Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE)
Abstract
:1. Background
2. Methods
2.1. Participants and Study Design
2.2. Outcomes
2.3. Adverse Effects
2.4. Statistical Analysis
2.5. Missing Data Analysis
2.6. Propensity Score Matching
2.7. Analysis of ICU Admission and Mortality
2.8. Analysis of Secondary Outcomes
2.9. Patient and Public Involvement
3. Results
3.1. Description of the Sample and Missing Data Analysis
3.2. Propensity Score Matching
3.3. Effects of Tocilizumab on Mortality and ICU Admission
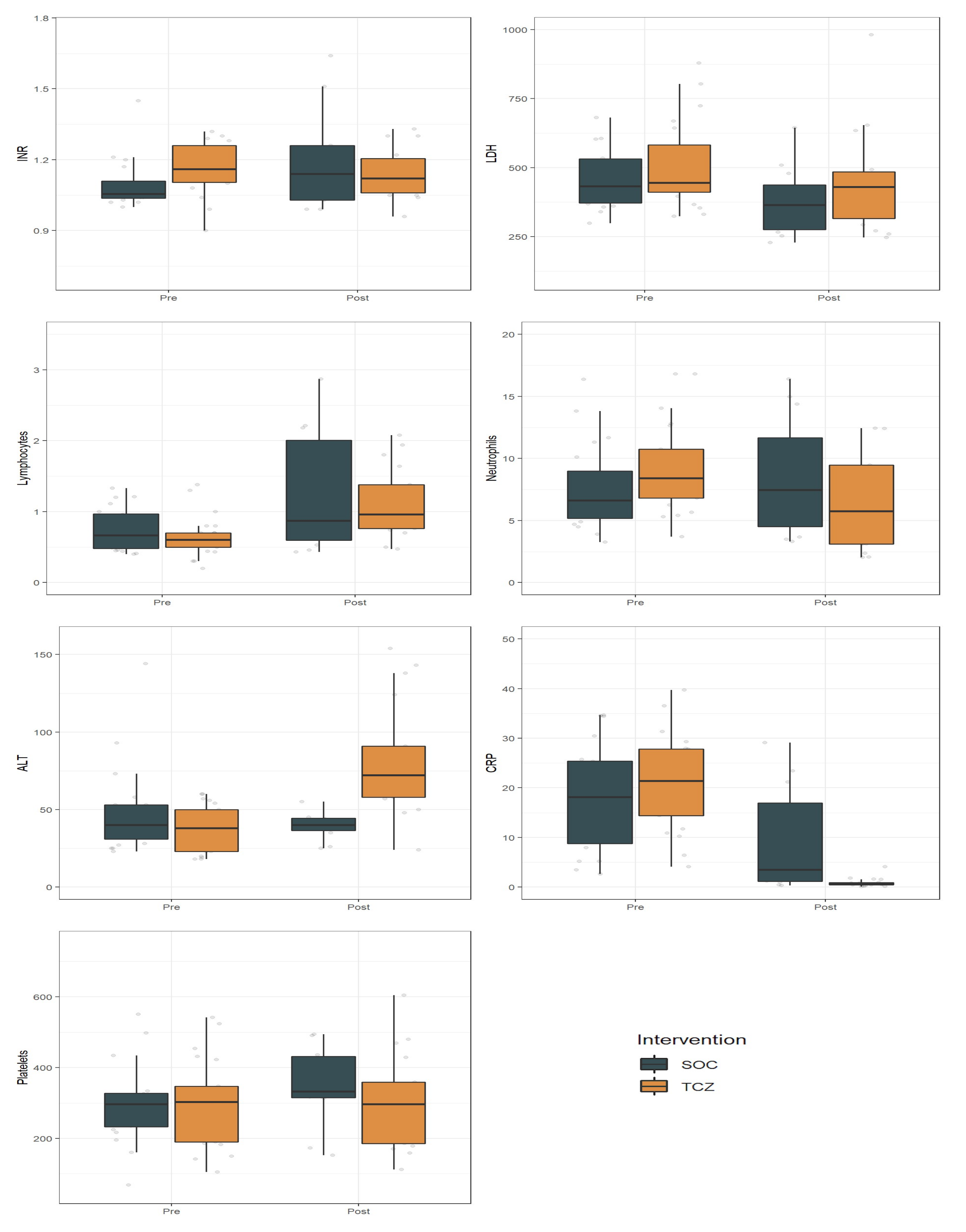
3.4. Effect of Tocilizumab on Laboratory Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus (COVID-19) Events as They Happen. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 20 April 2020).
- Ghebreyesus, T.A. WHO Director-General’s Opening REMARKS at the media Briefing on COVID-19–11 March 2020. Available online: https://www.whoint/dg/speeches/detail/who-director-general-s-opening-remarks-atthe-media-briefing-on-covid-19–11-March-2020 (accessed on 20 April 2020).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. JAMA 2020, 232, 1582–1589. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 2020, 105954. [Google Scholar] [CrossRef]
- Park, W.Y.; Goodman, R.B.; Steinberg, K.P.; Ruzinski, J.T.; Radella, F.; Park, D.R.; Pugin, J.; Skerrett, S.J.; Hudson, L.D.; Martin, T.R. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 1896–1903. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W. Biomarkers of Cytokine Release Syndrome and Neurotoxicity Related to CAR-T Cell Therapy. Biomarker Res. 2018, 6, 29387417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoels, M.M.; Van Der Heijde, D.; Breedveld, F.C.; Burmester, G.R.; Dougados, M.; Emery, P.; Ferraccioli, G.; Gabay, C.; Gibofsky, A.; Gomez-Reino, J.J.; et al. Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: Systematic literature review and meta-analysis informing a consensus statement. Ann. Rheum. Dis. 2013, 72, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kotch, C.; Barrett, D.; Teachey, D.T. Tocilizumab for the Treatment of Chimeric Antigen Receptor T Cell-Induced Cytokine Release Syndrome. Expert Rev. Clin. Immunol. 2019, 15, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 45, 32350134. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Tidyverse: Easily Install and Load the “Tidyverse”. R Package Version 2017; GitHub: San Francisco, CA, USA, 2017. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. {mice}: Multivariate Imputation by Chained Equations in, R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Pishgar, F.; Greifer, N. MatchThem: Matching and Weighting Multiply Imputed Datasets. Available online: https://cran.r-project.org/web/packages/MatchThem/MatchThem.pdf (accessed on 20 April 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kalil, A.C. Treating COVID-19—Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA 2020. [Google Scholar] [CrossRef]
- Wilson, K.C.; Chotirmall, S.H.; Bai, C.R.J. COVID19: Interim Guidance on Management Pending Empirical Evidence. Available online: https://www.thoracic.org/covid/covid-19-guidance.pdf (accessed on 20 April 2020).
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Nishimoto, N.; Hamano, Y.; Asanuma, N.; Miki, S.; Kasayama, S.; Suemura, M. Masked early symptoms of pneumonia in patients with rheumatoid arthritis during tocilizumab treatment: A report of two cases. Mod. Rheumatol. 2009, 19, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, E.B.; Reade, M.C.; Lee, M.; Shook, S.L.; Angus, D.C.; Kong, L.; Carter, M.; Yealy, D.M.; Kellum, J.A.; GenIMS Investigators. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol. Med. 2009, 15, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Ling, Y.; Zhang, Y.H.; Wei, L.Y.; Chen, X.; Li, X.; Liu, X.Y.; Liu, H.M.; Guo, Z.; Ren, H.; et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Genovese, M.C.; Kremer, J.M.; van Vollenhoven, R.F.; Alten, R.; Scali, J.J.; Kelman, A.; Dimonaco, S.; Brockwell, L. Transaminase Levels and Hepatic Events During Tocilizumab Treatment: Pooled Analysis of Long-Term Clinical Trial Safety Data in Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 1751–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayburn, A. Covid-19: Trials of four potential treatments to generate “robust data” of what works. BMJ 2020. [Google Scholar] [CrossRef] [Green Version]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020. [Google Scholar] [CrossRef]
- Coomes, E.A.H.H. Interleukin-6 in COVID-19: A Systematic Review and Meta-Analysis. 2020. submited, under review. [Google Scholar]
- Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04317092 (accessed on 20 April 2020).
- A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04320615 (accessed on 20 April 2020).
- Yakoub-Agha, I.; Moreau, A.S.; Ahmad, I.; Borel, C.; Hadhoum, N.; Masouridi-Levrat, S.; Naudin, J.; Nicolas-Virelizier, E.; Ouachee-Chardin, M.; Platon, L.; et al. Management of cytokine release syndrome in adult and pediatric patients undergoing CAR-T cell therapy for hematological malignancies: Recommendation of the French Society of Bone Marrow and cellular Therapy (SFGM-TC). Bull. Cancer 2019, 106, S102–S109. [Google Scholar] [CrossRef]
- Rosado, F.G.N.; Kim, A.S. Hemophagocytic lymphohistiocytosis. Am. J. Clin. Pathol. 2013. [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; Walsh, M. Risks and Benefits of Glucocorticoids in ANCA-Associated Vasculitis. Curr. Treat Options Rheumatol. 2017, 3, 244–253. [Google Scholar] [CrossRef]
| Whole Sample | Stratified by Treatment | ||||
|---|---|---|---|---|---|
| (n = 112) | SOC (n = 91) | Tocilizumab (n = 21) | |||
| n | % Missing | n | n | ||
| Sex | Male | 82 | 0 | 63 | 19 |
| Female | 30 | 28 | 2 | ||
| Death day 7 | Yes | 24 | 0 | 19 | 5 |
| No | 88 | 72 | 16 | ||
| ICU admission day 7 | Yes | 15 | 0 | 12 | 3 |
| No | 97 | 79 | 18 | ||
| Interstitial lung disease day 0 | Yes | 53 | 49.1 | 41 | 12 |
| No | 4 | 3 | 1 | ||
| Past tumor | Yes | 4 | 50 | 3 | 1 |
| No | 52 | 40 | 12 | ||
| Heart diseases | Yes | 9 | 50 | 7 | 2 |
| No | 47 | 36 | 11 | ||
| Hypertension | Yes | 28 | 50 | 20 | 8 |
| No | 28 | 23 | 5 | ||
| Diabetes | Yes | 10 | 50 | 8 | 2 |
| No | 46 | 35 | 11 | ||
| Lung diseases | Yes | 4 | 50 | 4 | 0 |
| No | 52 | 39 | 13 | ||
| Obesity | Yes | 16 | 50 | 12 | 4 |
| No | 40 | 31 | 9 | ||
| Other comorbidities | Yes | 16 | 50 | 12 | 4 |
| No | 40 | 31 | 9 | ||
| Whole Sample | Stratified by Treatment | ||||||
|---|---|---|---|---|---|---|---|
| SOC | Tocilizumab | ||||||
| Median | IQR | Missing % | Median | IQR | Median | IQR | |
| Age (y) | 63.55 | 16.95 | 0.00 | 63.74 | 16.32 | 62.33 | 18.68 |
| Days of hospitalization | 14 | 5.25 | 0 | 14.00 | 4.00 | 2.00 | 6.00 |
| INR day 0 | 1.11 | 0.16 | 16.07 | 1.09 | 0.15 | 1.16 | 0.16 |
| INR day 7 | 1.17 | 0.21 | 55.35 | 1.20 | 0.26 | 1.12 | 0.15 |
| LDH, 100 U/L day 0 | 441 | 219 | 9 | 439 | 228 | 445 | 172 |
| LDH, 100 U/L day 7 | 414 | 228 | 48 | 397 | 237 | 430 | 169 |
| Lymphocytes, 109/mL day 0 | 0.74 | 0.50 | 4.46 | 0.80 | 0.50 | 0.60 | 0.20 |
| Lymphocytes, 109/mL day 7 | 0.93 | 0.69 | 42.86 | 0.90 | 0.80 | 0.96 | 0.62 |
| Neutrophils, 109/mL day 0 | 6.43 | 4.34 | 4.46 | 6.08 | 4.02 | 8.40 | 3.94 |
| Neutrophils, 109/mL day 7 | 7.25 | 6.73 | 42.86 | 7.44 | 6.70 | 5.73 | 6.37 |
| ALT, U/L day 0 | 41 | 34 | 6.25 | 43.00 | 38.75 | 38.00 | 27.00 |
| ALT, U/L day 7 | 56 | 53.25 | 46.43 | 40.00 | 44.50 | 72.00 | 33.00 |
| CRP, mg/L day 0 | 15.61 | 13.75 | 3.57 | 14.88 | 14.41 | 21.38 | 13.40 |
| CRP, mg/L day 7 | 2.37 | 14.02 | 40.18 | 6.07 | 16.42 | 0.63 | 0.45 |
| PCT, ng/mL day 0 | 0.27 | 0.81 | 11.61 | 0.31 | 1.37 | 0.24 | 0.14 |
| PLT, 109/mL day 0 | 270 | 141 | 4.46 | 252.50 | 139.75 | 303.00 | 157.00 |
| PLT, 109/mL day 7 | 310 | 139.50 | 42.86 | 313 | 128.50 | 296 | 174.00 |
| P/F ratio day 0 | 197.5 | 194.33 | 60.71 | 144.00 | 222.05 | 224.80 | 62.00 |
| Mortality | ICU Admission | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | Statistic | DF | p | OR | 95% CI | Estimate (SE) | Statistic | DF | p | OR | 95% CI | |
| Intercept | −15.63 (6.21) | −2.52 | 29.19 | 0.02 | 0 | [0.00, 0.03] | −1.90 (3.00) | −0.63 | 3.22 | 0.53 | 0.15 | [0.00, 53.80] |
| Age | 0.21 (.09) | 2.43 | 28.54 | 0.02 | 1.24 | [1.04, 1.47] | −0.06 (.04) | −1.35 | 30.09 | 0.18 | 0.94 | [0.86,1.02] |
| Days of hospitalization | 0.25 (.12) | 2.08 | 33.32 | 0.04 | 1.29 | [1.01, 1.64] | ||||||
| TCZ | −0.25 (1.27) | −0.2 | 24.18 | 0.84 | 0.78 | [0.06, 9.34] | −2.18 (1.74) | −1.26 | 29.54 | 0.22 | 0.11 | [0.00, 3.38] |
| INR | LDH | Lymphocytes | Neutrophils | ALT | CRP | PLT | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | |
| Intercept | 0.92 | 0.04 | 0.00 | 486.59 | 42.89 | 0.00 | 1.62 | 0.17 | 0.00 | 7.92 | 2.10 | 0.00 | 47.78 | 22.01 | 0.03 | 18.23 | 2.25 | 0.00 | 322.60 | 32.11 | 0.00 |
| Time | −0.09 | 0.04 | 0.03 | −75.89 | 63.24 | 0.24 | −0.34 | 0.13 | 0.01 | 0.44 | 3.23 | 0.89 | 48.01 | 34.67 | 0.17 | −8.45 | 2.83 | 0.00 | −3.32 | 44.32 | 0.94 |
| TCZ | −0.09 | 0.05 | 0.09 | 25.30 | 58.81 | 0.67 | 0.13 | 0.22 | 0.57 | 1.27 | 2.97 | 0.67 | −11.02 | 31.10 | 0.72 | 2.56 | 3.16 | 0.42 | −17.55 | 44.90 | 0.70 |
| Time × TCZ | 0.17 | 0.05 | 0.00 | 22.80 | 86.15 | 0.79 | −0.16 | 0.18 | 0.36 | 1.69 | 4.54 | 0.71 | −9.30 | 46.82 | 0.84 | −7.84 | 3.60 | 0.03 | 1.23 | 62.51 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaneri, M.; Bogliolo, L.; Valsecchi, P.; Sacchi, P.; Zuccaro, V.; Brandolino, F.; Montecucco, C.; Mojoli, F.; Giusti, E.M.; Bruno, R.; et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020, 8, 695. https://doi.org/10.3390/microorganisms8050695
Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, Montecucco C, Mojoli F, Giusti EM, Bruno R, et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020; 8(5):695. https://doi.org/10.3390/microorganisms8050695
Chicago/Turabian StyleColaneri, Marta, Laura Bogliolo, Pietro Valsecchi, Paolo Sacchi, Valentina Zuccaro, Fabio Brandolino, Carlomaurizio Montecucco, Francesco Mojoli, Emanuele Maria Giusti, Raffaele Bruno, and et al. 2020. "Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE)" Microorganisms 8, no. 5: 695. https://doi.org/10.3390/microorganisms8050695





