Evaluation of a Newly Developed Vacuum Dried Microtiter Plate for Rapid Biocide Susceptibility Testing of Clinical Enterococcus faecium Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Biocides
2.3. Biocide Susceptibility Testing
2.3.1. MIC Determination by Wet Plate Procedure
2.3.2. MIC and MBC Determination by Dried Plate Procedure
2.4. Comparative Analysis of Both MIC Testing Methods
3. Results and Discussion
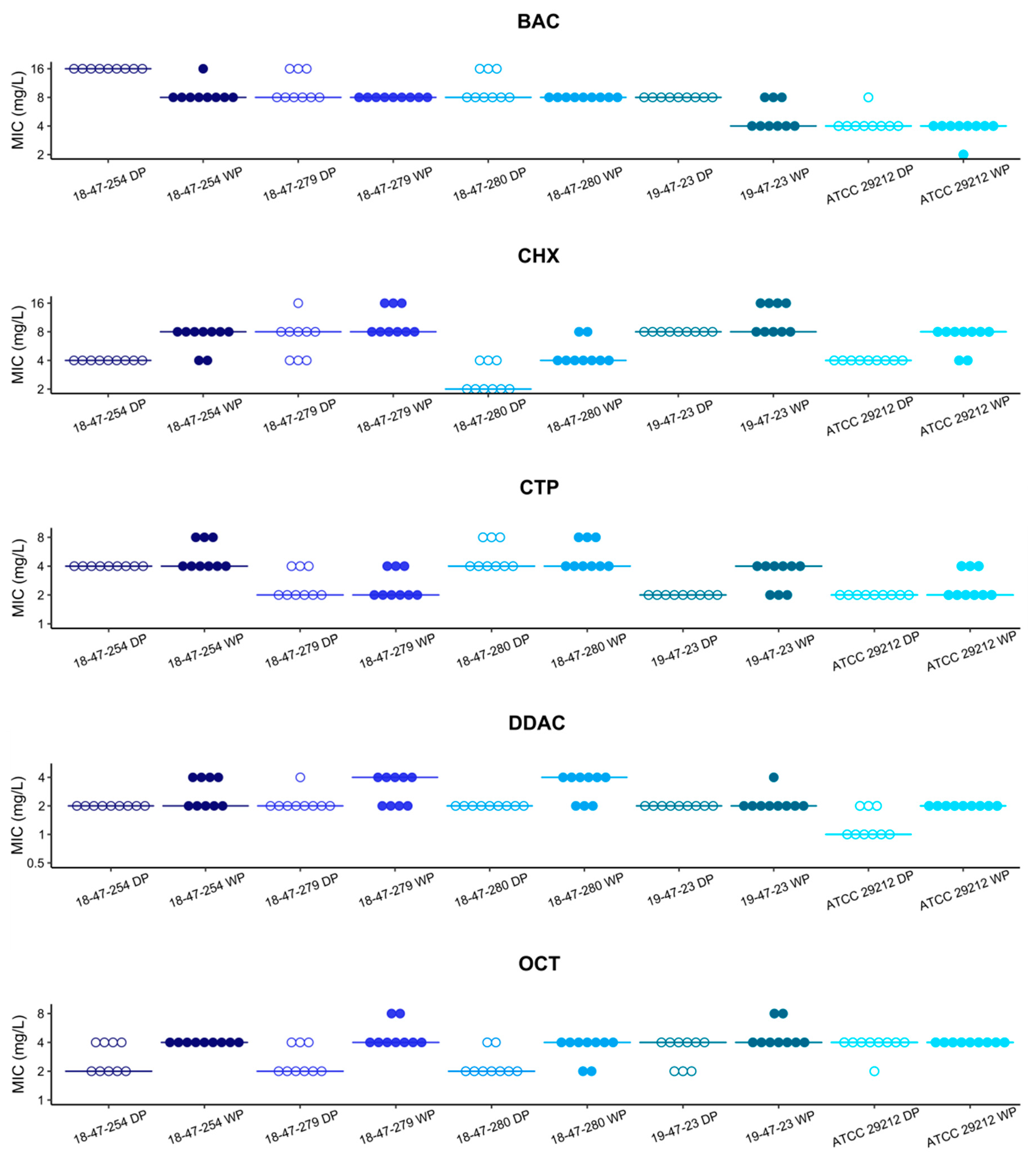
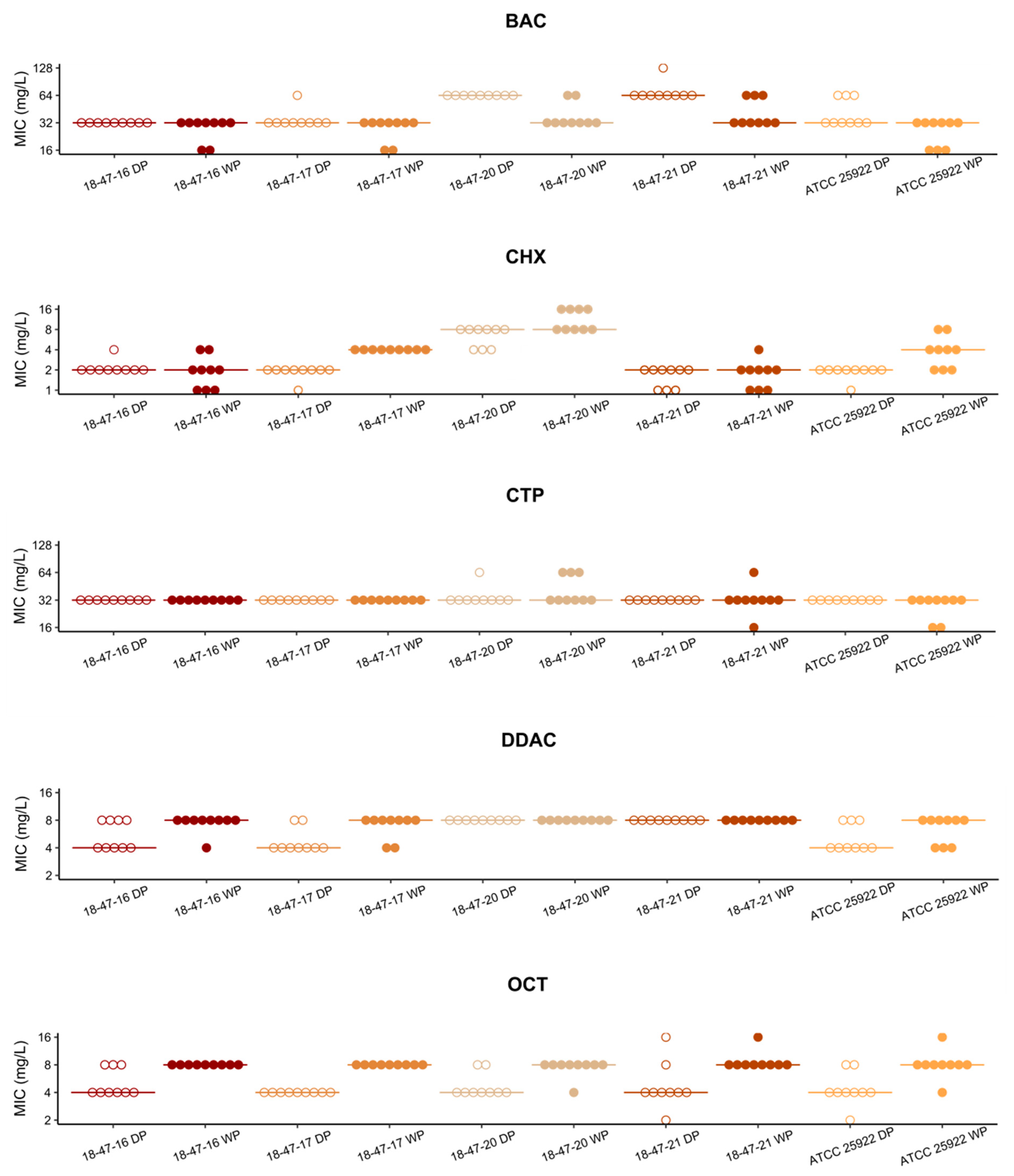
3.1. MIC Values Determined with Vacuum Dried Biocide Microtiter Plates Are in Agreement with Results Obtained with Broth Microdilution According to ISO 20776-1
3.2. Cationic Biocide Susceptibility Profiles of Vancomycin Resistant and Susceptible E. faecium Are Similar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; p. 28. [Google Scholar]
- Maillard, J.Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16s–27s. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.Y. Resistance of Bacteria to Biocides. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, J.A.; Jagadeesan, B.; Goodburn, K.; Grunwald, L.; Jensen, O.N.; Jespers, A.D.; Kanagachandran, K.; Lafforgue, H.; Seefelder, W.; Quentin, M.C. Relationship of Sanitizers, Disinfectants, and Cleaning Agents with Antimicrobial Resistance. J. Food Prot. 2019, 82, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Hardy, K.; Sunnucks, K.; Gil, H.; Shabir, S.; Trampari, E.; Hawkey, P.; Webber, M. Increased Usage of Antiseptics Is Associated with Reduced Susceptibility in Clinical Isolates of Staphylococcus aureus. MBio 2018, 9. [Google Scholar] [CrossRef]
- Stein, C.; Vincze, S.; Kipp, F.; Makarewicz, O.; Al Dahouk, S.; Pletz, M.W. Carbapenem-resistant Klebsiella pneumoniae with low chlorhexidine susceptibility. Lancet Infect. Dis. 2019, 19, 932–933. [Google Scholar] [CrossRef]
- Dawczynski, K.; Proquitte, H.; Roedel, J.; Edel, B.; Pfeifer, Y.; Hoyer, H.; Dobermann, H.; Hagel, S.; Pletz, M.W. Intensified colonisation screening according to the recommendations of the German Commission for Hospital Hygiene and Infectious Diseases Prevention (KRINKO): Identification and containment of a Serratia marcescens outbreak in the neonatal intensive care unit, Jena, Germany, 2013–2014. Infection 2016, 44, 739–746. [Google Scholar] [CrossRef]
- Fessler, A.T.; Schug, A.R.; Geber, F.; Scholtzek, A.D.; Merle, R.; Brombach, J.; Hensel, V.; Meurer, M.; Michael, G.B.; Reinhardt, M.; et al. Development and evaluation of a broth macrodilution method to determine the biocide susceptibility of bacteria. Vet. Microbiol. 2018, 223, 59–64. [Google Scholar] [CrossRef]
- Knapp, L.; Amezquita, A.; McClure, P.; Stewart, S.; Maillard, J.Y. Development of a protocol for predicting bacterial resistance to microbicides. Appl. Environ. Microbiol. 2015, 81, 2652–2659. [Google Scholar] [CrossRef][Green Version]
- Bock, L.J.; Hind, C.K.; Sutton, J.M.; Wand, M.E. Growth media and assay plate material can impact on the effectiveness of cationic biocides and antibiotics against different bacterial species. Lett. Appl. Microbiol. 2018, 66, 368–377. [Google Scholar] [CrossRef]
- ISO. ISO 20776-1:2019. Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial susceptibility test devices—Part 1: Broth micro-dilution reference method for testing the in vitro activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Alotaibi, S.M.I.; Ayibiekea, A.; Pedersen, A.F.; Jakobsen, L.; Pinholt, M.; Gumpert, H.; Hammerum, A.M.; Westh, H.; Ingmer, H. Susceptibility of vancomycin-resistant and -sensitive Enterococcus faecium obtained from Danish hospitals to benzalkonium chloride, chlorhexidine and hydrogen peroxide biocides. J. Med. Microbiol. 2017, 66, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Ignak, S.; Nakipoglu, Y.; Gurler, B. Frequency of antiseptic resistance genes in clinical staphycocci and enterococci isolates in Turkey. Antimicrob. Resist. Infect. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteri, 3rd ed.; M45; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Roedel, A.; Dieckmann, R.; Brendebach, H.; Hammerl, J.A.; Kleta, S.; Noll, M.; Al Dahouk, S.; Vincze, S. Biocide-Tolerant Listeria monocytogenes Isolates from German Food Production Plants Do Not Show Cross-Resistance to Clinically Relevant Antibiotics. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 20776-2:2007. Clinical Laboratory Testing and in vitro Diganostic Test Systems—Susceptibility Testing of Infectious agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 2: Evaluation of Performance of Antimicrobial Susceptibility Test Devices; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Deus, D.; Krischek, C.; Pfeifer, Y.; Sharifi, A.R.; Fiegen, U.; Reich, F.; Klein, G.; Kehrenberg, C. Comparative analysis of the susceptibility to biocides and heavy metals of extended-spectrum beta-lactamase-producing Escherichia coli isolates of human and avian origin, Germany. Diagn. Microbiol. Infect. Dis. 2017, 88, 88–92. [Google Scholar] [CrossRef]
- Ghazisaeedi, F.; Ciesinski, L.; Bednorz, C.; Johanns, V.; Pieper, L.; Tedin, K.; Wieler, L.H.; Günther, S. Phenotypic zinc resistance does not correlate with antimicrobial multi-resistance in fecal E. coli isolates of piglets. Gut Pathog. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Kernberger-Fischer, I.A.; Krischek, C.; Strommenger, B.; Fiegen, U.; Beyerbach, M.; Kreienbrock, L.; Klein, G.; Kehrenberg, C. Susceptibility of Methicillin-Resistant and-Susceptible Staphylococcus aureus Isolates of Various Clonal Lineages from Germany to Eight Biocides. Appl. Environ. Microb. 2018, 84. [Google Scholar] [CrossRef]
- Morrissey, I.; Oggioni, M.R.; Knight, D.; Curiao, T.; Coque, T.; Kalkanci, A.; Martinez, J.L.; Consortium, B. Evaluation of Epidemiological Cut-Off Values Indicates that Biocide Resistant Subpopulations Are Uncommon in Natural Isolates of Clinically-Relevant Microorganisms. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Schwaiger, K.; Harms, K.S.; Bischoff, M.; Preikschat, P.; Molle, G.; Bauer-Unkauf, I.; Lindorfer, S.; Thalhammer, S.; Bauer, J.; Holzel, C.S. Insusceptibility to disinfectants in bacteria from animals, food and humans-is there a link to antimicrobial resistance? Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Wieland, N.; Boss, J.; Lettmann, S.; Fritz, B.; Schwaiger, K.; Bauer, J.; Holzel, C.S. Susceptibility to disinfectants in antimicrobial-resistant and -susceptible isolates of E. coli, Enterococcus faecalis and Enterococcus faecium from poultry-ESBL/AmpC-phenotype of E. coli is not associated with resistance to a quaternary ammonium compound, DDAC. J. Appl. Microbiol. 2017, 122, 1508–1517. [Google Scholar] [CrossRef]
- Lavilla Lerma, L.; Benomar, N.; Valenzuela, A.S.; Casado Munoz Mdel, C.; Galvez, A.; Abriouel, H. Role of EfrAB efflux pump in biocide tolerance and antibiotic resistance of Enterococcus faecalis and Enterococcus faecium isolated from traditional fermented foods and the effect of EDTA as EfrAB inhibitor. Food Microbiol. 2014, 44, 249–257. [Google Scholar] [CrossRef]
- Prieto, A.M.G.; Wijngaarden, J.; Braat, J.C.; Rogers, M.R.C.; Majoor, E.; Brouwer, E.C.; Zhang, X.L.; Bayjanov, J.R.; Bonten, M.J.M.; Willems, R.J.L.; et al. The Two-Component System ChtRS Contributes to Chlorhexidine Tolerance in Enterococcus faecium. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial Multidrug Efflux Pumps: Much More Than Antibiotic Resistance Determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Exp. Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Phillips-Jones, M.K.; Harding, S.E. Antimicrobial resistance (AMR) nanomachines-mechanisms for fluoroquinolone and glycopeptide recognition, efflux and/or deactivation. Biophys. Rev. 2018, 10, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
| Biocide (Concentration Range Tested) | Species | Number of Isolates with MIC Value (mg/L) of | MIC95 | Number of Isolates with MBC Value (mg/L) of | MBC95 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ||||
| BAC (0.5–256 mg/L) | VSE | 1 | 11 | 36 | 8 | 41 | 7 | 16 | |||||||||
| VRE | 15 | 27 | 8 | 42 | 8 | ||||||||||||
| CHX (0.25–128 mg/L) | VSE | 1 | 2 | 35 | 10 | 4 | 2 | 1 | 45 | 16 | |||||||
| VRE | 1 | 41 | 2 | 3 | 1 | 3 | 35 | 16 | |||||||||
| CTP (1–256 mg/L) | VSE | 3 * | 17 | 28 | 4 | 48 | 4 | ||||||||||
| VRE | 11 | 31 | 4 | 42 | 4 | ||||||||||||
| DDAC (0.5–128 mg/L) | VSE | 1 # | 25 | 22 | 2 | 48 | 2 | ||||||||||
| VRE | 26 | 16 | 2 | 2 | 40 | 2 | |||||||||||
| OCT (0.125–32 mg/L) | VSE | 1 | 8 | 39 | 2 | 22 | 26 | 4 | |||||||||
| VRE | 4 | 38 | 2 | 22 | 20 | 4 | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roedel, A.; Dieckmann, R.; Makarewicz, O.; Hartung, A.; Noll, M.; Pletz, M.W.; Dahouk, S.A.; Vincze, S. Evaluation of a Newly Developed Vacuum Dried Microtiter Plate for Rapid Biocide Susceptibility Testing of Clinical Enterococcus faecium Isolates. Microorganisms 2020, 8, 551. https://doi.org/10.3390/microorganisms8040551
Roedel A, Dieckmann R, Makarewicz O, Hartung A, Noll M, Pletz MW, Dahouk SA, Vincze S. Evaluation of a Newly Developed Vacuum Dried Microtiter Plate for Rapid Biocide Susceptibility Testing of Clinical Enterococcus faecium Isolates. Microorganisms. 2020; 8(4):551. https://doi.org/10.3390/microorganisms8040551
Chicago/Turabian StyleRoedel, Alice, Ralf Dieckmann, Oliwia Makarewicz, Anita Hartung, Matthias Noll, Mathias W. Pletz, Sascha Al Dahouk, and Szilvia Vincze. 2020. "Evaluation of a Newly Developed Vacuum Dried Microtiter Plate for Rapid Biocide Susceptibility Testing of Clinical Enterococcus faecium Isolates" Microorganisms 8, no. 4: 551. https://doi.org/10.3390/microorganisms8040551
APA StyleRoedel, A., Dieckmann, R., Makarewicz, O., Hartung, A., Noll, M., Pletz, M. W., Dahouk, S. A., & Vincze, S. (2020). Evaluation of a Newly Developed Vacuum Dried Microtiter Plate for Rapid Biocide Susceptibility Testing of Clinical Enterococcus faecium Isolates. Microorganisms, 8(4), 551. https://doi.org/10.3390/microorganisms8040551








