Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Isolation and Identification of Endophytic Fungi
2.3. Fermentation Medium
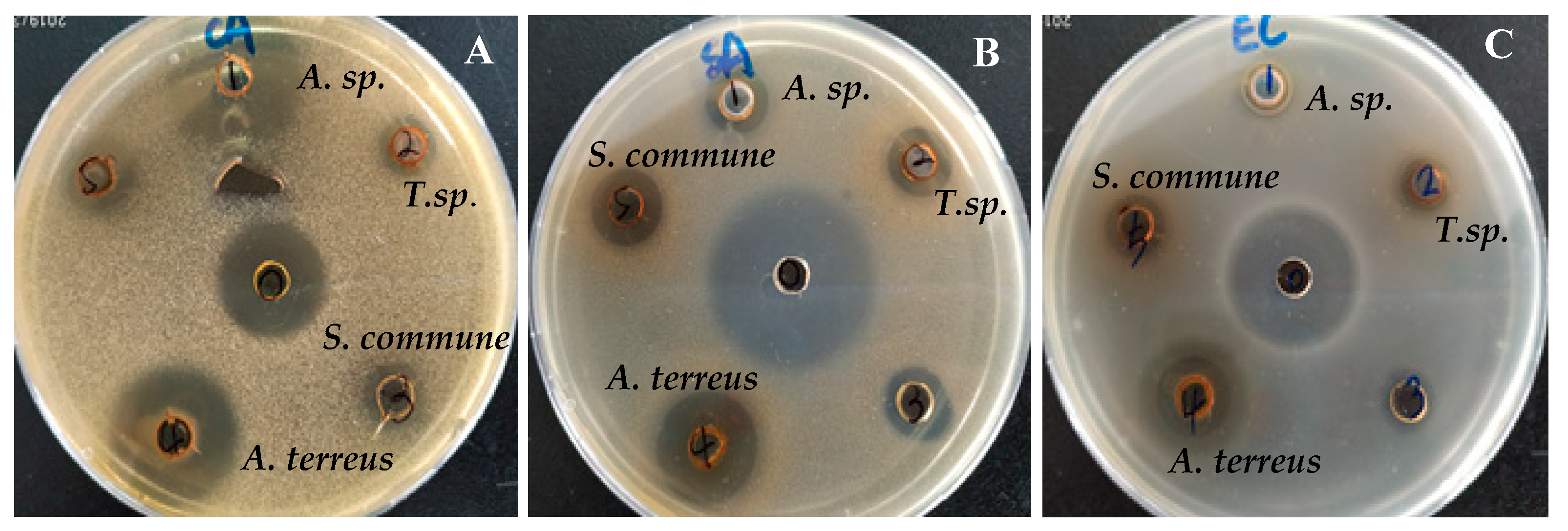
2.4. Antimicrobial Assay
2.5. Melanin Content Assay
2.5.1. Cell Culture
2.5.2. Melanin Measurement
2.5.3. Tyrosinase Activity Assay
2.6. Protein Tyrosine Phosphatase 1B (PTP1B) Inhibition Assay
2.7. Cytotoxic Activity (MTT Assay)
2.8. DPPH Radical Scavenging Activity Assay
2.9. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Endophytic Fungi
3.2. Chemical Composition of the Endophyic Fungus Aspergillus sp. XJA6 Extract by GC–MS
3.3. Antimicrobial Activity of Endophytic Fungi
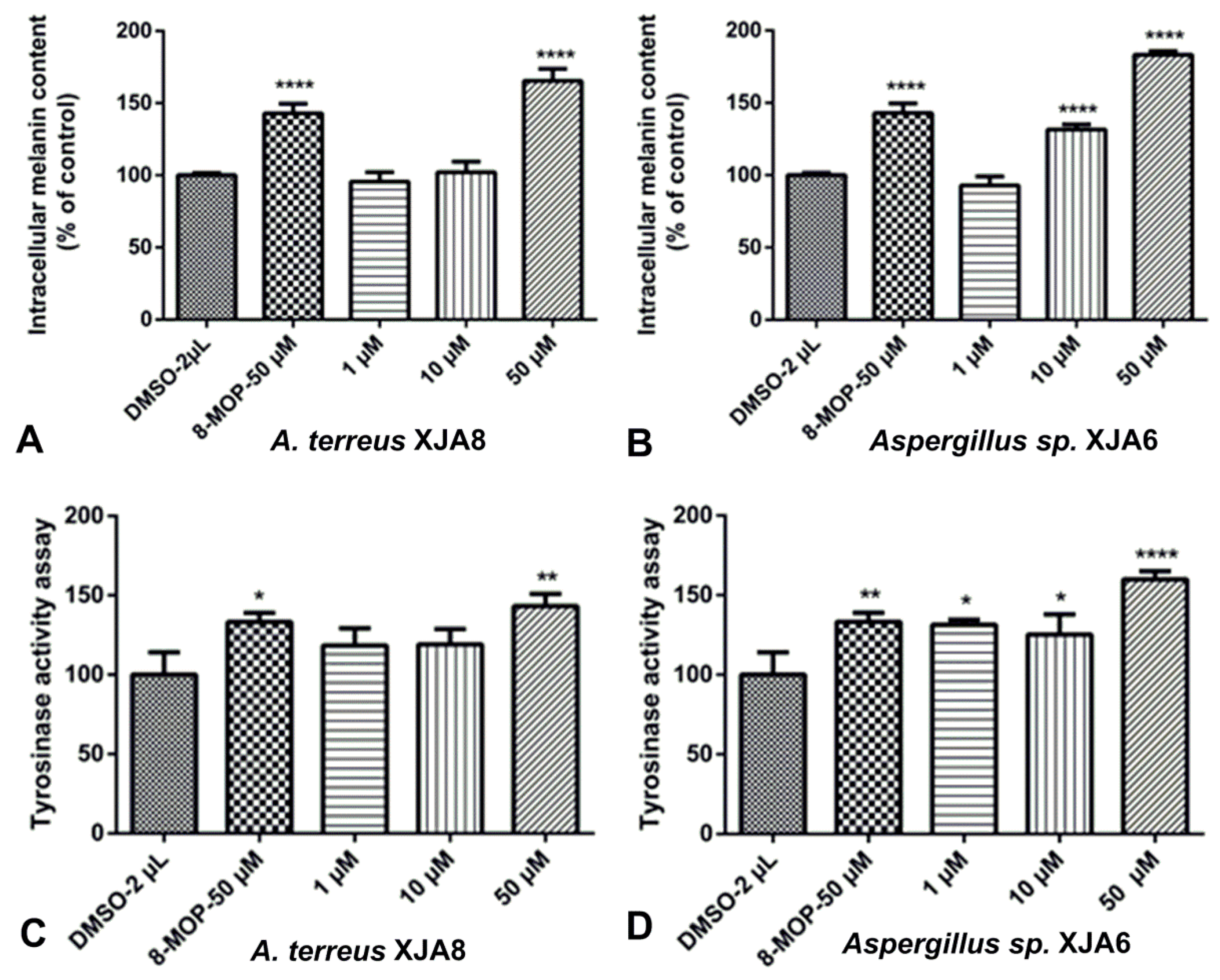
3.4. Effect of Crude Extracts of Endophytic Fungi Derived from V. anthelmintica Root on Melanin Content Assay and Tyrosinase Activity in B16 cells
3.5. Antidiabetic Activity (PTP1B Assay)
3.6. Cytotoxic Activity of Ethyl Acetate Extracts Obtained from Fungal Endophytes
3.7. DPPH Radical Scavenging Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asaf, S.; Khan, M.A.; Khan, A.L.; Waqas, M.; Shahzad, R.; Kim, A.-Y.; Kang, S.-M.; Lee, I.-J. Bacterial endophytes from arid land plants regulate endogenous hormone content and promote growth in crop plants: An example of Sphingomonas sp. and Serratia marcescens. J. Plant. Interact. 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Akhter, N.; Pan, C.; Liu, Y.; Shi, Y.; Wu, B. Isolation and structure determination of a new indene derivative from endophytic fungus Aspergillus flavipes Y-62. Nat. Prod. Res. 2019, 33, 2939–2944. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Hosni, K.; Kim, M.-J.; Adnan, M.; Lee, I.-J. Endophytes Aspergillus caespitosus LK12 and Phoma sp. LK13 of Moringa peregrina produce gibberellins and improve rice plant growth. J. Plant. Interact. 2014, 9, 731–737. [Google Scholar] [CrossRef]
- Xie, S.; Wu, Y.; Qiao, Y.; Guo, Y.; Wang, J.; Hu, Z.; Zhang, Q.; Li, X.; Huang, J.; Zhou, Q.; et al. Protoilludane, Illudalane, and Botryane Sesquiterpenoids from the Endophytic Fungus Phomopsis sp. TJ507A. J. Nat. Prod. 2018, 81, 1311–1320. [Google Scholar] [CrossRef]
- Liao, G.; Wu, P.; Xue, J.; Liu, L.; Li, H.; Wei, X. Asperimides A–D, anti-inflammatory aromatic butenolides from a tropical endophytic fungus Aspergillus terreus. Fitoterapia 2018, 131, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, T.; Zhang, Z.; Ding, M.; Long, Y.; She, Z. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus versicolor SYSU-SKS025. Fitoterapia 2018, 124, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Shen, W.; Wang, J.; Han, M.; Chai, F.; Duan, X.; Yan, X.; Guo, J.; Gao, T.; Zuo, S.; et al. Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4.1850. Phytochemistry 2019, 158, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, K.I.; Hirose, D.; Suzuki, T.; Nakayama, W.; Jiang, W.-P.; Monthakantirat, O.; Wu, J.-B.; Kitanaka, S.; Makino, T. Identification of Lycopodium Alkaloids Produced by an Ultraviolet-Irradiated Strain of Paraboeremia, an Endophytic Fungus from Lycopodium serratum var. longipetiolatum. J. Nat. Prod. 2018, 81, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-L.; Wang, G.; Xu, F.-Q.; Liu, J.-S.; Wang, J.-T.; Zhang, R.; Liu, H.-T.; Hu, J.-M.; Wang, G.-K.; Wu, P.-Y. Aspergilolide, a steroid lactone produced by an endophytic fungus Aspergillus sp. MBL1612 isolated from Paeonia ostii. Nat. Prod. Res. 2019, 33, 2133–2138. [Google Scholar] [CrossRef]
- Hu, H.-B.; Luo, Y.-F.; Wang, P.; Wang, W.-J.; Wu, J. Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21. Fitoterapia 2018, 131, 265–271. [Google Scholar] [CrossRef]
- Numonov, S.; Edirs, S.; Bobakulov, K.; Qureshi, M.N.; Bozorov, K.; Sharopov, F.; Setzer, W.N.; Zhao, H.; Habasi, M.; Sharofova, M.; et al. Evaluation of the Antidiabetic Activity and Chemical Composition of Geranium collinum Root Extracts—Computational and Experimental Investigations. Molecules 2017, 22, 983. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Choowong, W.; Rachtawee, P.; Boonyuen, N.; Kongthong, S.; Isaka, M.; Thongpanchang, C. Bioactive hydroanthraquinones from endophytic fungus Nigrospora sp. BCC 47789. Phytochem. Lett. 2018, 24, 46–50. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.-H.; Zhu, K.; Wang, Y.; Cheng, Z.-Q.; Jiang, C.-S.; Dai, J.-G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-J.; Chen, H.-Q.; Wang, H.; Cai, C.-H.; Mei, W.-L.; Dai, H.-F. New secondary metabolites from the endophytic fungus Fusarium sp. HP-2 isolated from “Qi-Nan” agarwood. Fitoterapia 2018, 130, 180–183. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Panda, S.K.; Luyten, W.; Cimmino, A.; Tayung, K.; Evidente, A. Antimicrobial secondary metabolites of an endolichenic Aspergillus niger isolated from lichen thallus of Parmotrema ravum. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Xin, X.-Q.; Chen, Y.; Zhang, H.; Li, Y.; Yang, M.-H.; Kong, L.-Y. Cytotoxic seco-cytochalasins from an endophytic Aspergillus sp. harbored in Pinellia ternata tubers. Fitoterapia 2019, 132, 53–59. [Google Scholar] [CrossRef]
- Sharma, V.; Singamaneni, V.; Sharma, N.; Kumar, A.; Arora, D.; Kushwaha, M.; Bhushan, S.; Jaglan, S.; Gupta, P. Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorg. Med. Chem. Lett. 2018, 28, 2217–2221. [Google Scholar] [CrossRef]
- Elissawy, A.M.; Ebada, S.S.; Ashour, M.L.; El-Neketi, M.; Ebrahim, W.; Singab, A.B. New secondary metabolites from the mangrove-derived fungus Aspergillus sp. AV-2. Phytochem. Lett. 2019, 29, 1–5. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, U.; Zhang, T.; Yang, F.; Ito, Y. Separation of flavonoids from the seeds of Vernonia anthelmintica Willd by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1049, 219–222. [Google Scholar] [CrossRef]
- Maimaiti, Z.; Turak, A.; Aisa, H.A. Two new compounds from the seeds of Vernonia anthelmintica. J. Asian Nat. Prod. Res. 2017, 19, 862–868. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.-Q.; Chen, T.; Xuan, L.-J. New flavonoid glycosides from seeds of Baccharoides anthelmintica. Nat. Prod. Res. 2020, 34, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Rakhymbay, L.; Turak, A.; Zhenis, Z.; Aisa, H.A. Phenolic Compounds from Vernonia anthelmintica Seeds. Chem. Nat. Compd. 2019, 55, 732–733. [Google Scholar] [CrossRef]
- Rigerte, L.; Blumenstein, K.; Terhonen, E. New R-Based Methodology to Optimize the Identification of Root Endophytes against Heterobasidion parviporum. Microorganisms 2019, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.P.D.; Silva, L.C.N.D.; Silva, M.V.D.; Araújo, J.M.D.; Cavalcanti, M.D.S.; Lima, V.L.D.M. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front. Micobiol. 2015, 6, 350. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Lamshöft, M.; Schüffler, A.; Laatsch, H.; Spiteller, M. Antibacterial Secondary Metabolites from an Endophytic Fungus, Eupenicillium sp. LG41. J. Nat. Prod. 2014, 77, 2335–2341. [Google Scholar] [CrossRef]
- Rustamova, N.; Wubulikasimu, A.; Mukhamedov, N.; Gao, Y.; Egamberdieva, D.; Yili, A. Endophytic bacteria associated with medicinal plant Vernonia anthelmintica—Diversity and characterization. Curr. Microbiol. 2020. [Google Scholar] [CrossRef]
- Rustamova, N.; Bobakulov, K.; Begmatov, N.; Turak, A.; Yili, A.; Aisa, H.A. Secondary metabolites produced by endophytic Pantoea ananatis derived from roots of Baccharoides anthelmintica and their effect on melanin synthesis in murine B16 cells. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Nie, L.F.; Bozorov, K.; Niu, C.; Huang, G.; Aisa, H.A. Synthesis and biological evaluation of novel sulfonamide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones on melanin synthesis in murine B16 cells. Res. Chem. Intermed. 2017, 43, 6835–6843. [Google Scholar] [CrossRef]
- Nie, L.F.; Bozorov, K.; Huang, G.; Zhao, J.; Niu, C.; Aisa, H.A. Design, synthesis, and toward a side-ring optimization of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and their effect on melanin synthesis in murine B16 cells. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 656–667. [Google Scholar] [CrossRef]
- Nie, L.F.; Huang, G.; Bozorov, K.; Zhao, J.; Niu, C.; Sagdullaev, S.S.; Aisa, H.A. Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells. Heterocycl. Commun. 2018, 24, 43–50. [Google Scholar] [CrossRef]
- Niu, C.; Yin, L.; Aisa, H.A. Novel Furocoumarin Derivatives Stimulate Melanogenesis in B16 Melanoma Cells by Up-Regulation of MITF and TYR Family via Akt/GSK3β/β-Catenin Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 746. [Google Scholar] [CrossRef] [PubMed]
- Salamet, E.; Ablajan, T.; Sodik, N.; Xuelei, X.; Akber, A.H. Optimization of Extraction Process for Antidiabetic and Antioxidant Activities of Kursi Wufarikun Ziyabit Using Response Surface Methodology and Quantitative Analysis of Main Components. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–14. [Google Scholar]
- Bozorov, K.; Ma, H.-R.; Zhao, J.-Y.; Zhao, H.-Q.; Chen, H.; Bobakulov, K.; Xin, X.-L.; Elmuradov, B.; Shakhidoyatov, K.; Aisa, H.A. Discovery of diethyl 2,5-diaminothiophene-3,4-dicarboxylate derivatives as potent anticancer and antimicrobial agents and screening of anti-diabetic activity: Synthesis and in vitro biological evaluation. Part 1. Eur. J. Med. Chem. 2014, 84, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.y.; Nie, L.F.; Ma, H.-R.; Bobakulov, K.; Hu, R.; Rustamova, N.; Huang, G.; Efferth, T.; Aisa, H.A. Synthesis and in vitro biological evaluation of novel diaminothiophene scaffolds as antitumor and anti-influenza virus agents. Part 2. RSC Adv. 2017, 7, 31417–31427. [Google Scholar] [CrossRef]
- Bozorov, K.A.; Mamadalieva, N.Z.; Elmuradov, B.Z.; Triggiani, D.; Egamberdieva, D.; Tiezzi, A.; Aisa, H.A.; Shakhidoyatov, K.M. Synthesis of substituted thieno[2,3-D]pyrimidin-4-ones and their testing for evaluation of cytotoxic activity on mammalian cell models. J. Chem. 2013, 1–6. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Abuduwaili, A.; Mutailifu, P.; Wubulikasimu, A.; Rustamova, N.; Jingxue, C.; Aisa, H.A.; Yili, A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int. J. Biol. Macromol. 2019, 141, 431–443. [Google Scholar] [CrossRef]
- Nishanbaev, S.; Bobakulov, K.; Okhundedaev, B.; Sasmakov, S.; Yusupova, E.; Azimova, S.; Abdullaev, N. Component composition of the extracts and essential oils from the Alhagi canescens, growing in Uzbekistan and their antimicrobial activity. Nat. Prod. Res. 2019, 33, 3417–3420. [Google Scholar] [CrossRef]
- Hua, L.; Li, Y.; Wang, F.; Lu, D.-F.; Gao, K. Biologically active steroids from the aerial parts of Vernonia anthelmintica Willd. Fitoterapia 2012, 83, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Rajneesh, R.; Ashok, K.; Vihan, V.S. In vitro antimicrobial property of Vernonia anthelmintica seeds against P. multocida. Indian. Vet. J. 2010, 87, 120–121. [Google Scholar]
- Kamel, R.A.; Abdel-Razek, A.S.; Hamed, A.; Ibrahim, R.R.; Stammler, H.G.; Frese, M.; Sewald, N.; Shaaban, M. Isoshamixanthone: A new pyrano xanthone from endophytic Aspergillus sp. ASCLA and absolute configuration of epiisoshamixanthone. Nat. Prod. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Turak, A.; Maimaiti, Z.; Ma, H.; Aisa, H.A. Pseudo-disesquiterpenoids from seeds of Vernonia anthelmintica and their biological activities. Phytochem. Lett. 2017, 21, 163–168. [Google Scholar] [CrossRef]
- Gubiani, J.R.; Wijeratne, E.M.K.; Shi, T.; Araujo, A.R.; Arnold, A.E.; Chapman, E.; Gunatilaka, A.A.L. An epigenetic modifier induces production of (10′S)-verruculide B, an inhibitor of protein tyrosine phosphatases by Phoma sp. nov. LG0217, a fungal endophyte of Parkinsonia microphylla. Bioorg. Med. Chem. 2017, 25, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, B.-B.; Li, H.-Z.; Meng, X.; Lin, X.; Jiang, Y.-Y.; Ahn, J.-S.; Cui, L. Four new neolignans isolated from Eleutherococcus senticosus and their protein tyrosine phosphatase 1B inhibitory activity (PTP1B). Fitoterapia 2017, 121, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Jang, J.-H.; Ko, W.; Kim, K.-S.; Sohn, J.H.; Kang, M.-S.; Ahn, J.S.; Kim, Y.-C.; Oh, H. PTP1B Inhibitory and Anti-Inflammatory Effects of Secondary Metabolites Isolated from the Marine-Derived Fungus Penicillium sp. JF-55. Mar. Drugs 2013, 11, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Wei, X.; Lin, X.; Sheng, L.; Wang, Z.; Tu, Z.; Yang, X.; Zhou, X.; Li, J.; Liu, Y. Guignardins A–F, spirodioxynaphthalenes from the endophytic fungus Guignardia sp. KcF8 as a new class of PTP1B and SIRT1 inhibitors. Tetrahedron 2014, 70, 5806–5814. [Google Scholar] [CrossRef]
- Gao, W.-B.; Han, L.-P.; Xie, F.-X.; Ma, Q.-Y.; Li, X.-F.; Zhang, J.; Zhao, Y.-X.; Luo, D.-Q. A New Polyketide-Derived Metabolite with PTP1B Inhibitory Activity from the Endophytic Fungus Pestalotiopsis neglecta. Chem. Nat. Compd. 2019, 55, 1056–1058. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.-Y.; Jia, M.; Ming, Q.-L.; Yue, W.; Rahman, K.; Qin, L.-P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473. [Google Scholar] [CrossRef]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Ito, T.; Aimaiti, S.; Win, N.N.; Kodama, T.; Morita, H. New sesquiterpene lactones, vernonilides A and B, from the seeds of Vernonia anthelmintica in Uyghur and their antiproliferative activities. Bioorg. Med. Chem. Lett. 2016, 26, 3608–3611. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Yan, G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. Food Chem. 2007, 105, 548–554. [Google Scholar] [CrossRef]
- Cui, C.-M.; Li, X.-M.; Li, C.-S.; Sun, H.-F.; Gao, S.-S.; Wang, B.-G. Benzodiazepine Alkaloids from Marine-Derived Endophytic Fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]


| No. | Composition | RT/min | Quantity(%) |
|---|---|---|---|
| 1 | 2-(1-Methylcyclopropyl) aniline | 17.435 | 0.76 |
| 2 | Methyl 12-methyltetradecanoate | 18.795 | 0.84 |
| 3 | Diisobutyl phthalate | 19.857 | 5.98 |
| 4 | Methyl 14-methylpentadecanoate | 20.443 | 1.00 |
| 5 | Di-sec-butyl phthalate | 20.850 | 0.25 |
| 6 | Dibutyl phthalate | 21.139 | 46.57 |
| 7 | Methyl 14-methylhexadecanoate | 21.360 | 0.75 |
| 8 | (9Z,15Z)-Methyl 9,15-linoleate | 22.473 | 0.67 |
| 9 | Methyl oleate | 22.533 | 0.71 |
| 10 | Bis(2-ethylhexyl) adipate | 25.752 | 4.21 |
| 11 | Bis(2-ethylhexyl) phthalate | 27.307 | 14.85 |
| 12 | 1,4-Benzenedicarboxylic acid | 29.048 | 1.75 |
| 13 | Bis(2-ethylhexyl) sebacate | 29.550 | 0.82 |
| No. | Composition | RT/min | Quantity(%) |
|---|---|---|---|
| 1 | Methyl 13-methyltetradecanoate | 18.650 | 0.70 |
| 2 | Methyl 12-methyltetradecanoate | 18.761 | 1.86 |
| 3 | Methyl 14-methylhexadecanoate | 21.360 | 1.11 |
| 4 | Undecanoic acid | 19.13/21.73 a | 27.33 |
| 5 | Oleic Acid | 20.604 | 0.85 |
| 6 | Palmitic acid | 20.859 | 11.63 |
| 7 | Di-sec-butyl phthalate | 20.927 | 3.55 |
| 8 | Methyl 14-methylhexadecanoate | 21.284 | 1.65 |
| 9 | Methyl (13Z)-octadecenoate | 22.499 | 1.08 |
| 10 | Stearic acid | 23.170 | 5.31 |
| 11 | Bis(2-ethylhexyl) phthalate | 27.197 | 2.94 |
| Samples | Sample Concentration (mg/mL) | Sample Amount (µL) | C. albicans(ZOI) | S. aureus(ZOI) | E.coli (ZOI) |
|---|---|---|---|---|---|
| Ampicillin sodium salt | 10 | 5 | 20 | ||
| Ampicillin sodium salt | 1 | 5 | 27 | ||
| Amphotericin B | 5 | 20 | 17 | ||
| Aspergillus sp. XJA6 | 50 | 20 | 23 | 11 | 8 |
| Talaromyces sp. XJA4 | 50 | 20 | 9 | 11 | 9 |
| A. terreus XJA8 | 50 | 20 | 20 | 16 | 12 |
| S. commune XJA1 | 20 | 11 | 12 | 9.5 |
| Sample | IC50 (μg/mL) |
|---|---|
| Aspergillus sp. XJA6 | 5.662 ± 1.099 |
| Talaromyces sp. XJA4 | 4.789 ± 1.222 |
| A. terreus XJA8 | 23.439 ± 0.734 |
| S.commune XJA1 | 11.964 ± 0.484 |
| PTP1B | 1.46 ± 0.40 |
| Samples | Cell Lines | ||
|---|---|---|---|
| IC50 (μg/mL) | |||
| HT-29 | MDA-MB-231 | Hela | |
| Aspergillus sp. XJA6 | 19.31 ± 0.8 | 33.55 ± 0.1 | 9.99 ± 0.8 |
| A. terreus XJA8 | 5.73 ± 0.6 | 56.3 ± 0.6 | 24.69 ± 0.2 |
| Talaromyces sp. XJA4 | 90.43 ± 0.01 | >100 | 85.46 ± 0.3 |
| S. commune XJA1 | ND (not determined) | >100 | 89.8 ± 0.4 |
| DOX | 0.08 ± 0.2 | 0.1 ± 0.02 | 0.19 ± 0.01 |
| Crude Extracts | IC50 (±SD, µg/mL) | SI (HT-29) | SI (Hela) |
|---|---|---|---|
| Aspergillus sp. XJA6 | 92.70.3 ± 1.4 | nd | 9.3 |
| A. terreus XJA8 | 79.34 ± 0.7 | 13.8 | nd |
| Sample | IC50 (μg/mL) |
|---|---|
| Aspergillus sp. XJA6 | No effect |
| Talaromyces sp. XJA4 | 171.78 ± 8.06 |
| A. terreus XJA8 | 215.838 ± 7.25 |
| S. commune XJA1 | 55.21 ± 0.3 |
| Vitamin C | 5.34 ± 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustamova, N.; Gao, Y.; Zhang, Y.; Yili, A. Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica. Microorganisms 2020, 8, 586. https://doi.org/10.3390/microorganisms8040586
Rustamova N, Gao Y, Zhang Y, Yili A. Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica. Microorganisms. 2020; 8(4):586. https://doi.org/10.3390/microorganisms8040586
Chicago/Turabian StyleRustamova, Nigora, Yanhua Gao, Yong Zhang, and Abulimiti Yili. 2020. "Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica" Microorganisms 8, no. 4: 586. https://doi.org/10.3390/microorganisms8040586
APA StyleRustamova, N., Gao, Y., Zhang, Y., & Yili, A. (2020). Biological Activity of Endophytic Fungi from the Roots of the Medicinal Plant Vernonia anthelmintica. Microorganisms, 8(4), 586. https://doi.org/10.3390/microorganisms8040586






