Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media, Growth Conditions and Inoculum Preparation
2.2. Experimental Design of Winemaking Process
2.3. Microbiological Analyses
2.4. Physico-Chemical Analyses
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
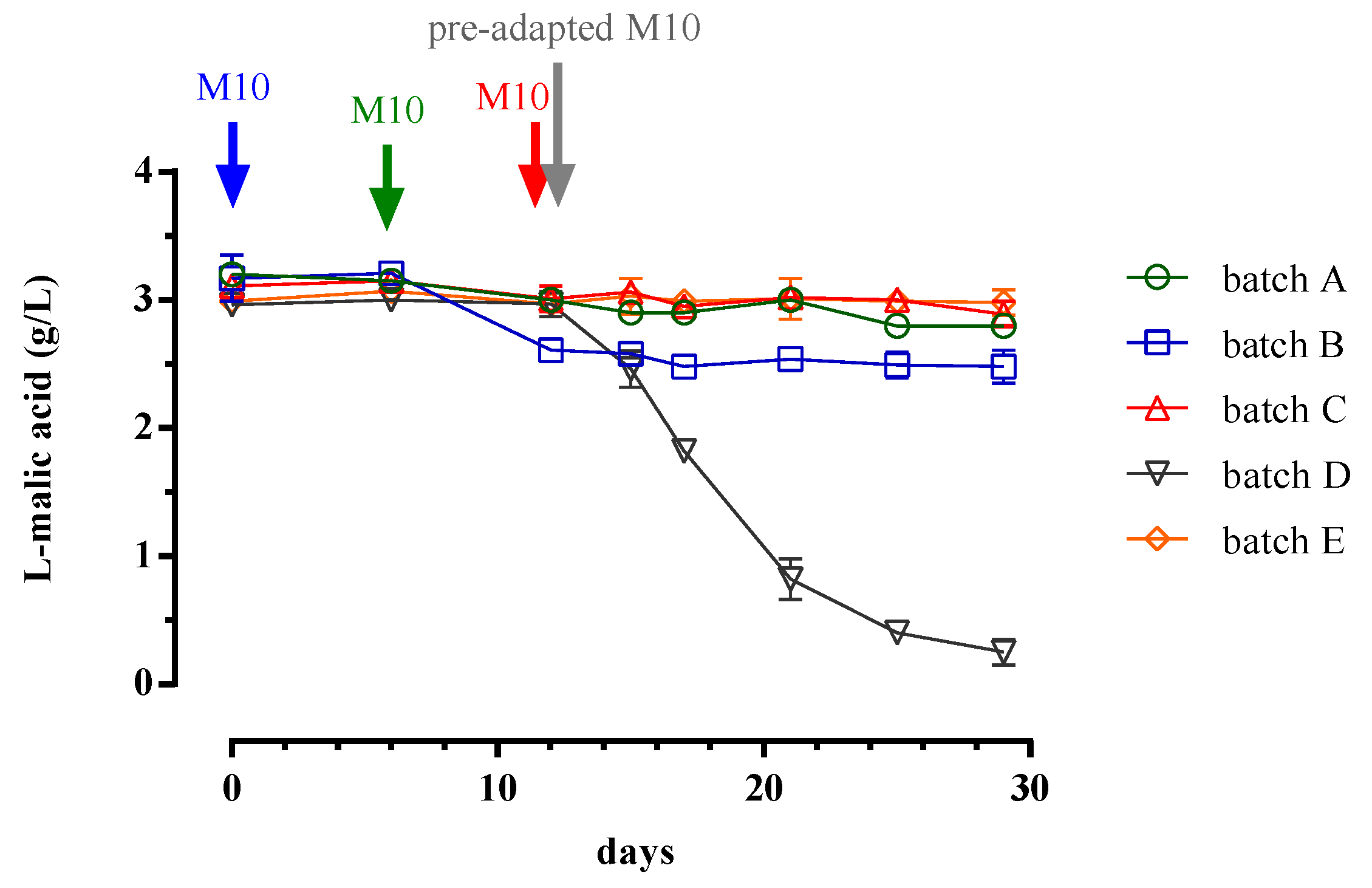
3.1. Trend of Cell Biomass, Alcoholic and Malolactic Fermentation
3.2. Wine Chemical Analysis
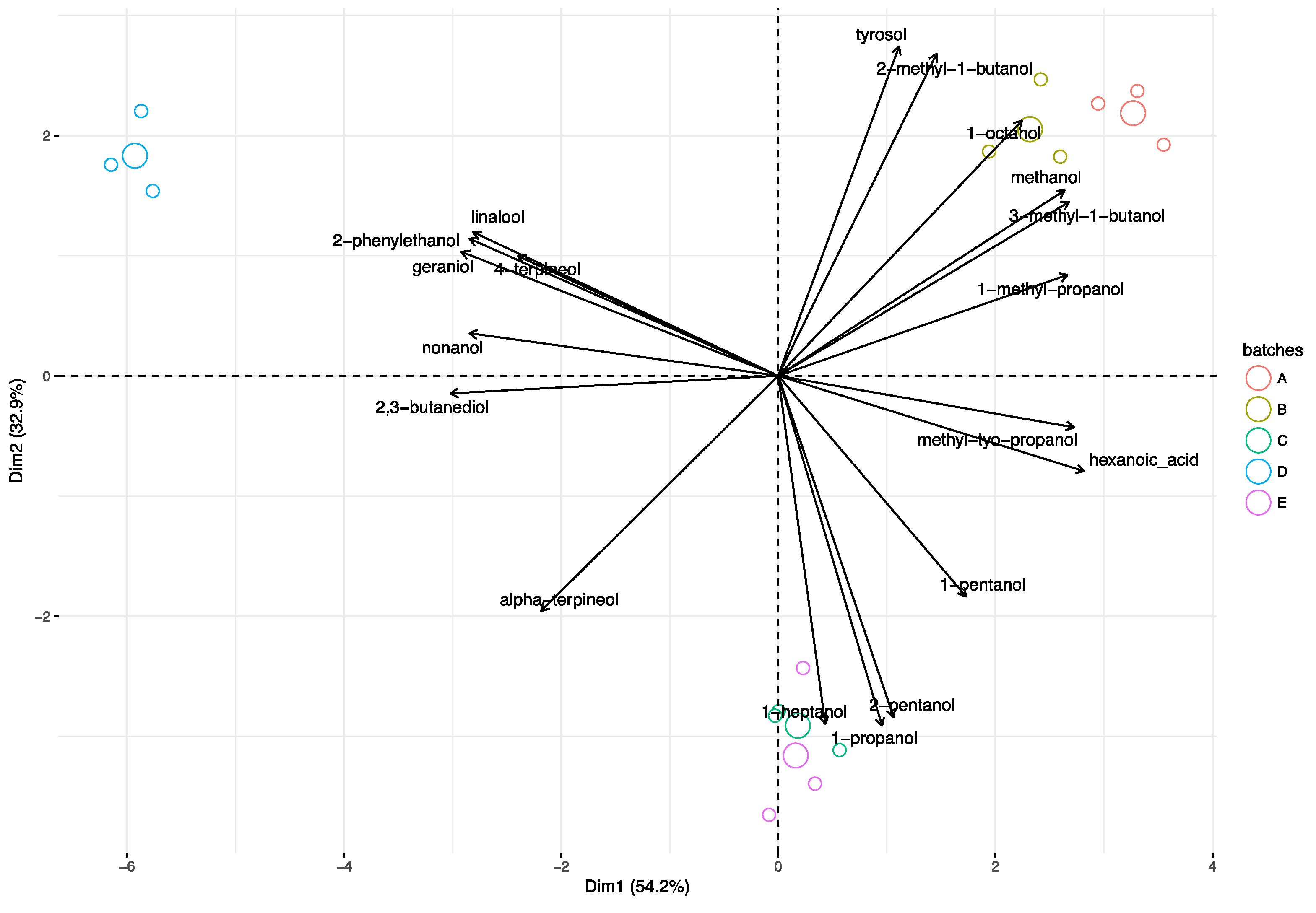
3.3. Principal Component Analysis
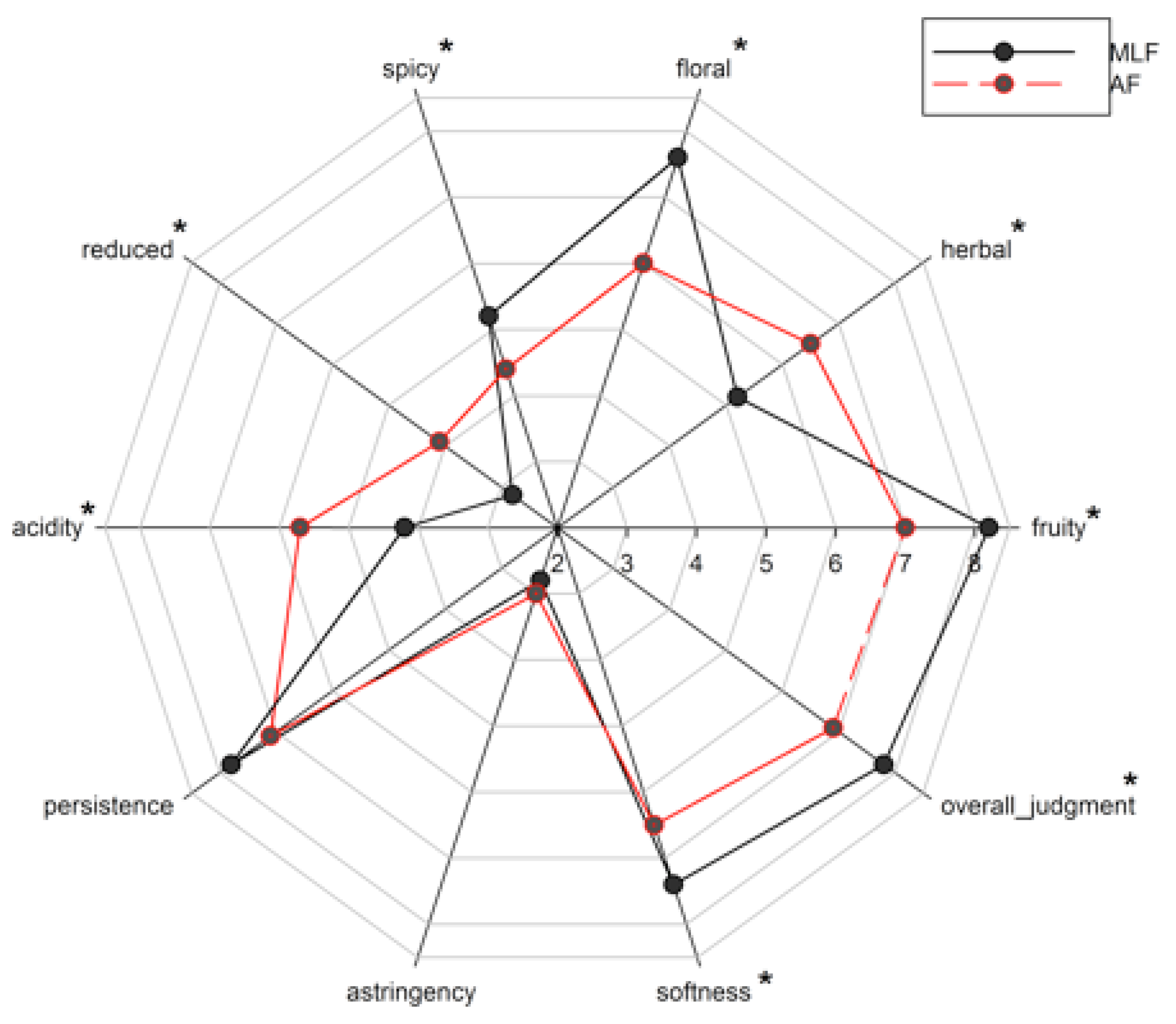
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the knowledge of malolactic fermentation influence on wine aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q. Malolactic fermentation in wine—Beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine R. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Teixeira, H.; Goncalves, M.G.; Rozes, N.; Ramos, A.; San Romao, M.V. Lactobacillic acid accumulation in the plasma membrane of Oenococcus oeni: A response to ethanol stress? Microbial Ecol. 2002, 43, 146–153. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Brizuela, N.; Gerbino, E.; Gómez-Zavaglia, A.; Semorile, L.; Tymczyszyn, E.E. Effect of protective agents and previous acclimation on ethanol resistance of frozen and freeze-dried Lactobacillus plantarum strains. Cryobiology 2015, 71, 522–528. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; La Hens, D.V.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Pannella, G.; Lippolis, R.; Ricciardi, A.; Mazzeo, M.F.; Zotta, T. Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int. J. Food Microbiol. 2019, 298, 51–62. [Google Scholar] [CrossRef]
- Sieiro, C.; Cansado, J.; Agrelo, D.; Velázquez, J.B.; Villa, T.G. Isolation and enological characterization of malolactic bacteria from the vineyards of northwestern. Spain. Appl. Environ. Microbiol. 1990, 56, 2936–2938. [Google Scholar] [CrossRef]
- Vailiant, H.; Formisyn, P.; Gerbaux, V. Malolactic fermentation of wine: Study of the influence of some physico-chemical factors by experimental design assays. J. Appl. Bacteriol. 1995, 79, 640–650. [Google Scholar] [CrossRef]
- Zapparoli, G.; Moser, M.; Dellaglio, F.; Tourdot-Marechal, R.; Guzzo, J. Typical metabolic traits of two Oenococcus oeni strains isolated from Valpolicella wines. Lett. Appl. Microbiol. 2004, 39, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus plantarum from traditional Italian wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Moreno-Arribas, M.V.; Tremonte, P.; Succi, M.; Sorrentino, E.; Macciola, V.; Di Renzo, M.; Coppola, R. Sequential inoculum of Hanseniaspora guilliermondii and Saccharomyces cerevisiae for winemaking Campanino on an industrial scale. World J. Microb. Biot. 2018, 34, 161. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Lombardi, S.J.; Iorizzo, M.; Letizia, F.; Di Martino, C.; Di Renzo, M.; Strollo, D.; Tremonte, P.; Pannella, G.; Ianiro, M.; et al. Use of strain Hanseniaspora guilliermondii BF1 for winemaking process of white grapes Vitis vinifera cv Fiano. Eur. Food Res. Technol. 2020, 246, 549–561. [Google Scholar] [CrossRef]
- Zapparoli, G.; Tosi, E.; Azzolini, M.; Vagnoli, P.; Krieger, S. Bacterial inoculation strategies for the achievement of malolactic fermentation in high-alcohol wines. S. Afr. J. Enol. Vitic. 2009, 30, 49–55. [Google Scholar] [CrossRef]
- Succi, M.; Pannella, G.; Tremonte, P.; Tipaldi, L.; Coppola, R.; Iorizzo, M.; Lombardi, S.J.; Sorrentino, E. Sub-optimal pH preadaptation improves the survival of Lactobacillus plantarum strains and the malic acid consumption in wine-like medium. Front. Microbiol. 2017, 8, 470. [Google Scholar] [CrossRef]
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef]
- Alegría, G.E.; López, I.; Ruiz, J.I.; Sáenz, J.; Fernández, E.; Zarazaga, M.; Dizy, M.; Torres, C.; Ruiz-Larrea, F. High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol. Lett. 2004, 230, 53–61. [Google Scholar] [CrossRef]
- Du Plessis, H.W.; Dicks, L.M.T.; Pretorius, I.S.; Lambrechts, M.G.; du Toit, M. Identification of lactic acid bacteria isolated from South African brandy base wines. Int. J. Food Microbiol. 2004, 91, 19–29. [Google Scholar] [CrossRef]
- Lopez, I.; Lopez, R.; Santamaria, P.; Torres, C.; Ruiz-Larrea, F. Performance of malolactic fermentation by inoculation of selected Lactobacillus plantarum and Oenococcus oeni strains isolated from Rioja red wines. Vitis 2008, 47, 123–129. [Google Scholar]
- Lee, J.E.; Hong, Y.S.; Lee, C.H. Characterization of fermentative behaviors of lactic acid bacteria in grape wines through 1H NMR-and GC-based metabolic profiling. J. Agric. Food Chem. 2009, 57, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.S.; Krauß, S.; Huch, M.; Du Toit, M.; Franz, C.M. Development of a quantitative PCR for detection of Lactobacillus plantarum starters during wine malolactic fermentation. J. Microbiol. Biotechnol. 2011, 21, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Franz, C.; Cho, G.S.; du Toit, M. Expression of the malolactic enzyme gene from Lactobacillus plantarum under winemaking conditions. Curr. Microbiol. 2011, 62, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, J.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT-Food Sci. Technol. 2016, 73, 334–341. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl. Environ. Microb. 2004, 70, 5715–5731. [Google Scholar] [CrossRef]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. Screening of Lactobacillus spp. and Pediococcus spp. for glycosidase activities that are important in oenology. J. Appl. Microbiol. 2005, 99, 1061–1069. [Google Scholar] [CrossRef]
- Matthews, A.; Grbin, P.R.; Jiranek, V. Biochemical characterisation of the esterase activities of wine lactic acid bacteria. Appl. Microbiol. Biot. 2007, 77, 329–337. [Google Scholar] [CrossRef]
- Iorizzo, M.; Macciola, V.; Testa, B.; Lombardi, S.J.; De Leonardis, A. Physicochemical and sensory characteristics of red wines from the rediscovered autochthonous Tintilia grapevine grown in the Molise region (Italy). Eur. Food Res. Technol. 2014, 238, 1037–1048. [Google Scholar] [CrossRef]
- De Leonardis, A.; Testa, B.; Macciola, V.; Lombardi, S.J.; Iorizzo, M. Exploring enzyme and microbial technology for the preparation of green table olives. Eur. Food Res. Technol. 2016, 242, 363–370. [Google Scholar] [CrossRef]
- Ugliano, M.; Genovese, A.; Moio, L. Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2003, 51, 5073–5078. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta. 2008, 621, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Calabretti, A.; Volpe, M.G.; Sorrentino, A.; Ionata, E.; Santomauro, F.; La Cara, F. Aglianico and Fiano wines obtained with an autochthonous non-Saccharomyces yeast. Ann. Microbiol. 2011, 61, 131–136. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT-Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef]
- European Community. Methods for the analysis of wines. Off. J. Eur. Commun. Regul. 1990, 272, 1–192. [Google Scholar]
- OIV. Compendium of International Methods of Analysis of Spirituous Beverages of Vitivinicultural Origin; International Organisation of Vine and Wine: Paris, France, 2014. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Iorizzo, M.; Lombardi, S.J.; Lopez, F.; Marconi, E. Effective assay for olive vinegar production from olive oil mill wastewaters. Food Chem. 2018, 240, 437–440. [Google Scholar] [CrossRef]
- Lombardi, S.J.; De Leonardis, A.; Lustrato, G.; Testa, B.; Iorizzo, M. Yeast autolysis in sparkling wine aging: Use of killer and sensitive Saccharomyces cerevisiae strains in co-culture. Recent Patents Biot. 2015, 9, 223–230. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.r-project.org/index.html (accessed on 25 February 2020).
- Costello, P.J.; Henschke, P.A.; Markides, A.J. Standardised methodology for testing malolactic bacteria and wine yeast compatibility. Aust. J. Grape Wine R. 2003, 9, 127–137. [Google Scholar] [CrossRef]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef]
- Rossouw, D.; Du Toit, M.; Bauer, F.F. The impact of co-inoculation with Oenococcus oeni on the trancriptome of Saccharomyces cerevisiae and on the flavour-active metabolite profiles during fermentation in synthetic must. Food Microbiol. 2012, 29, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Jussier, D.; Morneau, A.D.; de Orduna, R.M. Effect of simultaneous inoculation with yeast and bacteria on fermentation kinetics and key wine parameters of cool-climate Chardonnay. Appl. Environ. Microbiol. 2006, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Taniasuri, F.; Lee, P.R.; Liu, S.Q. Induction of simultaneous and sequential malolactic fermentation in durian wine. Int. J. Food Microbiol. 2016, 230, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, R.; Moser, S.; Davide, S.; Villegas, T.R.; Malacarne, M.; Larcher, R.; Nardi, T.; Vagnoli, P.; Krieger-Weber, S. Exploitation of simultaneous alcoholic and malolactic fermentation of Incrocio Manzoni, a traditional Italian white wine. S. Afr. J. Enol. Vitic. 2016, 37, 124–131. [Google Scholar] [CrossRef][Green Version]
- Bauer, R.; Dicks, L.M.T. Control of malolactic fermentation in Wine. A Review. S. Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar] [CrossRef]
- Rosi, I.; Fia, G.; Canuti, V. Influence of different pH values and inoculation time on the growth and malolactic activity of a strain of Oenococcus oeni. Aust. J. Grape Wine R. 2003, 9, 194–199. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Semorile, L. Effect of acclimation medium on cell viability, membrane integrity and ability to consume malic acid in synthetic wine by oenological Lactobacillus plantarum strains. J. Appl. Microbiol. 2014, 116, 360–367. [Google Scholar] [CrossRef]
- Tremonte, P.; Succi, M.; Coppola, R.; Sorrentino, E.; Tipaldi, L.; Picariello, G.; Pannella, G.; Fraternali, F. Homology-based modeling of Universal stress protein from Listeria innocua up-regulated under acid stress conditions. Front. Microbiol. 2016, 7, 1998. [Google Scholar] [CrossRef]
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. 1979, 12, 59–111. [Google Scholar] [CrossRef]
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of different techniques of malolactic fermentation induction on diacetyl metabolism and biosynthesis of selected aromatic esters in cool-climate grape wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Petersen, M.; Liu, J.; Toldam-Andersen, T. Influence of pre-fermentation treatments on wine volatile and sensory profile of the new disease tolerant cultivar Solaris. Molecules 2015, 20, 21609–21625. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Gil, J.V.; Pardo, I.; Ferrer, S. Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Res. Int. 1999, 32, 491–496. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine R. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Samappito, S.; Butkhup, L. Effect of skin contact treatments on the aroma profile and chemical components of mulberry (Morus alba Linn.) wines. Afr. J. Food Sci. 2010, 4, 52–61. [Google Scholar]
- Tao, Y.S.; Li, H. Active volatiles of cabernet sauvignon wine from Changli County. Health 2009, 1, 176. [Google Scholar] [CrossRef]
- Pederson, C.S.; Albury, M.N.; Christensen, M.D. The Growth of Yeasts in Grape Juice Stored at Low Temperature: IV. Fungistatic Effects of Organic Acids1. Appl. Microbiol. 1961, 9, 162. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef]
- Woolford, M.K. Microbiological screening of the straight chain fatty acids (C1–C12) as potential silage additives. J. Sci. Food Agric. 1975, 26, 219–228. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A.; Desens, C.; Joyeux, A. Stimulation de la fermentation malolactique par l’addition au vin d’enveloppes cellulaires de levure et différents adjuvants de nature polysaccharidique et azotée. OENO One 1985, 19, 229–240. [Google Scholar] [CrossRef]
- Schwab, W.; Schreier, P. Studies on bound aroma compounds of sour cherry fruit (Prunus cerasus L.). Z. Lebensm.-Unters. und-Forsch. 1990, 190, 228–231. [Google Scholar] [CrossRef]


| Physico-Chemical Parameters | AF | MLF | ||||
|---|---|---|---|---|---|---|
| pH | 3.25 | ± | 0.08 * | 3.68 | ± | 0.09 |
| Total acidity (g/L) | 8.54 | ± | 0.16 * | 6.52 | ± | 0.23 |
| Reducing sugars (g/L) | 1.10 | ± | 0.05 * | 0.75 | ± | 0.17 |
| Alcohol (% vol) | 12.58 | ± | 0.17 | 12.55 | ± | 0.13 |
| Volatile acidity (g/L) | 0.31 | ± | 0.07 | 0.42 | ± | 0.04 |
| L-malic acid (g/L) | 3.18 | ± | 0.06 * | 0.25 | ± | 0.11 |
| L-lactic acid (g/L) | 0.13 | ± | 0.03 * | 2.23 | ± | 0.07 |
| Free SO2 (mg/L) | 11.21 | ± | 0.41 | 11.34 | ± | 0.44 |
| Total SO2 (mg/L) | 45.16 | ± | 1.13 | 48.23 | ± | 1.75 |
| Compounds | Odor Description | Batch A | Batch B | Batch C | Batch D | Batch E | Threshold | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher alcohols (µg/L) | ||||||||||||||||||
| 1-propanol | alcohol, pungent | 15.67 | ± | 1.55a | 13.47 | ± | 1.68ab | 23.89 | ± | 1.84c | 10.80 | ± | 1.08b | 24.25 | ± | 2.55c | 9 | [54] |
| 1-methyl-propanol | 26.97 | ± | 0.90a | 19.97 | ± | 1.83c | 19.14 | ± | 0.81c | 13.57 | ± | 1.27b | 17.42 | ± | 1.67c | |||
| 3-methyl-1-butanol | whiskey, malt, burnt | 255.87 | ± | 33.13a | 282.90 | ± | 11.56a | 138.62 | ± | 2.95c | 84.60 | ± | 2.75b | 143.70 | ± | 2.24c | 30 | [54] |
| 2-pentanol | - | 22.67 | ± | 1.94a | 22.87 | ± | 1.70a | 36.77 | ± | 3.15c | 16.23 | ± | 2.08b | 35.46 | ± | 2.41c | ||
| 1-pentanol | balsamic | 11.17 | ± | 1.46a | 10.32 | ± | 1.93a | 11.80 | ± | 1.56a | 7.63 | ± | 1.10a | 12.71 | ± | 2.62a | 64 | [54] |
| 2-methyl-1-butanol | whiskey | 10.13 | ± | 0.85a | 9.90 | ± | 1.65a | 4.23 | ± | 0.51b | 6.43 | ± | 0.57b | 4.35 | ± | 1.00b | 65 | |
| 1-octanol | chestnut flowers, mushroomy | 0.033 | ± | 0.00075a | 0.030 | ± | 0.00081b | 0.020 | ± | 0.00130c | 0.021 | ± | 0.00079c | 0.023 | ± | 0.00035c | 0.001 | [14] |
| tyrosol | - | 58.1 | ± | 2.79a | 56.30 | ± | 3.22a | 43.5 | ± | 2.93bc | 49.43 | ± | 4.79ac | 39.3 | ± | 4.00b | ||
| 1-heptanol | lemon, orange, copper | 114.6 | ± | 2.52a | 113.77 | ± | 6.66a | 132.2 | ± | 2.93b | 111.93 | ± | 2.54a | 134.1 | ± | 3.80b | 0.2–0.3 | [58] |
| methyl-tyo-propanol | - | 85.9 | ± | 1.72a | 84.80 | ± | 1.78a | 84.1 | ± | 2.39a | 77.67 | ± | 3.00b | 84.1 | ± | 1.90a | ||
| 2,3-butanediol | butter, creamy | 382.5 | ± | 10.58a | 378.10 | ± | 12.60a | 418.5 | ± | 17.77b | 520.60 | ± | 11.59c | 434.5 | ± | 9.76b | 150 | [58] |
| 2-nonanol | fruity, green | 2.2 | ± | 0.19a | 2.12 | ± | 0.24a | 2.5 | ± | 0.21a | 3.47 | ± | 0.20b | 2.5 | ± | 0.24a | 0.058 | [14] |
| 2-phenylethanol | rose, honey, spice, lilac | 53.9 | ± | 2.35a | 55.33 | ± | 2.12a | 55.5 | ± | 2.91a | 87.71 | ± | 2.14b | 53.9 | ± | 4.77a | 10 | [54] |
| methanol | 27.8 | ± | 1.40a | 21.73 | ± | 1.70a | 13.4 | ± | 1.82b | 9.77 | ± | 0.81b | 14.1 | ± | 2.11b | 200 | [14] | |
| TOTAL | 1067.5 | 1071.61 | 984.17 | 999.86 | 1000.28 | |||||||||||||
| Esters (µg/L) | ||||||||||||||||||
| linalool | muscat, flowery, fruity | 53.46 | ± | 5.20a | 48.50 | ± | 4.66a | 49.11 | ± | 4.38a | 124.50 | ± | 9.81b | 51.05 | ± | 7.80a | 25 | [58] |
| 4-terpineol | light aroma, wood, soil | 8.47 | ± | 1.63a | 12.92 | ± | 1.84a | 10.96 | ± | 1.81a | 18.07 | ± | 3.42b | 10.30 | ± | 0.92a | 110–400 | [58] |
| alpha-terpineol | oil, anise, mint | 29.78 | ± | 2.93a | 28.43 | ± | 3.85a | 43.63 | ± | 2.84b | 44.68 | ± | 2.25b | 43.17 | ± | 3.40b | 250 | [54] |
| geraniol | fresh, citrus, floral, green, sweet, lemon/lime | 4.63 | ± | 0.32a | 4.00 | ± | 0.26a | 5.31 | ± | 0.19a | 22.42 | ± | 3.07b | 4.81 | ± | 0.17a | 15 | [61] |
| TOTAL | 96.3 | 93.85 | 109.0 | 209.67 | 109.3 | 3.40 | ||||||||||||
| Others (µg/L) | ||||||||||||||||||
| hexanoic_acid | cheese, rancid, fruity | 120.9 | ± | 5.65a | 124.73 | ± | 7.07a | 121.76 | ± | 6.90a | 88.94 | ± | 5.84b | 119.12 | ± | 4.82a | 670000 | [60] |
| TOTAL | 121.00 | 124.73 | 121.80 | 88.97 | 119.10 | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms 2020, 8, 516. https://doi.org/10.3390/microorganisms8040516
Lombardi SJ, Pannella G, Iorizzo M, Testa B, Succi M, Tremonte P, Sorrentino E, Di Renzo M, Strollo D, Coppola R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms. 2020; 8(4):516. https://doi.org/10.3390/microorganisms8040516
Chicago/Turabian StyleLombardi, Silvia Jane, Gianfranco Pannella, Massimo Iorizzo, Bruno Testa, Mariantonietta Succi, Patrizio Tremonte, Elena Sorrentino, Massimo Di Renzo, Daniela Strollo, and Raffaele Coppola. 2020. "Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine" Microorganisms 8, no. 4: 516. https://doi.org/10.3390/microorganisms8040516
APA StyleLombardi, S. J., Pannella, G., Iorizzo, M., Testa, B., Succi, M., Tremonte, P., Sorrentino, E., Di Renzo, M., Strollo, D., & Coppola, R. (2020). Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms, 8(4), 516. https://doi.org/10.3390/microorganisms8040516









