.L. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2000, 30, S5–S14. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Eng. J. Med. 1994, 331, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hoxie, N.J.; Davis, J.P.; Vergeront, J.M.; Nashold, R.D.; Blair, K.A. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am. J. Public Health 1997, 87, 2032–2035. [Google Scholar] [CrossRef]
- Corso, P.S.; Kramer, M.H.; Blair, K.A.; Addiss, D.G.; Davis, J.P.; Haddix, A.C. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infec. Dis. 2003, 9, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.-M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Mead, J.R. Prospects for immunotherapy and vaccines against Cryptosporidium. Hum. Vaccin. Immunother. 2014, 10, 1505–1513. [Google Scholar] [CrossRef]
- Sears, C.L.; Kirkpatrick, B.D. Is nitazoxanide an effective treatment for patients with acquired immune deficiency syndrome-related cryptosporidiosis? Nat. Rev. Gastroenterol. Hepatol. 2007, 4, 136–137. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, S.; Pawlowic, M.C.; Sateriale, A.; Brooks, C.F.; Studstill, C.J.; Bar-Peled, Y.; Cipriano, M.J.; Striepen, B. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 2015, 523, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Soares-Weiser, K.; Bergman, H.; Henschke, N.; Pitan, F.; Cunliffe, N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2019, 3, CD008521. [Google Scholar] [PubMed]
- Qadri, F.; Svennerholm, A.-M.; Faruque, A.S.G.; Sack, R.B. Enterotoxigenic Escherichia coli in developing countries: Epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 2005, 18, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.C.M.; Berkley, J.A. Guidelines for the treatment of dysentery (shigellosis): A systematic review of the evidence. Paediatr. Int. Child. Health 2018, 38, S50–S65. [Google Scholar] [CrossRef]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef]
- Giang, T.T.; Pollack, G.; Kotler, D.P. Cryptosporidiosis of the nasal mucosa in a patient with AIDS. AIDS 1994, 8, 555. [Google Scholar] [CrossRef]
- Shrikhande, S.; Chande, C.; Shegokar, V.; Powar, R. Pulmonary cryptosporidiosis in HIV negative, immunocompromised host. Indian J. Pathol. Microbiol. 2009, 52, 267–268. [Google Scholar] [CrossRef]
- Kibbler, C.C.; Smith, A.; Hamilton-Dutoit, S.J.; Milburn, H.; Pattinson, J.K.; Prentice, H.G. Pulmonary cryptosporidiosis occurring in a bone marrow transplant patient. Scand. J. Infect. Dis. 1987, 19, 581–584. [Google Scholar] [CrossRef]
- Sponseller, J.K.; Griffiths, J.K.; Tzipori, S. The evolution of respiratory cryptosporidiosis: Evidence for transmission by inhalation. Clin. Microbiol. Rev. 2014, 27, 575–586. [Google Scholar] [CrossRef]
- Widmer, G.; Klein, P.; Bonilla, R. Adaptation of Cryptosporidium oocysts to different excystation conditions. Parasitology 2007, 134, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Tandel, J.; English, E.D.; Sateriale, A.; Gullicksrud, J.A.; Beiting, D.P.; Sullivan, M.C.; Pinkston, B.; Striepen, B. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019, 4, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Davies, A.P. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hijjawi, N.S.; Meloni, B.P.; Ryan, U.M.; Olson, M.E.; Thompson, R.C.A. Successful in vitro cultivation of Cryptosporidium andersoni: Evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. Int. J. Parasitol. 2002, 32, 1719–1726. [Google Scholar] [CrossRef]
- Manwell, R.D. Gregarines and haemogregarines. In Parasitic Protozoa; Academic Press: New York, NY, USA, 1977; Volume III, pp. 1–32. [Google Scholar]
- Levine, N.D. Phylum II. Apicomplexa Levine 1970. In Illustrated Guide to the Protoza; American Society of Parasitologists: Lawrence, KA, USA, 1985; pp. 322–374. [Google Scholar]
- Rosales, M.J.; Cordón, G.P.; Moreno, M.S.; Sánchez, C.M.; Mascaró, C. Extracellular like-gregarine stages of Cryptosporidium parvum. Acta Trop. 2005, 95, 74–78. [Google Scholar] [CrossRef]
- Hijjawi, N.S.; Meloni, B.P.; Ng’anzo, M.; Ryan, U.M.; Olson, M.E.; Cox, P.T.; Monis, P.T.; Thompson, R.C.A. Complete development of Cryptosporidium parvum in host cell-free culture. Int. J. Parasitol. 2004, 34, 769–777. [Google Scholar] [CrossRef]
- Schaefer, D.A.; Betzer, D.P.; Smith, K.D.; Millman, Z.G.; Michalski, H.C.; Menchaca, S.E.; Zambriski, J.A.; Ojo, K.K.; Hulverson, M.A.; Arnold, S.L.M.; et al. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J. Infect. Dis. 2016, 214, 1856–1864. [Google Scholar] [CrossRef]
- Sheoran, A.; Wiffin, A.; Widmer, G.; Singh, P.; Tzipori, S. Infection with Cryptosporidium hominis provides incomplete protection of the host against Cryptosporidium parvum. J. Infect. Dis. 2012, 205, 1019–1023. [Google Scholar] [CrossRef]
- McDonald, V.; Deer, R.; Uni, S.; Iseki, M.; Bancroft, G.J. Immune responses to Cryptosporidium muris and Cryptosporidium parvum in adult immunocompetent or immunocompromised (nude and SCID) mice. Infect. Immun. 1992, 60, 3325. [Google Scholar] [CrossRef]
- Sateriale, A.; Šlapeta, J.; Baptista, R.; Engiles, J.B.; Gullicksrud, J.A.; Herbert, G.T.; Brooks, C.F.; Kugler, E.M.; Kissinger, J.C.; Hunter, C.A.; et al. A genetically tractable, natural mouse model of cryptosporidiosis offers insights into host protective immunity. Cell Host Microbe. 2019, 26, 135–146.e135. [Google Scholar] [CrossRef]
- Karanis, P.; Aldeyarbi, H.M. Evolution of Cryptosporidium in vitro culture. Int. J. Parasitol. 2011, 41, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.J.; Jossé, L.; More, C.; Miller, C.N.; Michaelis, M.; Tsaousis, A.D. Past and future trends of Cryptosporidium in vitro research. Exp. Parasitol. 2019, 196, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hijjawi, N.S.; Meloni, B.P.; Morgan, U.M.; Thompson, R.C.A. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 2001, 31, 1048–1055. [Google Scholar] [CrossRef]
- Miller, C.N.; Jossé, L.; Brown, I.; Blakeman, B.; Povey, J.; Yiangou, L.; Price, M.; Cinatl, J.; Xue, W.-F.; Michaelis, M.; et al. A cell culture platform for Cryptosporidium that enables long-term cultivation and new tools for the systematic investigation of its biology. Int. J. Parasitol. 2018, 48, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Current, W.L.; Haynes, T.B. Complete development of Cryptosporidium in cell culture. Science 1984, 224, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.M.; Upton, S.J. In vitro development of Cryptosporidium parvum in serum-free media. Lett. Appl. Microbiol. 2007, 44, 520–523. [Google Scholar] [CrossRef]
- Petry, F.; Kneib, I.; Harris, J.R. Morphology and in vitro infectivity of sporozoites of Cryptosporidium parvum. J. Parasitol. 2009, 95, 1243–1246. [Google Scholar] [CrossRef]
- Girouard, D.; Gallant, J.; Akiyoshi, D.E.; Nunnari, J.; Tzipori, S. Failure to propagate Cryptosporidium spp. In cell-free culture. J. Parasitol. 2006, 92, 399–400. [Google Scholar] [CrossRef]
- Karanis, P.; Kimura, A.; Nagasawa, H.; Igarashi, I.; Suzuki, N. Observations on Cryptosporidium life cycle stages during excystation. J. Parasitol. 2008, 94, 298–300. [Google Scholar] [CrossRef]
- Zhang, L.; Sheoran, A.S.; Widmer, G. Cryptosporidium parvum DNA replication in cell-free culture. J. Parasitol. 2009, 95, 1239–1242. [Google Scholar] [CrossRef]
- Hijjawi, N.; Estcourt, A.; Yang, R.; Monis, P.; Ryan, U. Complete development and multiplication of Cryptosporidium hominis in cell-free culture. Vet. Parasitol. 2010, 169, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Boxell, A.; Hijjawi, N.; Monis, P.; Ryan, U. Comparison of various staining methods for the detection of Cryptosporidium in cell-free culture. Exp. Parasitol. 2008, 120, 67–72. [Google Scholar] [CrossRef]
- Yang, R.; Elankumaran, Y.; Hijjawi, N.; Ryan, U. Validation of cell-free culture using scanning electron microscopy (SEM) and gene expression studies. Exp. Parasitol. 2015, 153, 55–62. [Google Scholar] [CrossRef]
- Aldeyarbi, H.M.; Karanis, P. Electron microscopic observation of the early stages of Cryptosporidium parvum asexual multiplication and development in in vitro axenic culture. Eur. J. Protistol. 2016, 52, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Aldeyarbi, H.M.; Karanis, P. The ultra-structural similarities between Cryptosporidium parvum and the gregarines. J. Eukaryot. Microbiol. 2016, 63, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Aldeyarbi, H.M.; Karanis, P. The fine structure of sexual stage development and sporogony of Cryptosporidium parvum in cell-free culture. Parasitology 2016, 143, 749–761. [Google Scholar] [CrossRef]
- Edwinson, A.; Widmer, G.; McEvoy, J. Glycoproteins and gal-galnac cause Cryptosporidium to switch from an invasive sporozoite to a replicative trophozoite. Int. J. Parasitol. 2016, 46, 67–74. [Google Scholar] [CrossRef]
- Paziewska-Harris, A.; Singer, M.; Schoone, G.; Schallig, H. Quantitative analysis of Cryptosporidium growth in in vitro culture-the impact of parasite density on the success of infection. Parasitol. Res. 2016, 115, 329–337. [Google Scholar] [CrossRef]
- Koh, W.; Thompson, A.; Edwards, H.; Monis, P.; Clode, P.L. Extracellular excystation and development of Cryptosporidium: Tracing the fate of oocysts within Pseudomonas aquatic biofilm systems. BMC Microbiol. 2014, 14, 281. [Google Scholar] [CrossRef]
- Matsubayashi, M.; Ando, H.; Kimata, I.; Nakagawa, H.; Furuya, M.; Tani, H.; Sasai, K. Morphological changes and viability of Cryptosporidium parvum sporozoites after excystation in cell-free culture media. Parasitology 2010, 137, 1861–1866. [Google Scholar] [CrossRef]
- Morada, M.; Lee, S.; Gunther-Cummins, L.; Weiss, L.M.; Widmer, G.; Tzipori, S.; Yarlett, N. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 2016, 46, 21–29. [Google Scholar] [CrossRef] [PubMed]
- DeCicco RePass, M.A.; Chen, Y.; Lin, Y.; Zhou, W.; Kaplan, D.L.; Ward, H.D. Novel bioengineered three-dimensional human intestinal model for long-term infection of Cryptosporidium parvum. Infect. Immun. 2017, 85, e00731-16. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, M.; Vanneste, S.B.; Creusy, C.; Guyot, K.; Gantois, N.; Chabe, M.; Delaire, B.; Mouray, A.; Baydoun, A.; Forzy, G.; et al. Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept. Sci. Rep. 2017, 7, 17288. [Google Scholar] [CrossRef] [PubMed]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018, 3, 814–823. [Google Scholar] [CrossRef]
- Wilke, G.; Funkhouser-Jones, L.J.; Wang, Y.; Ravindran, S.; Wang, Q.; Beatty, W.L.; Baldridge, M.T.; VanDussen, K.L.; Shen, B.; Kuhlenschmidt, M.S.; et al. A stem-cell-derived platform enables complete Cryptosporidium development in vitro and genetic tractability. Cell Host Microbe. 2019, 26, 123–134. [Google Scholar] [CrossRef]
- Alcantara Warren, C.; Destura, R.V.; Sevilleja, J.E.A.D.; Barroso, L.F.; Carvalho, H.; Barrett, L.J.; O’Brien, A.D.; Guerrant, R.L. Detection of epithelial-cell injury, and quantification of infection, in the hct-8 organoid model of cryptosporidiosis. J. Infect. Dis. 2008, 198, 143–149. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab. Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Castellanos-Gonzalez, A.; Cabada, M.M.; Nichols, J.; Gomez, G.; White, A.C. Human primary intestinal epithelial cells as an improved in vitro model for Cryptosporidium parvum infection. Infect. Immun. 2013, 81, 1996. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- Yang, Q.; Bermingham, N.A.; Finegold, M.J.; Zoghbi, H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001, 294, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Yamamoto, T.; Sakai, H.; Sakai, Y.; Fujii, T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab. Chip. 2008, 8, 741–746. [Google Scholar] [CrossRef]
- Imura, Y.; Asano, Y.; Sato, K.; Yoshimura, E. A microfluidic system to evaluate intestinal absorption. Anal. Sci. 2009, 25, 1403–1407. [Google Scholar] [CrossRef]
- Mahler, G.J.; Esch, M.B.; Glahn, R.P.; Shuler, M.L. Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol. Bioeng. 2009, 104, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Sung, J.H.; Yang, J.; Yu, C.; Yu, J.; March, J.C.; Shuler, M.L. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 2012, 14, 895–906. [Google Scholar] [CrossRef]
- Delon, L.C.; Guo, Z.; Oszmiana, A.; Chien, C.-C.; Gibson, R.; Prestidge, C.; Thierry, B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials 2019, 225, 119521. [Google Scholar] [CrossRef]
- Grassart, A.; Malardé, V.; Gobaa, S.; Sartori-Rupp, A.; Kerns, J.; Karalis, K.; Marteyn, B.; Sansonetti, P.; Sauvonnet, N. Bioengineered human organ-on-chip reveals intestinal microenvironment and mechanical forces impacting Shigella infection. Cell Host Microbe. 2019, 26, 435–444.e434. [Google Scholar] [CrossRef]
- Van Midwoud, P.M.; Merema, M.T.; Verpoorte, E.; Groothuis, G.M.M. A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices. Lab. Chip. 2010, 10, 2778–2786. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.-A.; Sonntag, F.; Hayden, P.; Ayehunie, S.; et al. Chip-based human liver–intestine and liver–skin co-cultures—A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Gleeson, J.P.; Troisi, E.J.; Estrada, H.Q.; Kerns, S.J.; Hinojosa, C.D.; Hamilton, G.A.; Targan, S.R.; Svendsen, C.N.; Barrett, R.J. Enhanced utilization of induced pluripotent stem cell–derived human intestinal organoids using microengineered chips. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 669–677.e662. [Google Scholar] [CrossRef] [PubMed]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 2018, 8, 2871. [Google Scholar] [CrossRef] [PubMed]
| In Vitro Culture Method | Full Multiplication Cycle Supported | Maximum Time Growth is Supported | Uses | Reference | |
|---|---|---|---|---|---|
| Host–Pathogen Interactions | Large Scale Oocyst Production | ||||
| HCT-8 cell lines | No | 25 days | Partially | No | Hijjawi et al. [35] |
| Cell-free culture | Yes | 46 days | No | Partially | Hijjawi et al. [28] |
| Hollow fiber technology | Yes | <180 days | Partially | Yes | Morada et al. [53] |
| Silk-protein scaffold model | Yes | 15 days | Partially | No | DeCicco RePass et al. [54] |
| Colon explants | Yes | 27 days | Yes | No | Baydoun et al. [55] |
| Lung and small intestine organoids | Yes | 28 days | Yes | No | Heo et al. [56] |
| Stem cell-derived cultures | Yes | <20 days | Yes | No | Wilke et al. [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekera, S.; Zahedi, A.; O’Dea, M.; King, B.; Monis, P.; Thierry, B.; M. Carr, J.; Ryan, U. Organoids and Bioengineered Intestinal Models: Potential Solutions to the Cryptosporidium Culturing Dilemma. Microorganisms 2020, 8, 715. https://doi.org/10.3390/microorganisms8050715
Gunasekera S, Zahedi A, O’Dea M, King B, Monis P, Thierry B, M. Carr J, Ryan U. Organoids and Bioengineered Intestinal Models: Potential Solutions to the Cryptosporidium Culturing Dilemma. Microorganisms. 2020; 8(5):715. https://doi.org/10.3390/microorganisms8050715
Chicago/Turabian StyleGunasekera, Samantha, Alireza Zahedi, Mark O’Dea, Brendon King, Paul Monis, Benjamin Thierry, Jillian M. Carr, and Una Ryan. 2020. "Organoids and Bioengineered Intestinal Models: Potential Solutions to the Cryptosporidium Culturing Dilemma" Microorganisms 8, no. 5: 715. https://doi.org/10.3390/microorganisms8050715
APA StyleGunasekera, S., Zahedi, A., O’Dea, M., King, B., Monis, P., Thierry, B., M. Carr, J., & Ryan, U. (2020). Organoids and Bioengineered Intestinal Models: Potential Solutions to the Cryptosporidium Culturing Dilemma. Microorganisms, 8(5), 715. https://doi.org/10.3390/microorganisms8050715






