New Approaches for Cryptococcosis Treatment
Abstract
1. Introduction
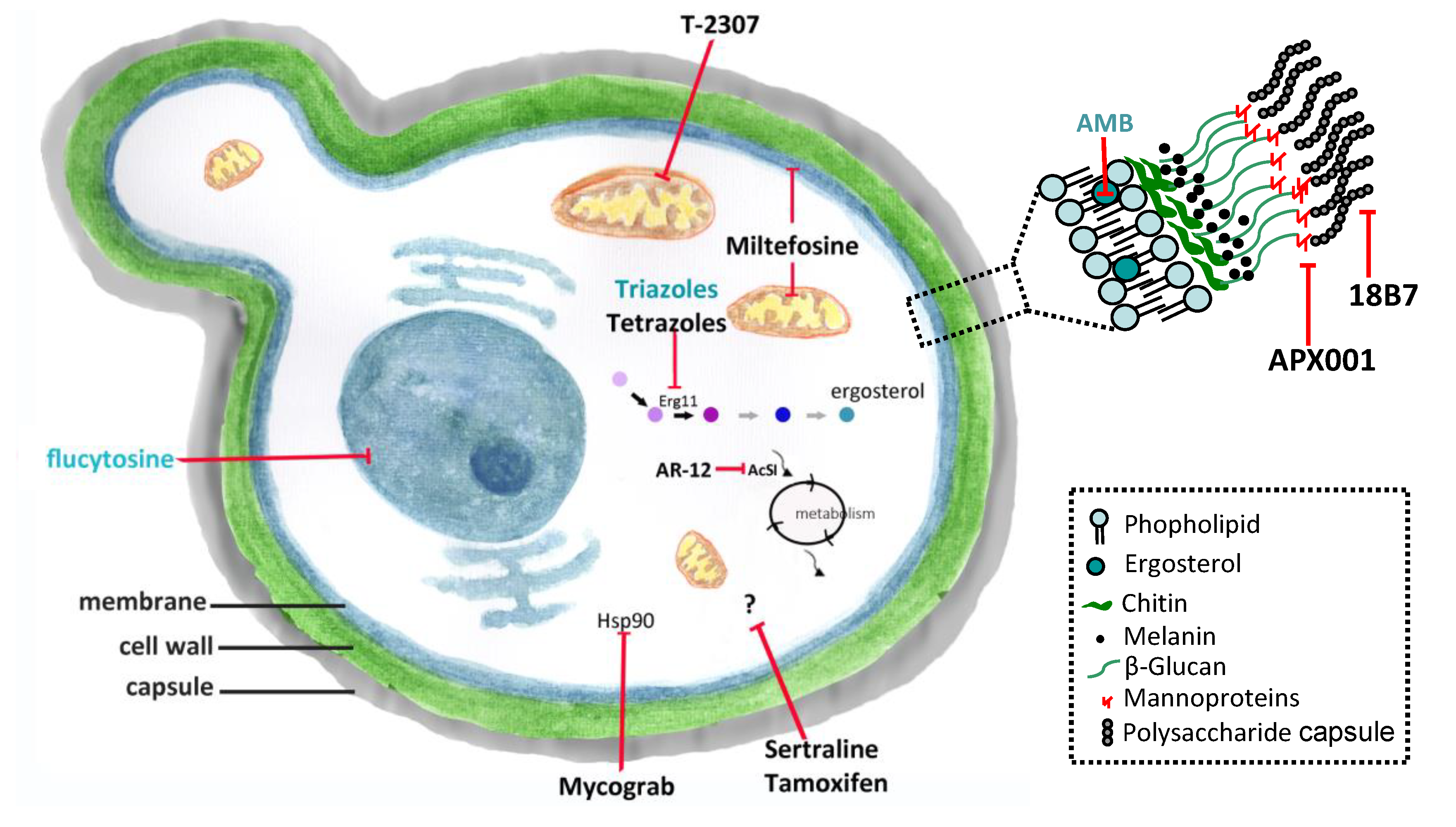
2. Current Therapy
2.1. Amphotericin B (AMB)
2.2. Flucytosine (5-FC)
2.3. Fluconazole (FLC)
2.4. Voriconazole (VRC)
3. New Molecules and Drug Repurposing
3.1. Interferon-Gamma (IFN-γ)
3.2. Mycograb
3.3. 18B7
3.4. APX001 (Fosmanogepix)/APX001A (Manogepix)
3.5. T-2307
3.6. Sertraline
3.7. Tamoxifen
3.8. AR-12
3.9. Miltefosine (MFS)
3.10. Tetrazoles
4. Other Molecules and Targets
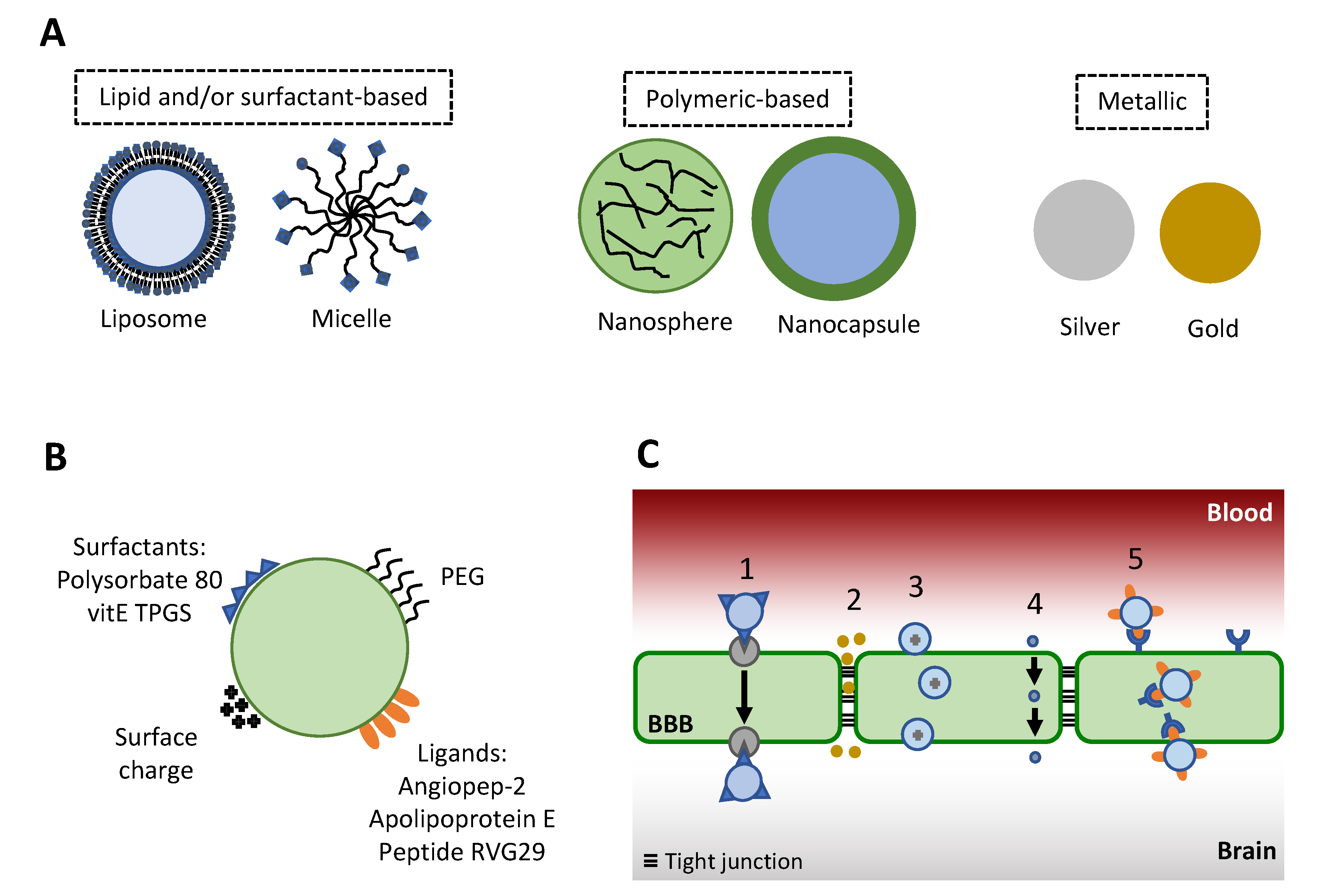
5. Drug Delivery Systems
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Andrew Alspaugh, J. The Cryptococcus neoformans capsule: A sword and a shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 3099, 1–10. [Google Scholar] [CrossRef]
- Loyse, A.; Thangaraj, H.; Easterbrook, P.; Ford, N.; Roy, M.; Chiller, T.; Govender, N.; Harrison, T.S.; Bicanic, T. Cryptococcal meningitis: Improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 2013, 13, 629–637. [Google Scholar] [CrossRef]
- Loyse, A.; Burry, J.; Cohn, J.; Ford, N.; Chiller, T.; Ribeiro, I.; Koulla-Shiro, S.; Mghamba, J.; Ramadhani, A.; Nyirenda, R.; et al. Leave no one behind: Response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries. Lancet Infect. Dis. 2019, 19, e143–e147. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Diagnosis, Prevention, and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. 2019. Available online: https://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/ (accessed on 20 February 2019).
- Cheong, J.W.S.; Mccormack, J. Fluconazole resistance in cryptococcal disease: Emerging or intrinsic? Med. Mycol. 2013, 51, 261–269. [Google Scholar] [CrossRef]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wirth, F.; de Azevedo, M.I.; Pilla, C.; Aquino, V.R.; Neto, G.W.; Goldani, L.Z. Relationship between intracranial pressure and antifungal agents levels in the CSF of patients with cryptococcal meningitis. Med. Mycol. 2018, 56, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kethireddy, S.; Andes, D. CNS pharmacokinetics of antifungal agents. Expert Opin. Drug Metab. Toxicol. 2007, 3, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier drug Targeting: The future of brain drug development. Mol. Interv. 2003, 3, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.D. Antifungal Drugs: Special problems treating Central Nervous System infections. J. Fungi 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; World Health Organization: Geneva, Switzerland, 2018; pp. 1–51. [Google Scholar]
- Perfect, J.R.; Bicanic, T. Cryptococcosis diagnosis and treatment: What do we know now. Fungal Genet. Biol. 2015, 78, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49, 7–10. [Google Scholar] [CrossRef]
- Wilcock, B.C.; Endo, M.M.; Uno, B.E.; Burke, M.D. C2′-OH of Amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 2013, 135, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, M.; Yang, Z. Design of amphotericin B oral formulation for antifungal therapy. Drug Deliv. 2017, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, G.; Wasan, E.K.; Di, Y.; Wasan, K.M. The development of oral amphotericin B to treat systemic fungal and parasitic infections: Has the myth been finally realized? Pharmaceutics 2019, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Chiu, M.; Rex, J.H. Resistance to antifungal agents. In Topley & Wilson’s Microbiology and Microbial Infections, 9th ed.; Arnold: London, UK, 1998; pp. 177–187. [Google Scholar]
- Posch, W.; Blatzer, M.; Wilflingseder, D.; Lass-Flörl, C. Aspergillus terreus: Novel lessons learned on amphotericin B resistance. Med. Mycol. 2018, 56, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, E.M.; Lyman, C.A.; Walsh, T.J. Amphotericin B: Polyene resistance mechanisms. In Antimicrobial Drug Resistance; Humana Press: Totowa, NJ, USA, 2009; pp. 295–305. [Google Scholar]
- Duschinsky, R.; Pleven, E.; Heidelberger, C. The synthesis of 5-Fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Tassel, D.; Madoff, M. Treatment of Candida sepsis and Cryptococcus meningitis with 5-Fluorocytosine. JAMA 1968, 206, 830. [Google Scholar] [CrossRef]
- Polak, A.; Scholer, H.J. Mode of action of 5-Fluorocytosine and mechanisms of resistance. Chemotherapy 1975, 21, 113–130. [Google Scholar] [CrossRef]
- Normark, S.; Schonebeck, J. In Vitro studies of 5-Fluorocytosine resistance in Candida albicans and Torulopsis glabrata. Antimicrob. Agents Chemother. 1972, 2, 114–121. [Google Scholar] [CrossRef]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef]
- Richardson, K. The discovery and profile of Fluconazole. J. Chemother. 1990, 2, 51–54. [Google Scholar] [CrossRef]
- Kartalija, M.; Kaye, K.; Tureen, J.H.; Liu, Q.; Tauber, M.G.; Elliott, B.R.; Sande, M.A. Treatment of experimental Cryptococcal meningitis with Fluconazole: Impact of dose and addition of Flucytosine on mycologic and pathophysiologic outcome. J. Infect. Dis. 1996, 173, 1216–1221. [Google Scholar] [CrossRef]
- Brammer, K.W.; Farrow, P.R.; Faulkner, J.K. Pharmacokinetics and tissue penetration of Fluconazole in humans. Clin. Infect. Dis. 1990, 12, S318–S326. [Google Scholar] [CrossRef] [PubMed]
- Goa, K.L.; Barradell, L.B. Fluconazole. Drugs 1995, 50, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Gago, S.; Serrano, C.; Alastruey-Izquierdo, A.; Cuesta, I.; Martín-Mazuelos, E.; Aller, A.I.; Gómez-López, A.; Mellado, E. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses 2017, 60, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Rodero, L.; Mellado, E.; Rodriguez, A.C.; Salve, A.; Guelfand, L.; Cahn, P.; Cuenca-Estrella, M.; Davel, G.; Rodriguez-Tudela, J.L. G484S amino acid substitution in Lanosterol 14-α Demethylase (ERG11) is related to Fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob. Agents Chemother. 2003, 47, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Sionov, E.; Chang, Y.C.; Garraffo, H.M.; Kwon-Chung, K.J. Heteroresistance to Fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob. Agents Chemother. 2009, 53, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Groll, A.H.; Chiou, C.C.; Walsh, T.J. Newer systemic antifungal agents. Drugs 2004, 64, 1997–2020. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/Pharmacodynamic profile of Voriconazole. Clin. Pharmacokinet. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- Mondon, P.; Petter, R.; Amalfitano, G.; Luzzati, R.; Concia, E.; Polacheck, I.; Kwon-Chung, K.J. Heteroresistance to Fluconazole and Voriconazole in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1999, 43, 1856–1861. [Google Scholar] [CrossRef]
- Mandras, N.; Roana, J.; Tullio, V.; Allizond, V.; Banche, G.; Scalas, D.; Fucale, G.; Cuffini, A. A case of Fluconazole, Voriconazole-resistant Cryptococcus neoformans isolated from an immunocompetent patient. J. Chemother. 2011, 23, 379–380. [Google Scholar] [CrossRef]
- Perkins, A.; Gomez-Lopez, A.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Rates of antifungal resistance among Spanish clinical isolates of Cryptococcus neoformans var. neoformans. J. Antimicrob. Chemother. 2005, 56, 1144–1147. [Google Scholar] [CrossRef]
- Kano, R.; Okubo, M.; Hasegawa, A.; Kamata, H. Multi-azole-resistant strains of Cryptococcus neoformans var. grubii isolated from a FLZ-resistant strain by culturing in medium containing voriconazole. Med. Mycol. 2017, 55, 877–882. [Google Scholar] [PubMed]
- Tsubamoto, H.; Ueda, T.; Inoue, K.; Sakata, K.; Shibahara, H.; Sonoda, T. Repurposing itraconazole as an anticancer agent. Oncol. Lett. 2017, 14, 1240–1246. [Google Scholar] [CrossRef]
- Truong, M.; Monahan, L.G.; Carter, D.A.; Charles, I.G. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 2018, 6, e4761. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Bustamante, B.; Ticona, E.; Hamill, R.J.; Johnson, P.C.; Reboli, A.; Aberg, J.; Hasbun, R.; Hsu, H.H. Recombinant Interferon-γ1b as adjunctive therapy for AIDS-related acute Cryptococcal meningitis. J. Infect. Dis. 2004, 189, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Tohyama, M.; Teruya, K.; Kudeken, N.; Xie, Q.; Saito, A. Contribution of interferon-γ in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 1996, 13, 123–130. [Google Scholar]
- Jarvis, J.N.; Meintjes, G.; Rebe, K.; Williams, G.N.; Bicanic, T.; Williams, A.; Schutz, C.; Bekker, L.-G.; Wood, R.; Harrison, T.S. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis. AIDS 2012, 26, 1105–1113. [Google Scholar] [CrossRef]
- Matthews, R.C.; Rigg, G.; Hodgetts, S.; Carter, T.; Chapman, C.; Gregory, C.; Illidge, C.; Burnie, J. Preclinical assessment of the efficacy of Mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 2003, 47, 2208–2216. [Google Scholar] [CrossRef]
- de Aguiar Cordeiro, R.; de Jesus Evangelista, A.J.; Serpa, R.; de Farias Marques, F.J.; de Melo, C.V.S.; de Oliveira, J.S.; Sidrim, J.J.C. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology 2016, 162, 309–317. [Google Scholar] [PubMed]
- Nooney, L.; Matthews, R.C.; Burnie, J.P. Evaluation of Mycograb®, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn. Microbiol. Infect. Dis. 2005, 51, 19–29. [Google Scholar] [CrossRef]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.A.; Rivera, J.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [CrossRef]
- Mukherjee, J.; Feldmesser, M.; Scharff, M.D.; Casadevall, A. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob. Agents Chemother. 1995, 39, 1398–1405. [Google Scholar] [CrossRef]
- Feldmesser, M.; Mukherjee, J.; Casadevall, A. Combination of 5-flucytosine and capsule-binding monoclonal antibody in the treatment of murine Cryptococcus neoformans infections and in vitro. J. Antimicrob. Chemother. 1996, 37, 617–622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dromer, F.; Charreire, J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: Study during murine Cryptococcosis and mechanisms of action. J. Infect. Dis. 1991, 163, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, M.; Casadevall, A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 1997, 158, 790–799. [Google Scholar] [PubMed]
- Larsen, R.A.; Pappas, P.G.; Perfect, J.; Aberg, J.A.; Casadevall, A.; Cloud, G.A.; James, R.; Filler, S.; Dismukes, W.E. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated Cryptococcal meningitis. Antimicrob. Agents Chemother. 2005, 49, 952–958. [Google Scholar] [CrossRef]
- Zhao, M.; Lepak, A.J.; Marchillo, K.; Vanhecker, J.; Sanchez, H.; Ambrose, P.G.; Andes, D.R. APX001 Pharmacokinetic/Pharmacodynamic target determination against Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2019, 63, e02372-18. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Flamm, R.K.; Bien, P.A.; Castanheira, M. In Vitro activity of APX001A (Manogepix) and comparator agents against 1,706 fungal isolates collected during an International Surveillance Program (2017). Antimicrob. Agents Chemother. 2019, 63, e00840-19. [Google Scholar] [CrossRef]
- Shaw, K.J.; Schell, W.A.; Covel, J.; Duboc, G.; Giamberardino, C.; Kapoor, M.; Moloney, M.; Soltow, Q.A.; Tenor, J.L.; Toffaletti, D.L.; et al. In Vitro and In Vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob. Agents Chemother. 2018, 62, e00523-18. [Google Scholar] [CrossRef]
- Shibata, T.; Takahashi, T.; Yamada, E.; Kimura, A.; Nishikawa, H.; Hayakawa, H.; Nomura, N.; Mitsuyama, J. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 2012, 56, 5892–5897. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Nomura, N.; Hashimoto, K.; Yamada, E.; Nishikawa, H.; Kaeriyama, M.; Kimura, A.; Todo, Y.; Narita, H. In Vitro and In Vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 2008, 52, 1318–1324. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukuda, Y.; Mitsuyama, J.; Tashiro, M.; Tanaka, A.; Takazono, T.; Saijo, T.; Yamamoto, K.; Nakamura, S.; Imamura, Y.; et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: An emerging fungal pathogen. J. Antimicrob. Chemother. 2017, 72, 1709–1713. [Google Scholar] [CrossRef]
- DeVane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of Sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The Antidepressant Sertraline provides a promising therapeutic option for neurotropic Cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Trevinõ-Rangel, R.D.J.; Villanueva-Lozano, H.; Hernández-Rodríguez, P.; Martínez-Reséndez, M.F.; Garciá-Juárez, J.; Rodríguez-Rocha, H.; González, G.M. Activity of sertraline against Cryptococcus neoformans: In vitro and in vivo assays. Med. Mycol. 2016, 54, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Xu, J. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology 2010, 1, 99–105. [Google Scholar] [CrossRef][Green Version]
- Spitzer, M.; Griffiths, E.; Blakely, K.M.; Wildenhain, J.; Ejim, L.; Rossi, L.; De Pascale, G.; Curak, J.; Brown, E.; Tyers, M.; et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 2011, 7, 1–14. [Google Scholar] [CrossRef]
- Rhein, J.; Huppler Hullsiek, K.; Tugume, L.; Nuwagira, E.; Mpoza, E.; Evans, E.E.; Kiggundu, R.; Pastick, K.A.; Ssebambulidde, K.; Akampurira, A.; et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: A randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect. Dis. 2019, 19, 843–851. [Google Scholar] [CrossRef]
- Morello, K.C.; Wurz, G.T.; DeGregorio, M.W. Pharmacokinetics of selective estrogen receptor modulators. Clin. Pharmacokinet. 2003, 42, 361–372. [Google Scholar] [CrossRef]
- Hai, T.P.; Van, A.D.; Ngan, N.T.T.; Nhat, L.T.H.; Lan, N.P.H.; Van Vinh Chau, N.; Thwaites, G.E.; Krysan, D.; Day, J.N. The combination of tamoxifen with amphotericin B, but not with fluconazole, has synergistic activity against the majority of clinical isolates of Cryptococcus neoformans. Mycoses 2019, 62, 818–825. [Google Scholar] [CrossRef]
- Butts, A.; Koselny, K.; Chabrier-Roselló, Y.; Semighini, C.P.; Brown, J.C.S.; Wang, X.; Annadurai, S.; DiDone, L.; Tabroff, J.; Childers, W.E.; et al. Estrogen receptor antagonists are anti-Cryptococcal agents that directly bind EF Hand proteins and synergize with Fluconazole In Vivo. MBio 2014, 5, e00765-13. [Google Scholar] [CrossRef]
- Santos-Gandelman, J.; Rodrigues, M.L.; Machado Silva, A. Future perspectives for cryptococcosis treatment. Expert Opin. Ther. Pat. 2018, 28, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Green, J.; Favazzo, L.; Glazier, V.E.; DiDone, L.; Ransford, S.; Krysan, D.J. Antitumor/Antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme Acetyl CoA synthetase. ACS Infect. Dis. 2016, 2, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Green, J.; DiDone, L.; Halterman, J.P.; Fothergill, A.W.; Wiederhold, N.P.; Patterson, T.F.; Cushion, M.T.; Rappelye, C.; Wellington, M.; et al. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob. Agents Chemother. 2016, 60, 7115–7127. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Ravu, R.R.; Chen, Y.L.; Jacob, M.R.; Pan, X.; Agarwal, A.K.; Khan, S.I.; Heitman, J.; Clark, A.M.; Li, X.C. Synthesis and antifungal activities of miltefosine analogs. Bioorganic Med. Chem. Lett. 2013, 23, 4828–4831. [Google Scholar] [CrossRef] [PubMed]
- Widmer, F.; Wright, L.C.; Obando, D.; Handke, R.; Ganendren, R.; Ellis, D.H.; Sorrell, T.C. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 2006, 50, 414–421. [Google Scholar] [CrossRef]
- Spadari, C.C.; Vila, T.; Rozental, S.; Ishida, K. Miltefosine has a postantifungal effect and induces apoptosis in Cryptococcus yeasts. Antimicrob. Agents Chemother. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Spadari, C.C.; da Silva de Bastiani, F.W.M.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomedicine 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Bocanegra, R.; Kirkpatrick, W.R.; Sorrell, T.C.; Patterson, T.F. Limited activity of Miltefosine in murine models of Cryptococcal meningoencephalitis and disseminated Cryptococcosis. Antimicrob. Agents Chemother. 2013, 57, 745–750. [Google Scholar] [CrossRef]
- Qian, A.; Zheng, Y.; Wang, R.; Wei, J.; Cui, Y.; Cao, X.; Yang, Y. Design, synthesis, and structure-activity relationship studies of novel tetrazole antifungal agents with potent activity, broad antifungal spectrum and high selectivity. Bioorganic Med. Chem. Lett. 2018, 28, 344–350. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Wang, Y.-F.; Xu, Z. Tetrazole hybrids and their antifungal activities. Eur. J. Med. Chem. 2019, 170, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. The antifungal arsenal: Alternative drugs and future targets. Int. J. Antimicrob. Agents 2018, 51, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Fothergill, A.W.; Iqbal, N.; Bolden, C.B.; Grossman, N.T.; Garvey, E.P.; Brand, S.R.; Hoekstra, W.J.; Schotzinger, R.J.; Ottinger, E.; et al. Potent In Vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob. Agents Chemother. 2016, 60, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Vedula, P.; Smith, K.D.; Meya, D.B.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Boulware, D.R. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med. Mycol. 2017, 55, 453–456. [Google Scholar] [PubMed]
- Wiederhold, N.P.; Patterson, H.P.; Tran, B.H.; Yates, C.M.; Schotzinger, R.J.; Garvey, E.P. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J. Antimicrob. Chemother. 2018, 73, 404–408. [Google Scholar] [CrossRef]
- Garvey, E.P.; Sharp, A.D.; Warn, P.A.; Yates, C.M.; Schotzinger, R.J. The novel fungal CYP51 inhibitor VT-1598 is efficacious alone and in combination with liposomal amphotericin B in a murine model of cryptococcal meningitis. J. Antimicrob. Chemother. 2018, 73, 2815–2822. [Google Scholar] [CrossRef]
- Rossi, S.A.; de Oliveira, H.C.; Agreda-Mellon, D.; Lucio, J.; Mendes Giannini, M.J.S.; García-Cambero, J.P.; Zaragoza, O. Identification of off-patent drugs that show synergism with Amphotericin B or that present antifungal action against Cryptococcus neoformans and Candida spp. Antimicrob. Agents Chemother. 2020, 64, e01921-19. [Google Scholar] [CrossRef]
- Joffe, L.S.; Schneider, R.; Lopes, W.; Azevedo, R.; Staats, C.C.; Kmetzsch, L.; Schrank, A.; Del Poeta, M.; Vainstein, M.H.; Rodrigues, M.L. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front. Microbiol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Nixon, G.L.; McEntee, L.; Johnson, A.; Farrington, N.; Whalley, S.; Livermore, J.; Natal, C.; Washbourn, G.; Bibby, J.; Berry, N.; et al. Repurposing and reformulation of the antiparasitic agent flubendazole for treatment of Cryptococcal meningoencephalitis, a neglected fungal disease. Antimicrob. Agents Chemother. 2018, 62, e01909-17. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Ovalle, R.; Casadevall, A. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic Infection. J. Infect. Dis. 2001, 183, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef]
- Mor, V.; Rella, A.; Farnoud, A.M.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.B.; Ojima, I.; Bullesbach, E.; et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. MBio 2015, 6, e00647-15. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Zeeshan, M.; Mukhtar, M.; Ul Ain, Q.; Khan, S.; Ali, H. Nanopharmaceuticals: A boon to the brain-targeted drug delivery. In Pharmaceutical Formulation Design—Recent Practices; IntechOpen: London, UK, 2020; Volume I, pp. 1–21. [Google Scholar]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood–brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood–brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, M.; Singh, A. V Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2014, 14, 1201–1212. [Google Scholar] [CrossRef]
- Garcia-Garcia, E.; Andrieux, K.; Gil, S.; Couvreur, P. Colloidal carriers and blood–brain barrier (BBB) translocation: A way to deliver drugs to the brain? Int. J. Pharm. 2005, 298, 274–292. [Google Scholar] [CrossRef]
- Collnot, E.-M.; Baldes, C.; Schaefer, U.F.; Edgar, K.J.; Wempe, M.F.; Lehr, C.-M. Vitamin E TPGS P-Glycoprotein inhibition mechanism: Influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol. Pharm. 2010, 7, 642–651. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2012, 64, 213–222. [Google Scholar] [CrossRef]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.-W.; Mannino, R.; Zarif, L.; Perlin, D.S. Efficacy of oral cochleate-Amphotericin B in a mouse model of systemic Candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef]
- Lu, R.; Hollingsworth, C.; Qiu, J.; Wang, A.; Hughes, E.; Xin, X.; Konrath, K.M.; Elsegeiny, W.; Park, Y.-D.; Atakulu, L.; et al. Efficacy of oral encochleated Amphotericin B in a mouse model of Cryptococcal meningoencephalitis. MBio 2019, 10, e00724-19. [Google Scholar] [CrossRef]
- Ren, T.; Xu, N.; Cao, C.; Yuan, W.; Yu, X.; Chen, J.; Ren, J. Preparation and therapeutic efficacy of polysorbate-80-coated Amphotericin B/PLA-b-PEG Nanoparticles. J. Biomater. Sci. Polym. 2009, 20, 1369–1380. [Google Scholar] [CrossRef]
- Xu, N.; Gu, J.; Zhu, Y.; Wen, H.; Ren, Q.; Chen, J. Efficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in mice. Int. J. Nanomed. 2011, 6, 905–913. [Google Scholar] [CrossRef]
- Shao, K.; Huang, R.; Li, J.; Han, L.; Ye, L.; Lou, J.; Jiang, C. Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Control. Release 2010, 147, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Wu, J.; Chen, Z.; Huang, S.; Li, J.; Ye, L.; Lou, J.; Zhu, L.; Jiang, C. A brain-vectored angiopep-2 based polymeric micelles for the treatment of intracranial fungal infection. Biomaterials 2012, 33, 6898–6907. [Google Scholar] [CrossRef]
- Ćurić, A.; Keller, B.L.; Reul, R.; Möschwitzer, J.; Fricker, G. Development and lyophilization of itraconazole loaded poly(butyl cyanoacrylate) nanospheres as a drug delivery system. Eur. J. Pharm. Sci. 2015, 78, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ćurić, A.; Möschwitzer, J.P.; Fricker, G. Development and characterization of novel highly-loaded itraconazole poly(butyl cyanoacrylate) polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2017, 114, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Asghar, S.; Yang, L.; Hu, Z.; Chen, Z.; Shao, F.; Xiao, Y. Borneol and poly (ethylene glycol) dual modified BSA nanoparticles as an itraconazole vehicle for brain targeting. Int. J. Pharm. 2020, 575, 119002. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Fu, B.M.; Zhang, Z.-J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhan, C.; Gu, B.; Meng, Q.; Wang, H.; Lu, W.; Hou, H. Targeted brain delivery of itraconazole via RVG29 anchored nanoparticles. J. Drug Target. 2011, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadari, C.d.C.; Wirth, F.; Lopes, L.B.; Ishida, K. New Approaches for Cryptococcosis Treatment. Microorganisms 2020, 8, 613. https://doi.org/10.3390/microorganisms8040613
Spadari CdC, Wirth F, Lopes LB, Ishida K. New Approaches for Cryptococcosis Treatment. Microorganisms. 2020; 8(4):613. https://doi.org/10.3390/microorganisms8040613
Chicago/Turabian StyleSpadari, Cristina de Castro, Fernanda Wirth, Luciana Biagini Lopes, and Kelly Ishida. 2020. "New Approaches for Cryptococcosis Treatment" Microorganisms 8, no. 4: 613. https://doi.org/10.3390/microorganisms8040613
APA StyleSpadari, C. d. C., Wirth, F., Lopes, L. B., & Ishida, K. (2020). New Approaches for Cryptococcosis Treatment. Microorganisms, 8(4), 613. https://doi.org/10.3390/microorganisms8040613




