Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Trial
2.2. Inoculum Production and Inoculation Procedure
2.3. Mycotoxin LC-MS/MS Analyses
2.4. Statistical Analysis
3. Results
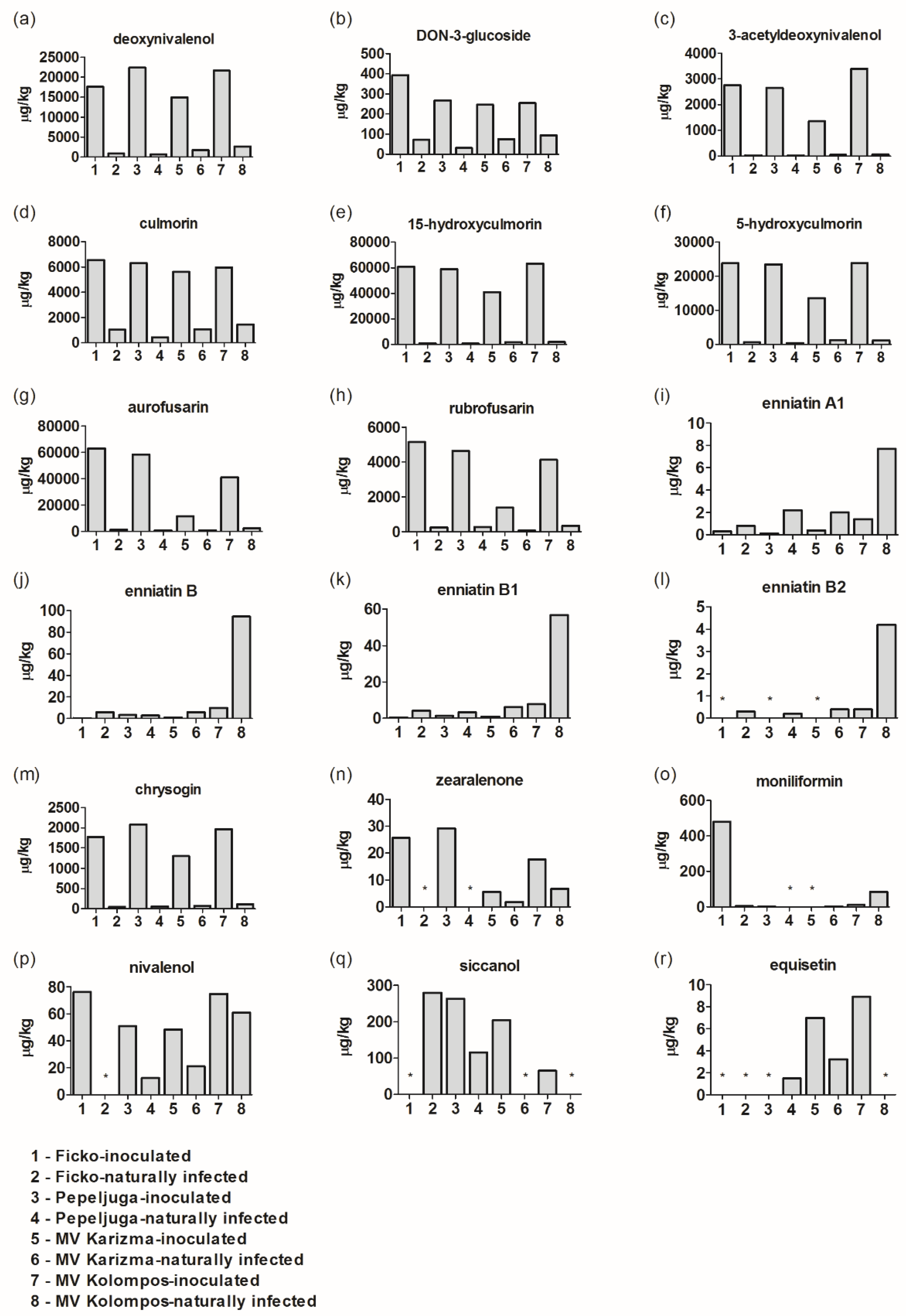
3.1. Fusarium Metabolites/Mycotoxins in Fusarium-Inoculated and Naturally-Infected Samples
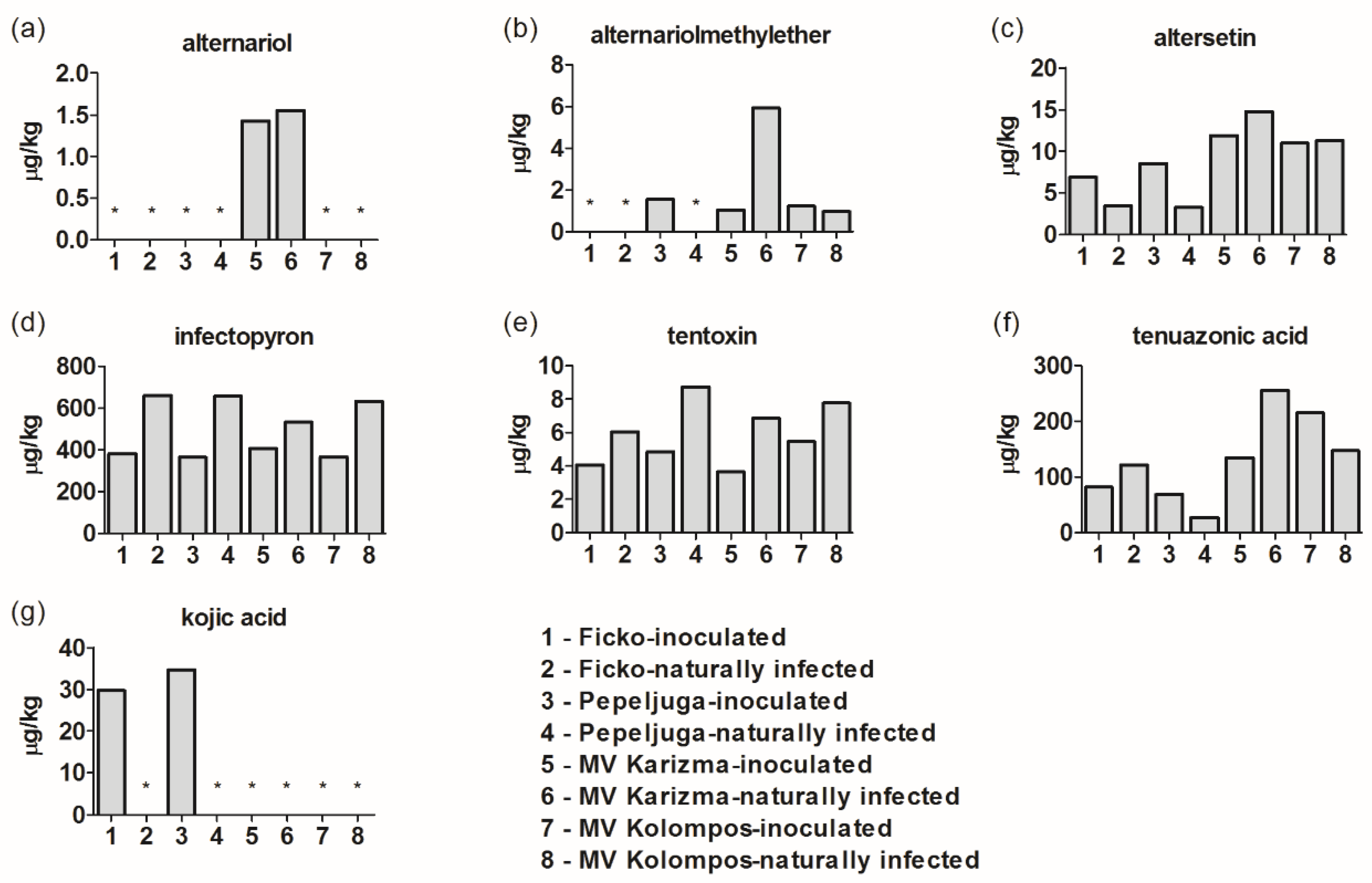
3.2. Alternaria and Aspergillus Metabolites/Mycotoxins in Fusarium-Inoculated and Naturally-Infected Samples
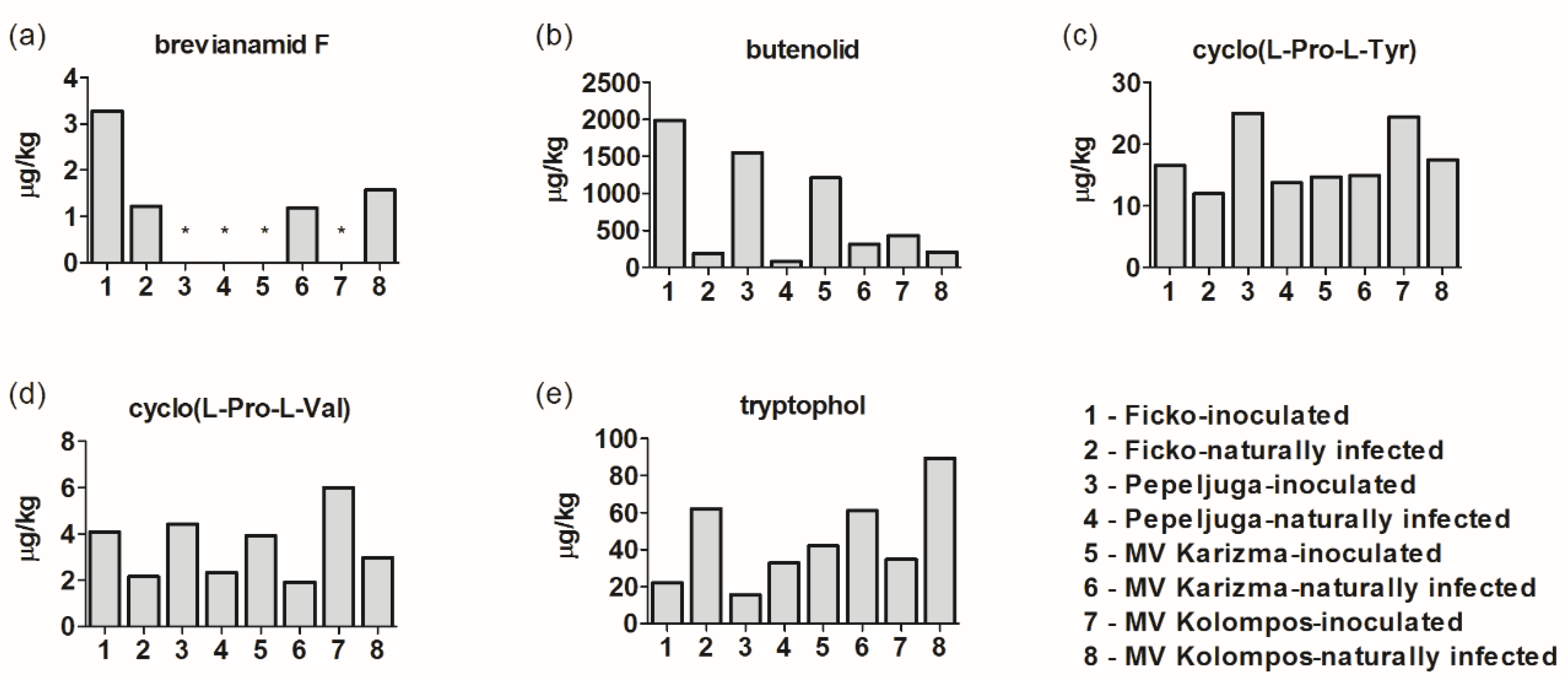
3.3. Other Metabolites in Fusarium-Inoculated and Naturally-Infected Samples
4. Discussion
4.1. Deoxynivalenol (DON), DON-3-Glucoside (D3G), and 3-Acetyldeoxynivalenol (3-ADON)
4.2. Culmorin (CULM), 15-Hydroxyculmorin, and 5-Hydroxyculmorin
4.3. Aurofusarin–Rubrofusarin
4.4. Enniantins (Enns)
4.5. Fumonisin
4.6. Zearalenone (ZEN), Moniliformin (MON), and Beauvericin (BEA)
4.7. Other Fusarium Metabolites
4.8. Alternaria, Aspergillus Mycotoxins and Other Metabolites
5. Conclusions
- -
- the presence of 36 wheat mycotoxins/metabolites under field conditions in Croatia under Fusarium inoculation and in a natural infection environment in the harvest year 2019;
- -
- the most abundant fungal metabolites were DON, CULM, 15-hydroxyculmorin, 5-hydroxyculmorin, and aurofusarin;
- -
- an increase in DON in inoculated (treated) compared to naturally-infected samples;
- -
- the absence of T2 and HT2 toxins in wheat produced commercially;
- -
- decreased values of Enns and Fumonisins in the inoculated samples—these are produced by other Fusarium species that probably out-competed the F. culmorum/F. graminearum in the inoculated samples.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization UN (FAO). FAOSTAT, Statistic Division, Database 2014. Available online: http://faostat.fao.org (accessed on 10 January 2020).
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxin profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.; Wild, C.; Baan, R.; Gelderblom, W.; Miller, J.; Riley, R.; Wu, F. Fungi producing significant mycotoxins. In Improving Public Health Through Mycotoxin Control; Pitt, J., Wild, C., Baan, R., Gelderblom, W., Miller, J., Riley, R., Wu, F., Eds.; IARC Scientific Publication: Lyon, France, 2012; Volume 158, pp. 1–30. [Google Scholar]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified fusarium mycotoxins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Campbell, K.W.; White, D.G. Evaluation of corn genotypes for resistance to Aspergillus ear rot, kernel infection, and aflatoxin production. Plant Dis. 1995, 79, 1039–1045. [Google Scholar] [CrossRef]
- Stanciu, O.; Banc, R.; Cozma, A.; Filip, L.; Miere, D.; Mañes, J.; Loghin, F. Occurrence of Fusarium mycotoxins in wheat from Europe—A review. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef]
- Binder, E.M. Managing the risk of mycotoxins in modern feed production. Anim. Feed Sci. Technol. 2007, 133, 149–166. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-Term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- European Union (EU). Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Consolidated Version from 28.07.2017 Including Amendments; European Union (EU): Brussels, Belgium, 2017; Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02006R1881-20170728 (accessed on 2 November 2019).
- World Health Organization. Available online: https://www.who.int/foodsafety/publications/monographs/en/ (accessed on 25 November 2019).
- Langseth, W.; Ghebremeskel, M.; Kosiak, B.; Kolsaker, P.; Miller, J.D. Production of culmorin compounds and other secondary metabolites by Fusarium culmorum and F. graminearum strains isolated from Norwegian cereals. Mycopathologia 2001, 152, 23–34. [Google Scholar] [CrossRef]
- Woelflingseder, L.; Warth, B.; Vierheilig, I.; Schwartz-Zimmermann, H.; Hametner, C.; Nagl, V.; Novak, B.; Šarkanj, Š.; Berthiller, F.; Adam, G.; et al. The Fusarium metabolite culmorin suppresses the In Vitro glucuronidation of deoxynivalenol. Arch. Toxicol. 2019, 93, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, R.; McCormick, S.P.; Proctor, R.H.; Teresi, J.M.; Hao, G.; Ward, T.J.; Alexander, H.J.; Vaughan, M.M. Synergistic phytotoxic effects of Culmorin and trichothecene mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Cheat, S.; Gerez, J.R.; Cognié, J.; Alassane-Kpembi, I.; Bracarense, A.P.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa In Vitro on explants and In Vivo on intestinal loops. Toxins 2015, 7, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Tančić, S.; Stanković, S.; Lević, J.; Krnjaja, V. Correlation of deoxynivalenol and zearalenone production by Fusarium species originating from wheat and maize grain. Pestic. Phytomed. 2015, 30, 99–105. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef]
- Liuzzi, V.; Mirabelli, V.; Cimmarusti, M.; Haidukowski, M.; Leslie, J.; Logrieco, A.; Caliandro, R.; Fanelli, F.; Mulè, G. Enniatin and beauvericin biosynthesis in Fusarium species: Production profiles and structural determinant prediction. Toxins 2017, 9, 45. [Google Scholar] [CrossRef]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the mycotoxin enniatin B. Front. Public Health 2017, 5, 304. [Google Scholar] [CrossRef]
- Schütt, F.; Nirenberg, H.I.; Demi, G. Moniliformin production in the genus Fusarium. Mycotoxin Res. 1998, 14, 35–40. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Roscoe, M.; Trelka, R.; Patrick, S.K.; Bamforth, J.M.; Gräfenhan, T.; Schlichting, L.; Fu, B.X. Fate of moniliformin during milling of Canadian durum wheat, processing, and cooking of spaghetti. Can. J. Plant Sci. 2014, 94, 555–563. [Google Scholar] [CrossRef]
- Ojuri, O.T.; Ezekiel, C.N.; Sulyok, M.; Ezeokoli, O.T.; Oyedele, O.A.; Ayeni, K.I.; Eskola, M.K.; Šarkanj, B.; Hajšlová, J.; Adeleke, R.A.; et al. Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in Nigeria. Food Chem. Toxicol. 2018, 121, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, A.; Tóth, B.; Varga, M.; Bartók, T.; Szabó-Hevér, A.; Farády, L.; Lehoczki-Krsjak, S. Role of fungicides, application of nozzle types, and the resistance level of wheat varieties in the control of Fusarium head blight and Deoxynivalenol. Toxins 2011, 3, 1453–1483. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Schmidt-Heydt, M.; Rodríguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 2607–2620. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Sulyok, M.; Ogara, I.M.; Abia, W.A.; Warth, B.; Šarkanj, B.; Turner, P.C.; Krska, R. Mycotoxins in uncooked and plate-ready household food from rural northern Nigeria. Food Chem. Toxicol. 2019, 128, 171–179. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of agronomic programmes with different susceptibility to deoxynivalenol risk on emerging contamination in winter wheat. Eur. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Lemmens, M.; Haim, K.; Lew, H.; Ruckenbauer, P. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. Phytopathology 2004, 152, 1–8. [Google Scholar] [CrossRef]
- Snijders, C.H.A.; Van Eeuwijk, F.A. Genotype X strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzac, F.C. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. Eur. J. Plant Pathol. 2010, 128, 511–516. [Google Scholar] [CrossRef]
- Priyanka, S.R.; Venkataramana, M.; Balakrishna, K.; Murali, H.S.; Batra, H.V. Development and evaluation of a multiplex PCR assay for simultaneous detection of major mycotoxigenic fungi from cereals. J. Food Sci. Technol. 2015, 52, 486–492. [Google Scholar] [CrossRef]
- Mishra, S.; Ansari, K.M.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Occurrence of deoxynivalenol in cereals and exposure risk assessment in Indian population. Food Control 2013, 30, 549–555. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Kovač, M.; Šubarić, D.; Bulaić, M.; Kovač, T.; Šarkanj, B. Yesterday masked, today modified: What do mycotoxins bring next? Arch. Ind. Hig. Toxicol. 2018, 69, 196–214. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.; Hofgaard, I.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-Quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of fusarium head blight of durum wheat (Triticum durum desf.) in central Italy and their In Vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Ghebremeskel, M.; Langseth, W. The occurrence of culmorin and hydroxy-culmorins in cereals. Mycopathologia 2000, 152, 103–108. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Kamani, M.H.; Fakhri, Y.; Coppa, C.F.S.C.; de Oliveira, C.A.F.; Sant’Ana, A.S. Changes in masked forms of deoxynivalenol and their co-occurrence with culmorin in cereal-based products: A systematic review and meta-analysis. Food Chem. 2019, 294, 587–596. [Google Scholar] [CrossRef]
- Mastanjević, K.; Šarkanj, B.; Rudolf, K.; Sulyok, M.; Warth, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef]
- Mastanjević, K.; Šarkanj, B.; Warth, B.; Krska, R.; Sulyok, M.; Mastanjević, K.; Šantek, B.; Krstanović, V. Fusarium culmorum multi-toxin screening in malting and brewing by-products. LWT 2018, 98, 642–645. [Google Scholar] [CrossRef]
- Habschied, K.; Krska, R.; Sulyok, M.; Lukinac, J.; Jukić, M.; Šarkanj, B.; Krstanović, V.; Mastanjević, K. The influence of steeping water change during malting on the multi-toxin content in Malt. Foods 2019, 8, 478. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl. Environ. Microbiol. 2010, 76, 136–141. [Google Scholar] [CrossRef]
- Rotter, R.G.; Trenholm, H.L.; Prelusky, D.B.; Hartin, K.E.; Thompson, B.K.; Miller, J.D. A preliminary examination of potential interactions between deoxynivalenol (DON) and other selected Fusarium metabolites in growing pigs. Can. J. Anim. Sci. 1992, 72, 107–116. [Google Scholar] [CrossRef]
- Ashley, J.N.; Hobbs, B.C.; Raistrick, H. Studies in the biochemistry of micro-organisms: The crystalline colouring matters of Fusarium culmorum (wg Smith) sacc. and related forms. Biochem. J. 1937, 31, 385. [Google Scholar] [CrossRef]
- Frandsen, R.J.; Schutt, C.; Lund, B.W.; Staerk, D.; Nielsen, J.; Olsson, S.; Giese, H. Two novel classes of enzymes are required for the biosynthesis of aurofusarin in Fusarium graminearum. J. Biol. Chem. 2011, 286, 10419–10428. [Google Scholar] [CrossRef]
- Malz, S.; Grell, M.N.; Thrane, C.; Maier, F.J.; Rosager, P.; Felk, A.; Albertsen, K.S.; Salomon, S.; Bohn, L.; Schäfer, W. Identification of a gene cluster responsible for the biosynthesis of aurofusarin in the Fusarium graminearum species complex. Fungal Genet. Biol. 2005, 42, 420–433. [Google Scholar] [CrossRef]
- Mortensen, A.; Granby, K.; Eriksen, F.D.; Cederberg, T.L.; Friis-Wandall, S.; Simonsen, Y.; Broesbøl-Jensen, B.; Bonnichsen, R. Levels and risk assessment of chemical contaminants in byproducts for animal feed in Denmark. J. Environ. Sci. Health B 2014, 49, 797–810. [Google Scholar] [CrossRef]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 69–380. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control 2017, 73, 147–155. [Google Scholar] [CrossRef]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of Beauvericin and Enniatins in wheat affected by Fusarium avenaceum head blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef]
- Amato, B.; Pfohl, K.; Tonti, S.; Nipoti, P.; Dastjerdi, R.; Pisi, A.; Karlovsky, P.; Prodi, A. Fusarium proliferatum and fumonisin B1 co-occur with Fusarium species causing Fusarium head blight in durum wheat in Italy. J. Appl. Bot. Food Qual. 2017, 88, 228–233. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Chehri, K.; Jahromi, S.T.; Reddy, K.R.N.; Abbasi, S.; Salleh, B. Occurrence of Fusarium spp. and Fumonisins in stored wheat grains marketed in Iran. Toxins 2010, 2, 2816–2823. [Google Scholar] [CrossRef]
- Da Rocha Lemos Mendes, G.; Alves dos Reis, T.; Corrêa, B.; Badiale-Furlong, E. Mycobiota and occurrence of Fumonisin B1 in wheat harvested in Southern Brazil. Cienc. Rural 2015, 45. [Google Scholar] [CrossRef]
- Beev, G.; Denev, S.; Bakalova, D. Zearalenone—Producing activity of Fusarium graminearum and Fusarium oxysporum isolated from Bulgarian wheat. Bulg. J. Agric. Sci. 2013, 19, 255–259. [Google Scholar]
- Habschied, K.; Šarkanj, B.; Klapec, T.; Krstanović, V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011, 129, 329–332. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, L. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Naturwissenschaften 2018, 105, 2. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Golinski, P.; Kostecki, M.; Lasocka, I.; Wisniewska, H.; Chelkowski, J.; Kaczmarek, Z. Moniliformin accumulation and other effects of Fusarium avenaceum (Fr.) Sacc. on kernels of winter wheat cultivars. J. Phytopathol. 1996, 144, 459–499. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Bentley, R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms 2020, 8, 578. https://doi.org/10.3390/microorganisms8040578
Spanic V, Katanic Z, Sulyok M, Krska R, Puskas K, Vida G, Drezner G, Šarkanj B. Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms. 2020; 8(4):578. https://doi.org/10.3390/microorganisms8040578
Chicago/Turabian StyleSpanic, Valentina, Zorana Katanic, Michael Sulyok, Rudolf Krska, Katalin Puskas, Gyula Vida, Georg Drezner, and Bojan Šarkanj. 2020. "Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples" Microorganisms 8, no. 4: 578. https://doi.org/10.3390/microorganisms8040578
APA StyleSpanic, V., Katanic, Z., Sulyok, M., Krska, R., Puskas, K., Vida, G., Drezner, G., & Šarkanj, B. (2020). Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms, 8(4), 578. https://doi.org/10.3390/microorganisms8040578





