Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Media
2.2. Imaging and the Image Analysis
2.3. Transmission Electron Microscopy (TEM)
2.4. Cell Death Assay
2.5. Reversibility in a Liquid Media
2.6. Primers and RT-qPCR
3. Results
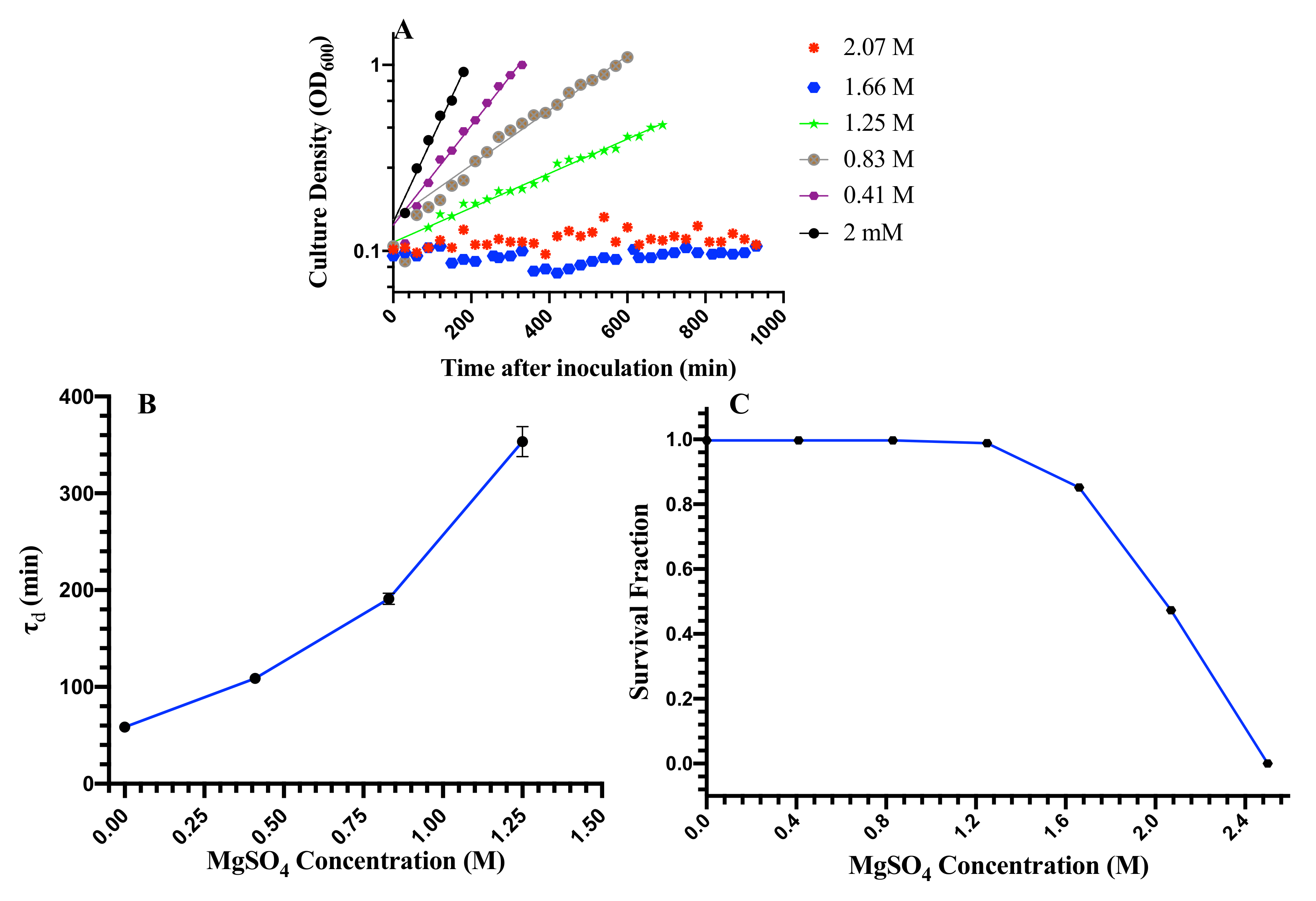
3.1. Cell Growth and Cell Death at Elevated MgSO Concentration
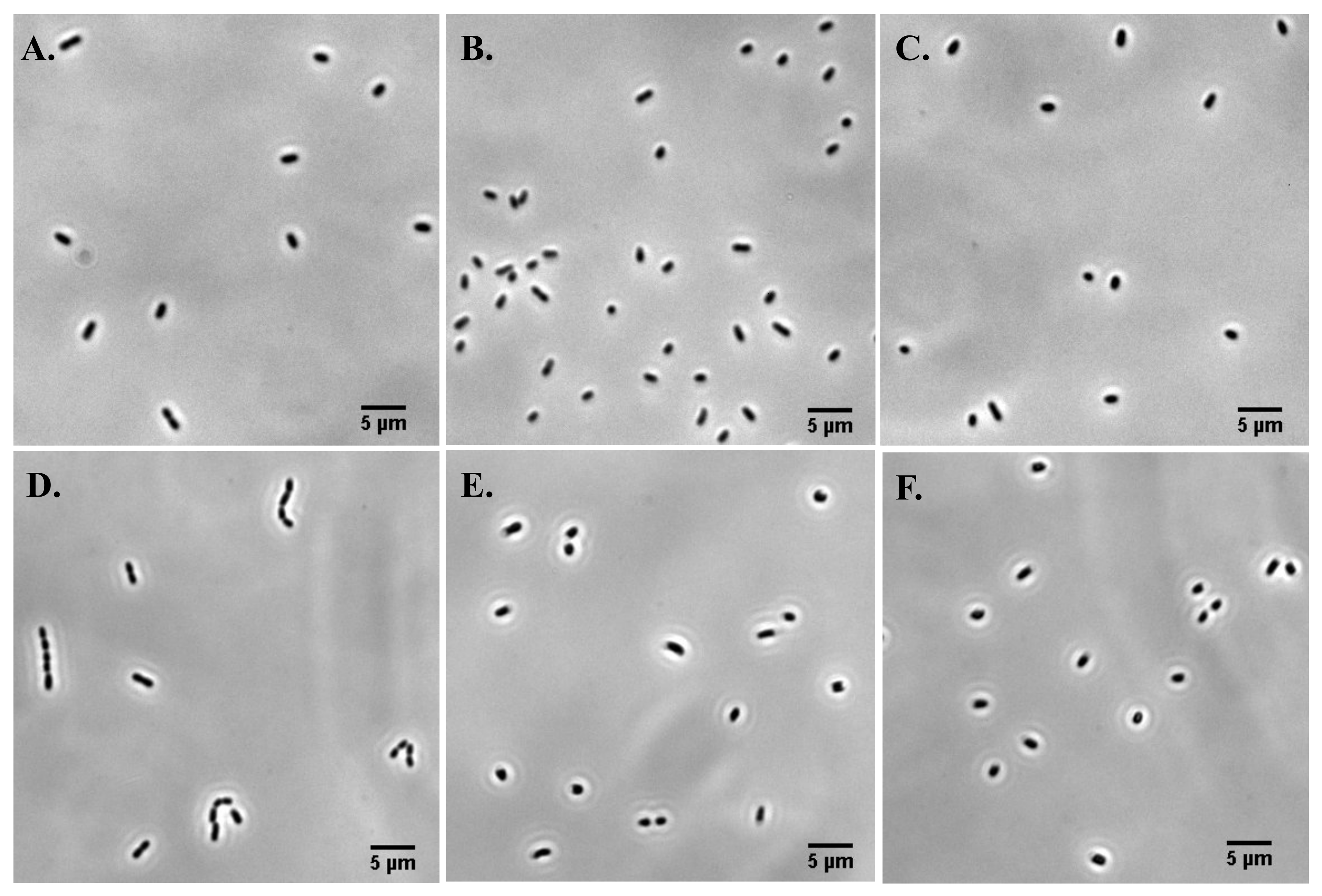
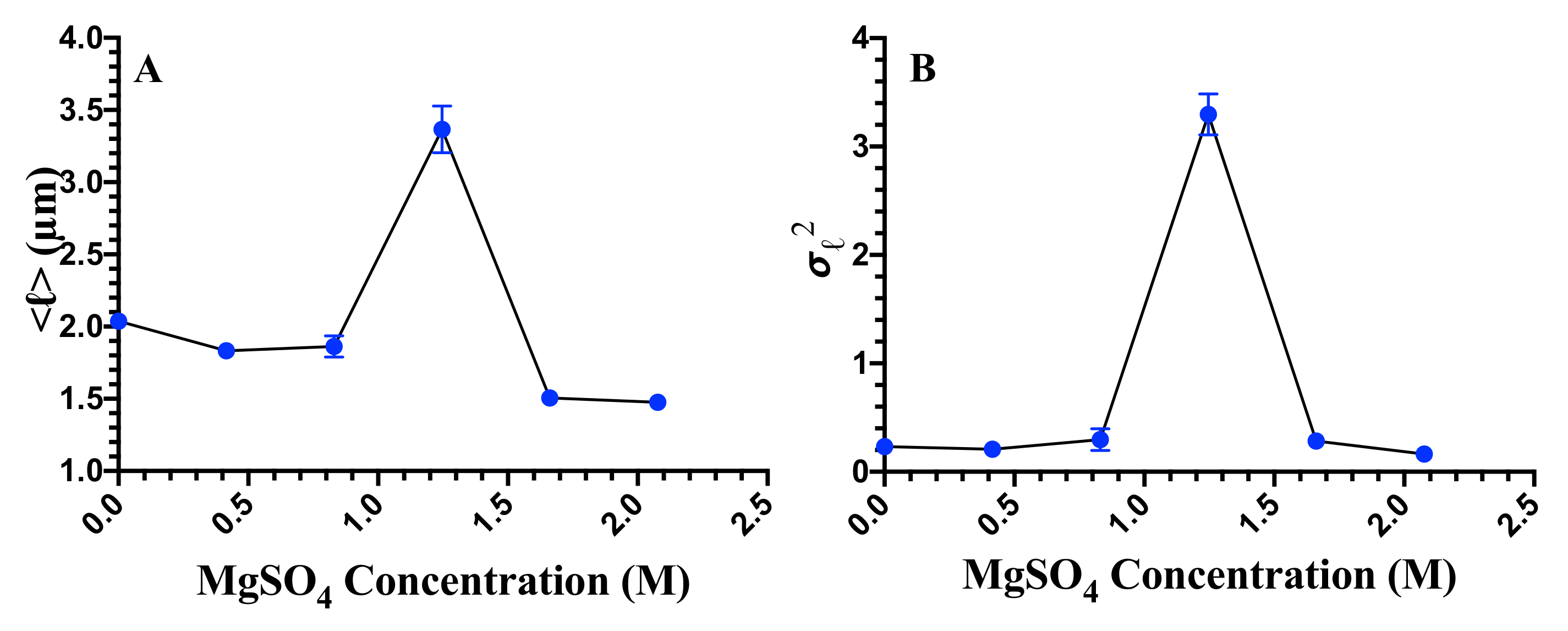
3.2. Cell Division, Cell Length, and Cellular Heterogeneity
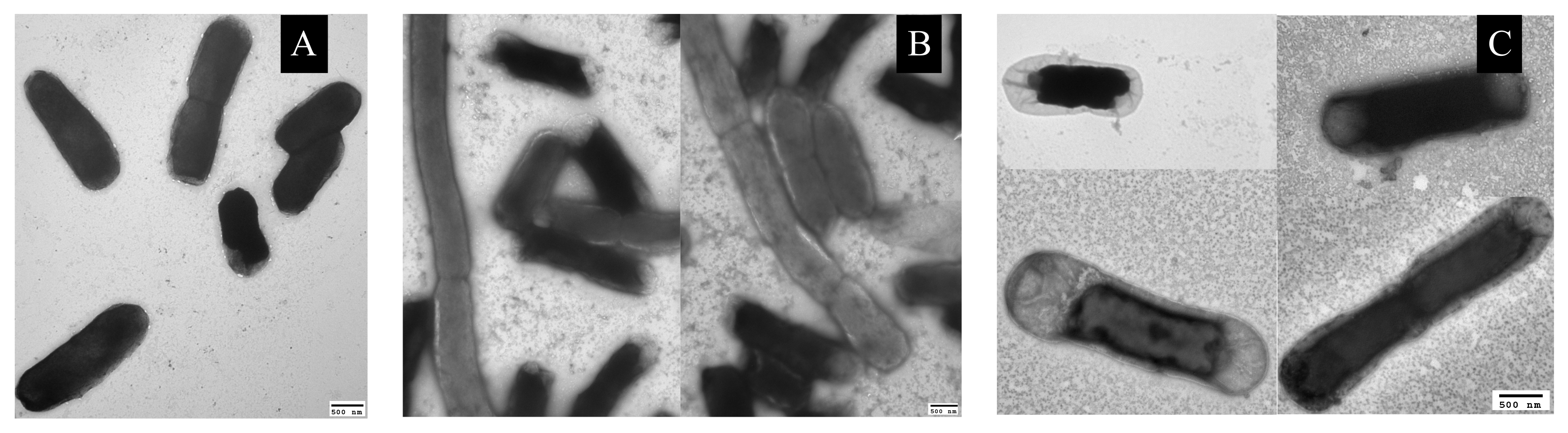
3.3. Plasmolysis of Cells
3.4. Expression of aqpZ, corA, cysP, and osmC
3.5. Reversibility of Population Doubling Time upon Removing the Applied Salinity Stress
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassen, P.; Reynolds, R.T.; Peale, S. Is there liquid water on Europa? Geophys. Res. Lett. 1979, 6, 731–734. [Google Scholar]
- Squyres, S.W.; Reynolds, R.T.; Cassen, P.M.; Peale, S.J. Liquid water and active resurfacing on Europa. Nature 1983, 301, 225. [Google Scholar]
- Schmidt, B.; Blankenship, D.D.; Patterson, G.; Schenk, P. Active formation of ’chaos terrain’ over shallow subsurface water on Europa. Nature 2011, 479, 502. [Google Scholar] [PubMed]
- Gaidos, E.J.; Nimmo, F. Planetary science: Tectonics and water on Europa. Nature 2000, 405, 637. [Google Scholar] [PubMed]
- Manga, M.; Wang, C.Y. Pressurized oceans and the eruption of liquid water on Europa and Enceladus. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Naganuma, T.; Uematsu, H. Dive Europa: A search-for-life initiative. Biol. Sci. Space 1998, 12, 126–130. [Google Scholar]
- Brown, M.; Hand, K. Salts and radiation products on the surface of Europa. Astron. J. 2013, 145, 110. [Google Scholar]
- Marion, G.M. A molal-based model for strong acid chemistry at low temperatures (<200 to 298 K). Geochim. Cosmochim. Acta 2002, 66, 2499–2516. [Google Scholar]
- Kargel, J.S.; Kaye, J.Z.; Head, J.W., III; Marion, G.M.; Sassen, R.; Crowley, J.K.; Ballesteros, O.P.; Grant, S.A.; Hogenboom, D.L. Europa’s crust and ocean: Origin, composition, and the prospects for life. Icarus 2000, 148, 226–265. [Google Scholar]
- Johnson, P.V.; Hodyss, R.; Vu, T.H.; Choukroun, M. Insights into Europa’s ocean composition derived from its surface expression. Icarus 2019, 321, 857–865. [Google Scholar]
- Clark, B.C. Implications of abundant hygroscopic minerals in the Martian regolith. Icarus 1978, 34, 645–665. [Google Scholar]
- Wänke, H.; Brückner, J.; Dreibus, G.; Rieder, R.; Ryabchikov, I. Chemical composition of rocks and soils at the Pathfinder site. In Chronology and Evolution of Mars; Springer: Dordrecht, The Netherlands, 2001; pp. 317–330. [Google Scholar]
- Clark, B.C.; Morris, R.; McLennan, S.; Gellert, R.; Jolliff, B.; Knoll, A.; Squyres, S.; Lowenstein, T.; Ming, D.; Tosca, N.; et al. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet. Sci. Lett. 2005, 240, 73–94. [Google Scholar]
- Gendrin, A.; Mangold, N.; Bibring, J.P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science 2005, 307, 1587–1591. [Google Scholar] [PubMed]
- Altheide, T.; Chevrier, V.; Nicholson, C.; Denson, J. Experimental investigation of the stability and evaporation of sulfate and chloride brines on Mars. Earth Planet. Sci. Lett. 2009, 282, 69–78. [Google Scholar]
- Vaniman, D.T.; Bish, D.L.; Chipera, S.J.; Fialips, C.I.; Carey, J.W.; Feldman, W.C. Magnesium sulphate salts and the history of water on Mars. Nature 2004, 431, 663–665. [Google Scholar] [PubMed]
- Kato, C.; Li, L.; Nogi, Y.; Nakamura, Y.; Tamaoka, J.; Horikoshi, K. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 1998, 64, 1510–1513. [Google Scholar] [PubMed]
- Takami, H.; Inoue, A.; Fuji, F.; Horikoshi, K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol. Lett. 1997, 152, 279–285. [Google Scholar]
- Das Sarma, S.; Das Sarma, P. Halophiles. eLS 2017, 1–13. [Google Scholar] [CrossRef]
- Huston, A.L.; Krieger-Brockett, B.B.; Deming, J.W. Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environ. Microbiol. 2000, 2, 383–388. [Google Scholar]
- Marion, G.M.; Fritsen, C.H.; Eicken, H.; Payne, M.C. The search for life on Europa: Limiting environmental factors, potential habitats, and Earth analogues. Astrobiology 2003, 3, 785–811. [Google Scholar]
- Preston, L.J.; Dartnell, L.R. Planetary habitability: Lessons learned from terrestrial analogues. Int. J. Astrobiol. 2014, 13, 81–98. [Google Scholar]
- Jenkins, O.P. Spotted lakes of epsomite in Washington and British Columbia. Am. J. Sci. 1918, 46, 638–644. [Google Scholar]
- Foster, I.S.; King, P.L.; Hyde, B.C.; Southam, G. Characterization of halophiles in natural MgSO4 salts and laboratory enrichment samples: Astrobiological implications for Mars. Planet. Space Sci. 2010, 58, 599–615. [Google Scholar]
- Pontefract, A.; Zhu, T.F.; Walker, V.K.; Hepburn, H.; Lui, C.; Zuber, M.T.; Ruvkun, G.; Carr, C.E. Microbial diversity in a hypersaline sulfate lake: A terrestrial analog of ancient Mars. Front. Microbiol. 2017, 8, 1819. [Google Scholar] [PubMed]
- Xing, R.; Gao, Q.B.; Zhang, F.Q.; Wang, J.L.; Chen, S.L. Large-scale distribution of bacterial communities in the Qaidam Basin of the Qinghai–Tibet Plateau. MicrobiologyOpen 2019, 8, e909. [Google Scholar] [PubMed]
- Crisler, J.D.; Newville, T.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Bacterial growth at the high concentrations of magnesium sulfate found in Martian soils. Astrobiology 2012, 12, 98–106. [Google Scholar] [PubMed]
- Kilmer, B.R.; Eberl, T.C.; Cunderla, B.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Molecular and phenetic characterization of the bacterial assemblage of Hot Lake, WA, an environment with high concentrations of magnesium sulphate, and its relevance to Mars. Int. J. Astrobiol. 2014, 13, 69–80. [Google Scholar]
- Fox-Powell, M.G.; Cockell, C.S. Building a geochemical view of microbial salt tolerance: Halophilic adaptation of Marinococcus in a natural magnesium sulfate brine. Front. Microbiol. 2018, 9, 739. [Google Scholar]
- Wilks, J.M.; Chen, F.; Clark, B.C.; Schneegurt, M.A. Bacterial growth in saturated and eutectic solutions of magnesium sulphate and potassium chlorate with relevance to Mars and the ocean worlds. Int. J. Astrobiol. 2019, 18, 502–509. [Google Scholar]
- Kumar, P.; Libchaber, A. Pressure and temperature dependence of growth and morphology of Escherichia coli: Experiments and stochastic model. Biophys. J. 2013, 105, 783–793. [Google Scholar]
- Nepal, S.; Kumar, P. Dynamics of phenotypic switching of bacterial cells with temporal fluctuations in pressure. Phys. Rev. E 2018, 97, 052411. [Google Scholar] [PubMed]
- Sinha, N.; Smith-Gill, S.J. Electrostatics in protein binding and function. Curr. Protein Pept. Sci. 2002, 3, 601–614. [Google Scholar] [PubMed]
- Sharp, K.A.; Honig, B. Salt effects on nucleic acids. Curr. Opin. Struct. Biol. 1995, 5, 323–328. [Google Scholar] [PubMed]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Mol. Biol. Rev. 1989, 53, 121–147. [Google Scholar]
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P.; et al. Is there a common water-activity limit for the three domains of life? ISME J. 2015, 9, 1333. [Google Scholar]
- Doudoroff, M. Experiments on the adaptation of Escherichia coli to sodium chloride. J. Gen. Physiol. 1940, 23, 585–611. [Google Scholar]
- Record, M.T., Jr.; Courtenay, E.S.; Cayley, D.S.; Guttman, H.J. Responses of E. coli to osmotic stress: Large changes in amounts of cytoplasmic solutes and water. Trends Biochem. Sci. 1998, 23, 143–148. [Google Scholar]
- Cota-Robles, E.H. Electron microscopy of plasmolysis in Escherichia coli. J. Bacteriol. 1963, 85, 499–503. [Google Scholar]
- Rojas, E.; Theriot, J.A.; Huang, K.C. Response of Escherichia coli growth rate to osmotic shock. Proc. Natl. Acad. Sci. USA 2014, 111, 7807–7812. [Google Scholar]
- Loewen, P.C.; Hu, B.; Strutinsky, J.; Sparling, R. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 1998, 44, 707–717. [Google Scholar]
- Gutierrez, C.; Devedjian, J.C. Osmotic induction of gene osmC expression in Escherichia coli K12. J. Mol. Biol. 1991, 220, 959–973. [Google Scholar] [PubMed]
- Delamarche, C.; Thomas, D.; Rolland, J.P.; Froger, A.; Gouranton, J.; Svelto, M.; Agre, P.; Calamita, G. Visualization of AqpZ-mediated water permeability in Escherichia coli by cryoelectron microscopy. J. Bacteriol. 1999, 181, 4193–4197. [Google Scholar] [PubMed]
- Calamita, G. The Escherichia coli aquaporin-Z water channel: MicroReview. Mol. Microbiol. 2000, 37, 254–262. [Google Scholar] [PubMed]
- Rao, N.V.; Shashidhar, R.; Bandekar, J.R. Comparative analysis of induction of osmotic-stress-dependent genes in Vibrio vulnificus exposed to hyper-and hypo-osmotic stress. Can. J. Microbiol. 2013, 59, 333–338. [Google Scholar] [PubMed]
- Smith, R.L.; Maguire, M.E. Microbial magnesium transport: Unusual transporters searching for identity. Mol. Microbiol. 1998, 28, 217–226. [Google Scholar] [PubMed]
- Payandeh, J.; Pfoh, R.; Pai, E.F. The structure and regulation of magnesium selective ion channels. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2778–2792. [Google Scholar]
- Hmiel, S.; Snavely, M.; Miller, C.; Maguire, M. Magnesium transport in Salmonella typhimurium: Characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1986, 168, 1444–1450. [Google Scholar]
- Karbonowska, H.; Wiater, A.; Hulanicka, D. Sulphate permease of Escherichia coli K12. Acta Biochim. Polonica 1977, 24, 329–334. [Google Scholar]
- Hryniewicz, M.; Sirko, A.; Pałucha, A.; Böck, A.; Hulanicka, D. Sulfate and thiosulfate transport in Escherichia coli K-12: Identification of a gene encoding a novel protein involved in thiosulfate binding. J. Bacteriol. 1990, 172, 3358–3366. [Google Scholar]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 2092–2100. [Google Scholar]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [PubMed]
- Nossal, N.G.; Heppel, L.A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J. Biol. Chem. 1966, 241, 3055–3062. [Google Scholar] [PubMed]
- Sledjeski, D.D.; Gottesman, S. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 1996, 178, 1204–1206. [Google Scholar] [PubMed]
- Anraku, Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J. Biol. Chem. 1967, 242, 793–800. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar]
- Wagle, B.R.; Upadhyay, A.; Arsi, K.; Shrestha, S.; Venkitanarayanan, K.; Donoghue, A.M.; Donoghue, D.J. Application of β-resorcylic acid as potential antimicrobial feed additive to reduce Campylobacter colonization in broiler chickens. Front. Microbiol. 2017, 8, 599. [Google Scholar]
- Bikandi, J.; Millán, R.S.; Rementeria, A.; Garaizar, J. In silico analysis of complete bacterial genomes: PCR, AFLP–PCR and endonuclease restriction. Bioinformatics 2004, 20, 798–799. [Google Scholar]
- Calamita, G.; Kempf, B.; Bonhivers, M.; Bishai, W.R.; Bremer, E.; Agre, P. Regulation of the Escherichia coli water channel gene aqpZ. Proc. Natl. Acad. Sci. USA 1998, 95, 3627–3631. [Google Scholar]
- Soupene, E.; King, N.; Lee, H.; Kustu, S. Aquaporin Z of Escherichia coli: Reassessment of its regulation and physiological role. J. Bacteriol. 2002, 184, 4304–4307. [Google Scholar]
- Mallo, R.C.; Ashby, M.T. AqpZ-mediated water permeability in Escherichia coli measured by stopped-flow spectroscopy. J. Bacteriol. 2006, 188, 820–822. [Google Scholar]
- ZoBell, C.E. Pressure effects on morphology and life processes of bacteria. High Press. Eff. Cell. Process. 1970, 11, 324–327. [Google Scholar]
- Jones, T.; Gill, C.; McMullen, L. The behaviour of log phase Escherichia coli at temperatures that fluctuate about the minimum for growth. Lett. Appl. Microbiol. 2004, 39, 296–300. [Google Scholar] [PubMed]
- Suzuki, H.; Pangborn, J.; Kilgore, W.W. Filamentous cells of Escherichia coli formed in the presence of mitomycin. J. Bacteriol. 1967, 93, 683–688. [Google Scholar]
- Rolinson, G.N. Effect of β-lactam antibiotics on bacterial cell growth rate. Microbiology 1980, 120, 317–323. [Google Scholar]
- Justice, S.S.; Hunstad, D.A.; Cegelski, L.; Hultgren, S.J. Morphological plasticity as a bacterial survival strategy. Nat. Rev. Microbiol. 2008, 6, 162–168. [Google Scholar] [PubMed]
- Nelson, C.M.; Schuppenhauer, M.R.; Clark, D.S. High-pressure, high-temperature bioreactor for comparing effects of hyperbaric and hydrostatic pressure on bacterial growth. Appl. Environ. Microbiol. 1992, 58, 1789–1793. [Google Scholar]
- Zwietering, M.; De Koos, J.; Hasenack, B.; De Witt, J.; Van’t Riet, K. Modeling of bacterial growth as a function of temperature. Appl. Environ. Microbiol. 1991, 57, 1094–1101. [Google Scholar]
- Bååth, E.; Arnebrant, K. Growth rate and response of bacterial communities to pH in limed and ash treated forest soils. Soil Biol. Biochem. 1994, 26, 995–1001. [Google Scholar]
- Rard, J.A.; Miller, D.G. Isopiestic determination of the Osmotic coefficients of aqueous sodium sulfate, magnesium sulfate, and sodium sulfate-magnesium sulfate at 25. degree.C. J. Chem. Eng. Data 1981, 26, 33–38. [Google Scholar]
- Chirife, J.; Resnik, S.L. Unsaturated solutions of sodium chloride as reference sources of water activity at various temperatures. J. Food Sci. 1984, 49, 1486–1488. [Google Scholar]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions; Courier Corporation: Chelmsford, MA, USA, 2002. [Google Scholar]
- Elowitz, M.B.; Levine, A.J.; Siggia, E.D.; Swain, P.S. Stochastic gene expression in a single cell. Science 2002, 297, 1183–1186. [Google Scholar] [PubMed]
- Kerr, R.A.; Levine, H.; Sejnowski, T.J.; Rappel, W.J. Division accuracy in a stochastic model of Min oscillations in Escherichia coli. Proc. Natl. Acade. Sci. USA 2006, 103, 347–352. [Google Scholar]
- Batada, N.N.; Shepp, L.A.; Siegmund, D.O. Stochastic model of protein–protein interaction: Why signaling proteins need to be colocalized. Proc. Natl. Acade. Sci. USA 2004, 101, 6445–6449. [Google Scholar]
- Raj, A.; Peskin, C.S.; Tranchina, D.; Vargas, D.Y.; Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006, 4, e309. [Google Scholar]
- Shahrezaei, V.; Swain, P.S. The stochastic nature of biochemical networks. Curr. Opin. Biotechnol. 2008, 19, 369–374. [Google Scholar] [PubMed]
- Bouvier, J.; Gordia, S.; Kampmann, G.; Lange, R.; Hengge-Aronis, R.; Gutierrez, C. Interplay between global regulators of Escherichia coli: Effect of RpoS, Lrp and H-NS on transcription of the gene osmC. Mol. Microbiol. 1998, 28, 971–980. [Google Scholar]
- Ueguchi, C.; Mizuno, T. The Escherichia coli nucleoid protein H-NS functions directly as a transcriptional repressor. EMBO J. 1993, 12, 1039–1046. [Google Scholar]
Sample Availability: Data will be made available upon publication. |

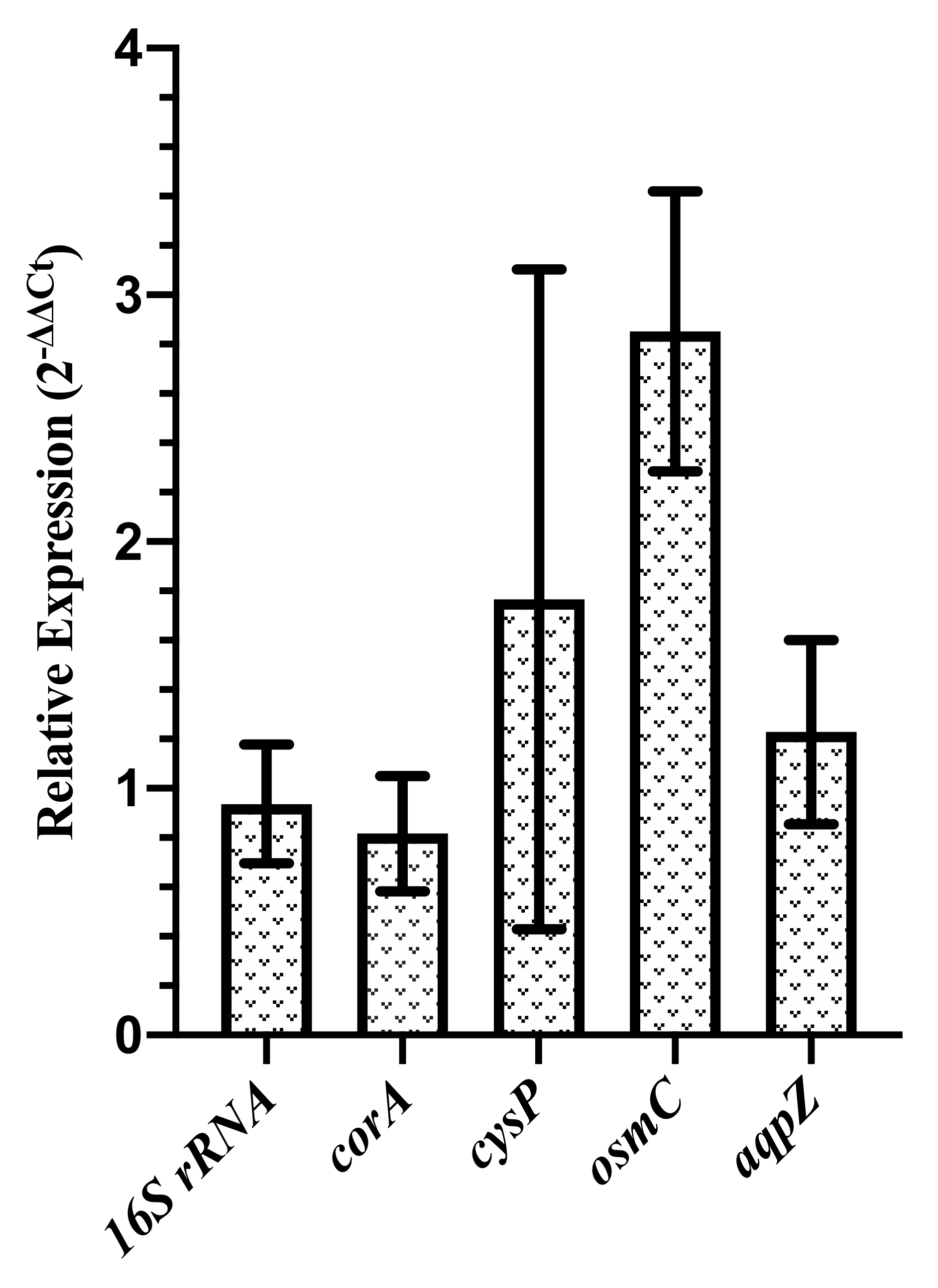
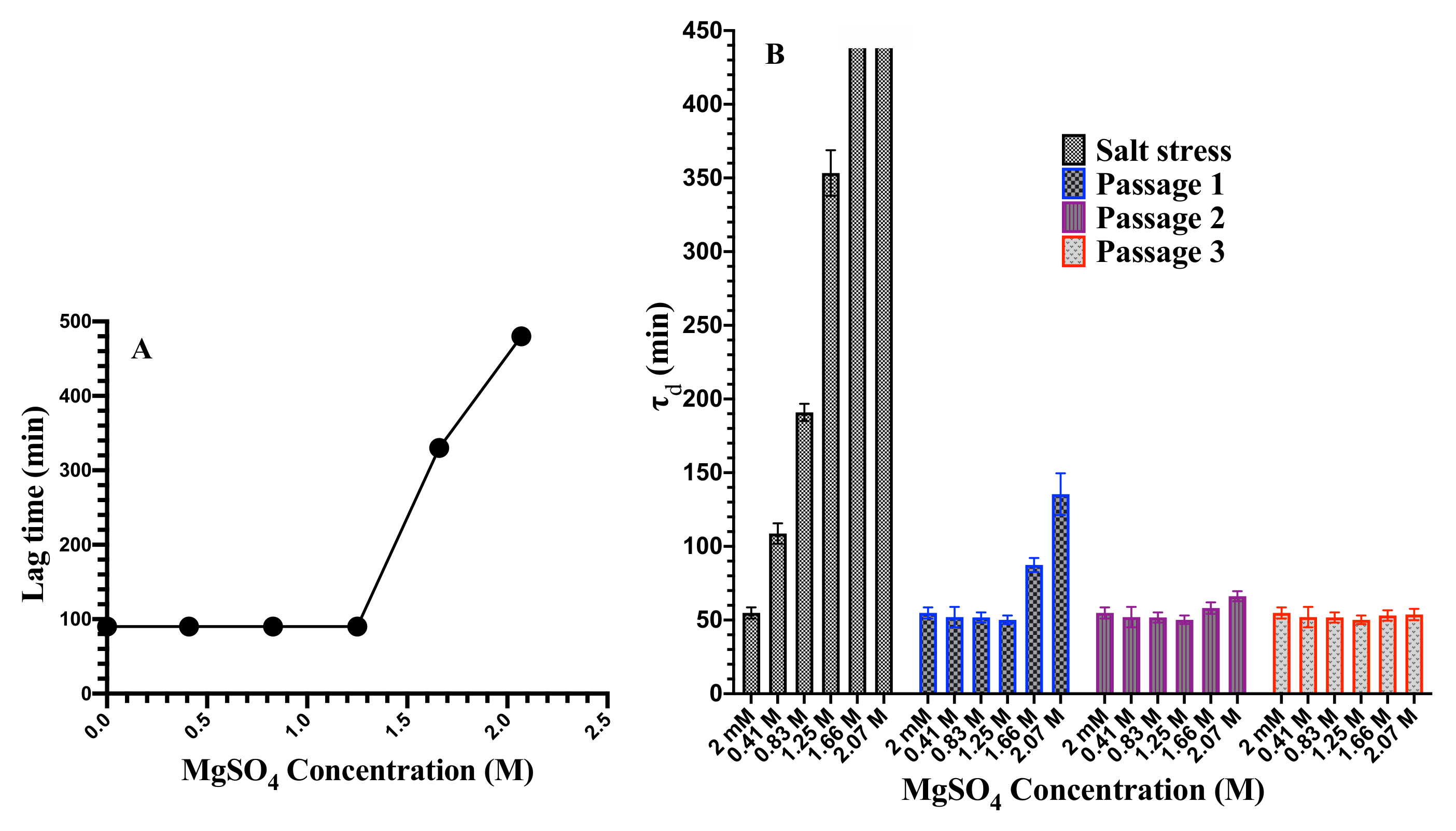
| Gene | Primer | Sequence (5–3) |
|---|---|---|
| 16S rRNA | Forward | TCGTCAGCTCGTGTTGTGAA |
| Reverse | AGGGCCATGATGACTTGACG | |
| corA | Forward | AACATCGAGCAGAACCGCAT |
| Reverse | AAAGATAATCGCGCCAGGGT | |
| cysP | Forward | CGCCGTTTGAGCAACAATGG |
| Reverse | TTTGTACGTCGGTCACCTGG | |
| osmC | Forward | ATCGATTGATACCACCGCCG |
| Reverse | GGGCATCCTGCTTTTGCTTT | |
| aqpZ | Forward | AGCATTCACCAGGCGGTTAT |
| Reverse | TCAGGGTTAAGGCCAGACCA |
| Concentration (M) | Water Activity | |
|---|---|---|
| MgSO | NaCl | |
| 0.1 | 0.9970 | 0.9967 |
| 0.2 | 0.9950 | 0.9934 |
| 0.3 | 0.9940 | 0.9901 |
| 0.4 | 0.9920 | 0.9868 |
| 0.5 | 0.9900 | 0.9836 |
| 0.6 | 0.9880 | 0.9803 |
| 0.7 | 0.9870 | 0.9769 |
| 0.8 | 0.9850 | 0.9736 |
| 0.9 | 0.9830 | 0.9702 |
| 1.0 | 0.9810 | 0.9669 |
| 1.2 | 0.9760 | 0.9601 |
| 1.25 | 0.9740 | 0.9552 |
| 1.4 | 0.9710 | 0.9532 |
| 1.6 | 0.9660 | 0.9461 |
| 1.8 | 0.9600 | 0.9389 |
| 2.0 | 0.9530 | 0.9316 |
| 2.5 | 0.9320 | 0.9127 |
| 3.0 | 0.9050 | 0.8932 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepal, S.; Kumar, P. Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars. Microorganisms 2020, 8, 637. https://doi.org/10.3390/microorganisms8050637
Nepal S, Kumar P. Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars. Microorganisms. 2020; 8(5):637. https://doi.org/10.3390/microorganisms8050637
Chicago/Turabian StyleNepal, Sudip, and Pradeep Kumar. 2020. "Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars" Microorganisms 8, no. 5: 637. https://doi.org/10.3390/microorganisms8050637
APA StyleNepal, S., & Kumar, P. (2020). Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars. Microorganisms, 8(5), 637. https://doi.org/10.3390/microorganisms8050637






