Changes in the Trophic Pathways within the Microbial Food Web in the Global Warming Scenario: An Experimental Study in the Adriatic Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth/Grazing Experiment
2.1.1. Experimental Settings
2.1.2. Determination of Growth and Grazing Parameters
2.1.3. Removal of Prey Standing Stock and Production
2.1.4. Ingestion Rate (I)
2.2. Bacterial Carbon Respiration, Carbon Demand, and Growth Efficiency
2.3. HNF and Ciliate Gross Growth Efficiency (GGE)
2.4. Calculation of Grazing Preference Index
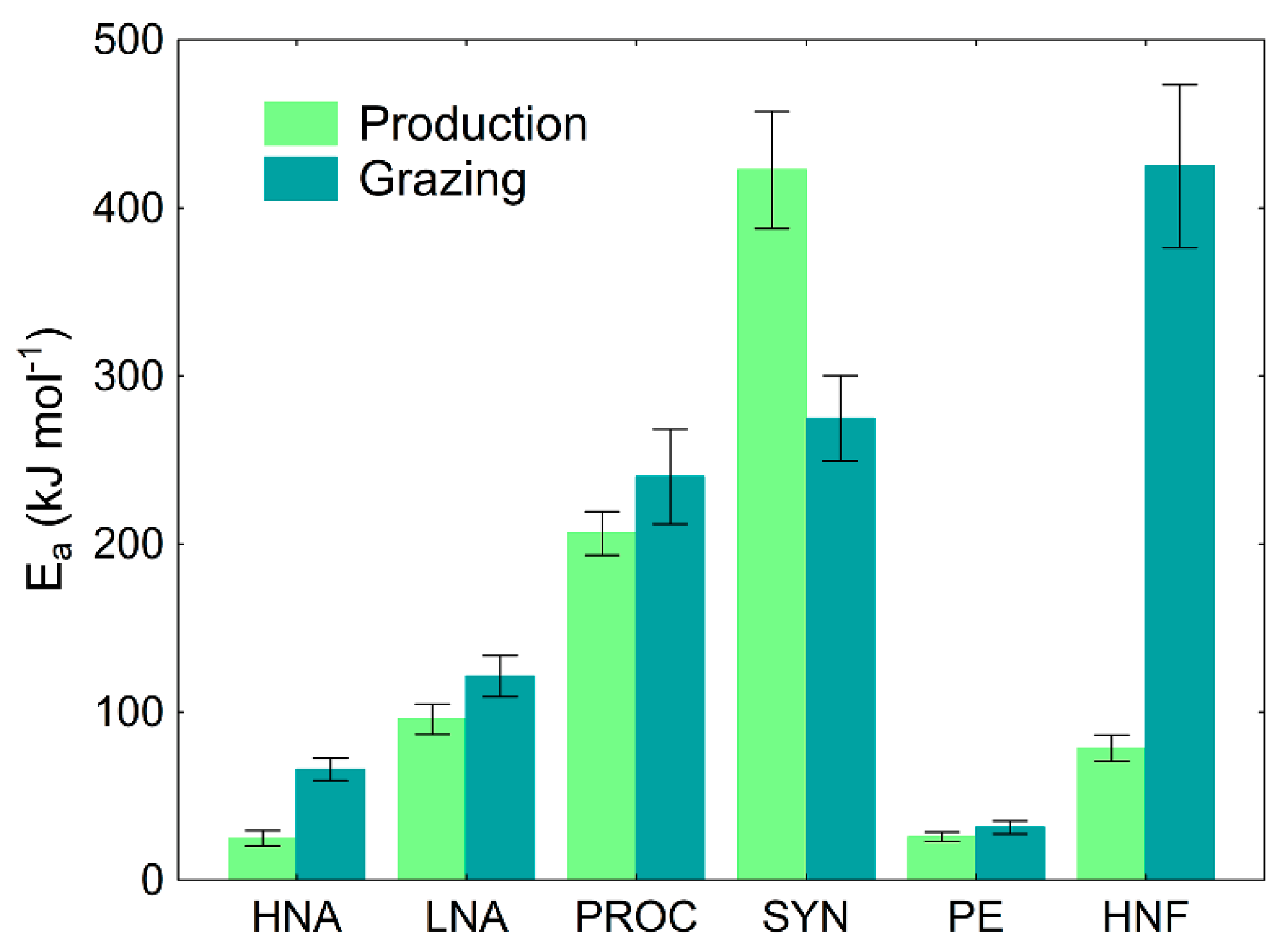
2.5. Sensitivity to Temperature Analyses
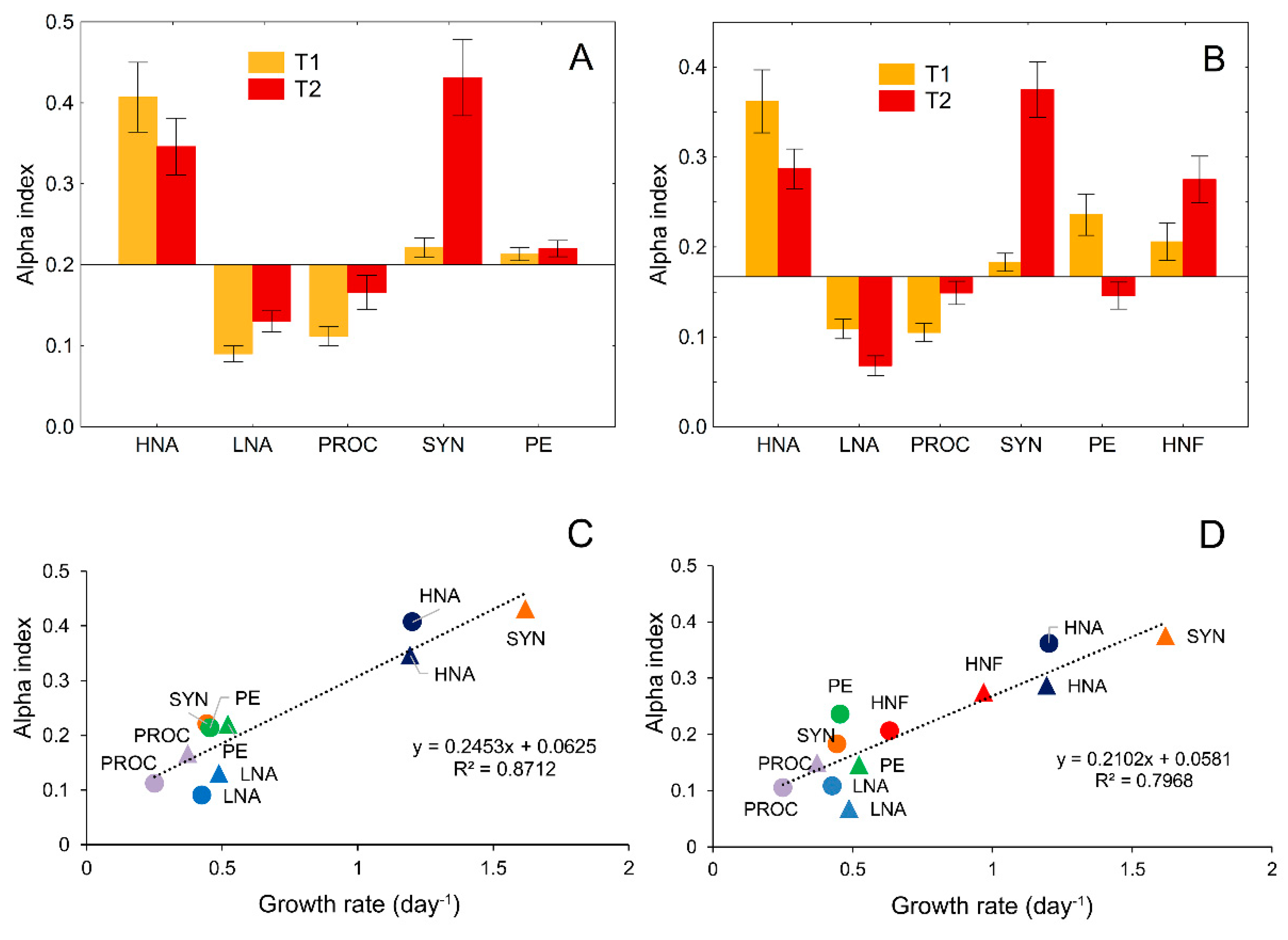
2.6. Niche Breadth and Niche Overlap Measures
2.7. Data Analysis
2.7.1. Environmental Parameters
2.7.2. Flow Cytometry Analysis
2.7.3. Ciliates
2.7.4. Abundance to Biomass Conversion
2.8. Statistical Analysis
3. Results
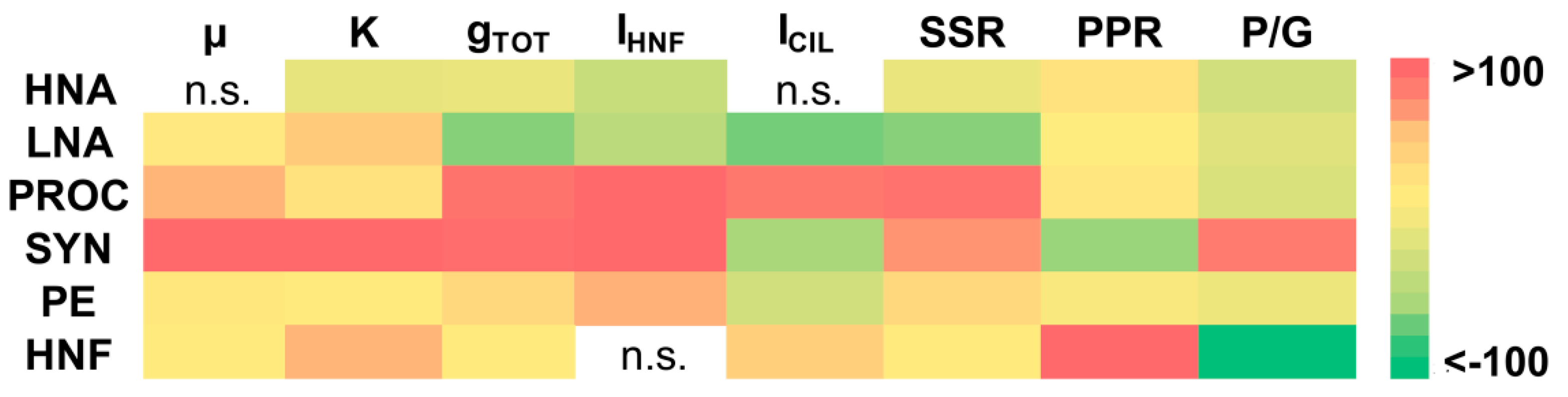
3.1. Impact of Temperature Rise on Microbial Growth and Grazing Parameters
3.2. The Sensitivity of Microbial Production and Losses-to-Grazing to Temperature
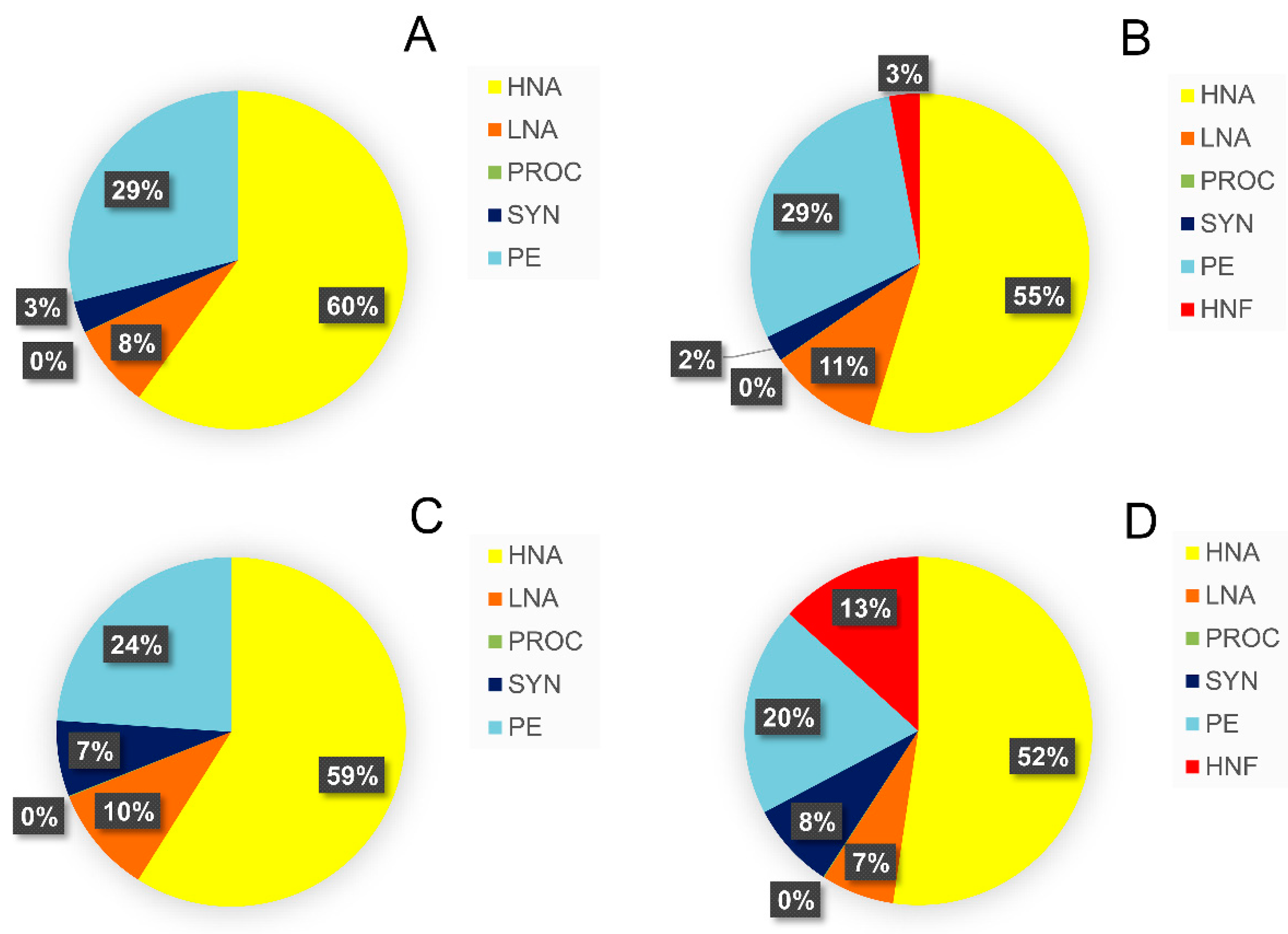
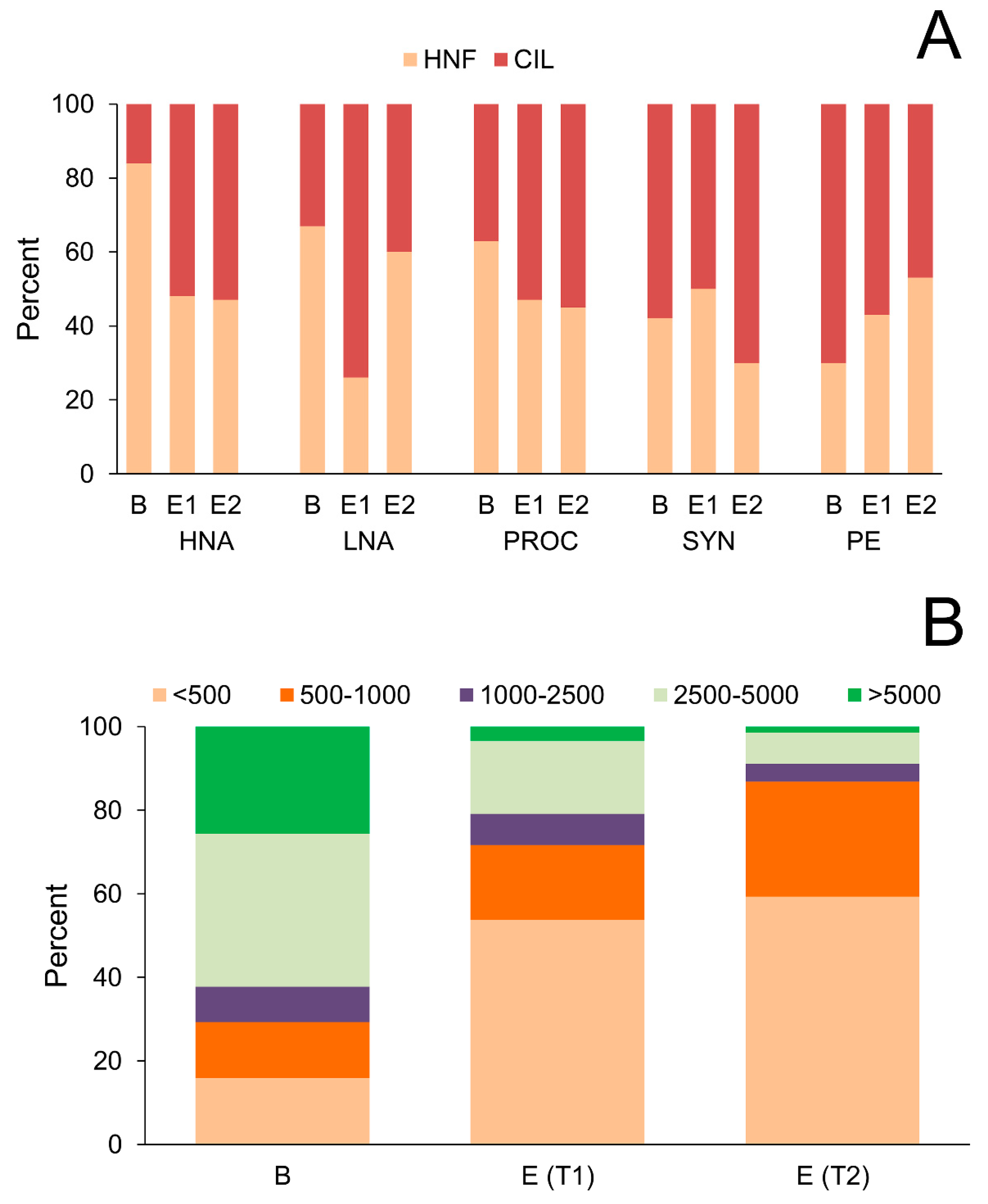
3.3. Relative Contribution of Prey Biomass to Total Biomass Ingested by Protistan Predators
3.4. Analysis of Prey Preference by Protistan Predators
3.5. Food-Niche Relationship among Protistan Predators
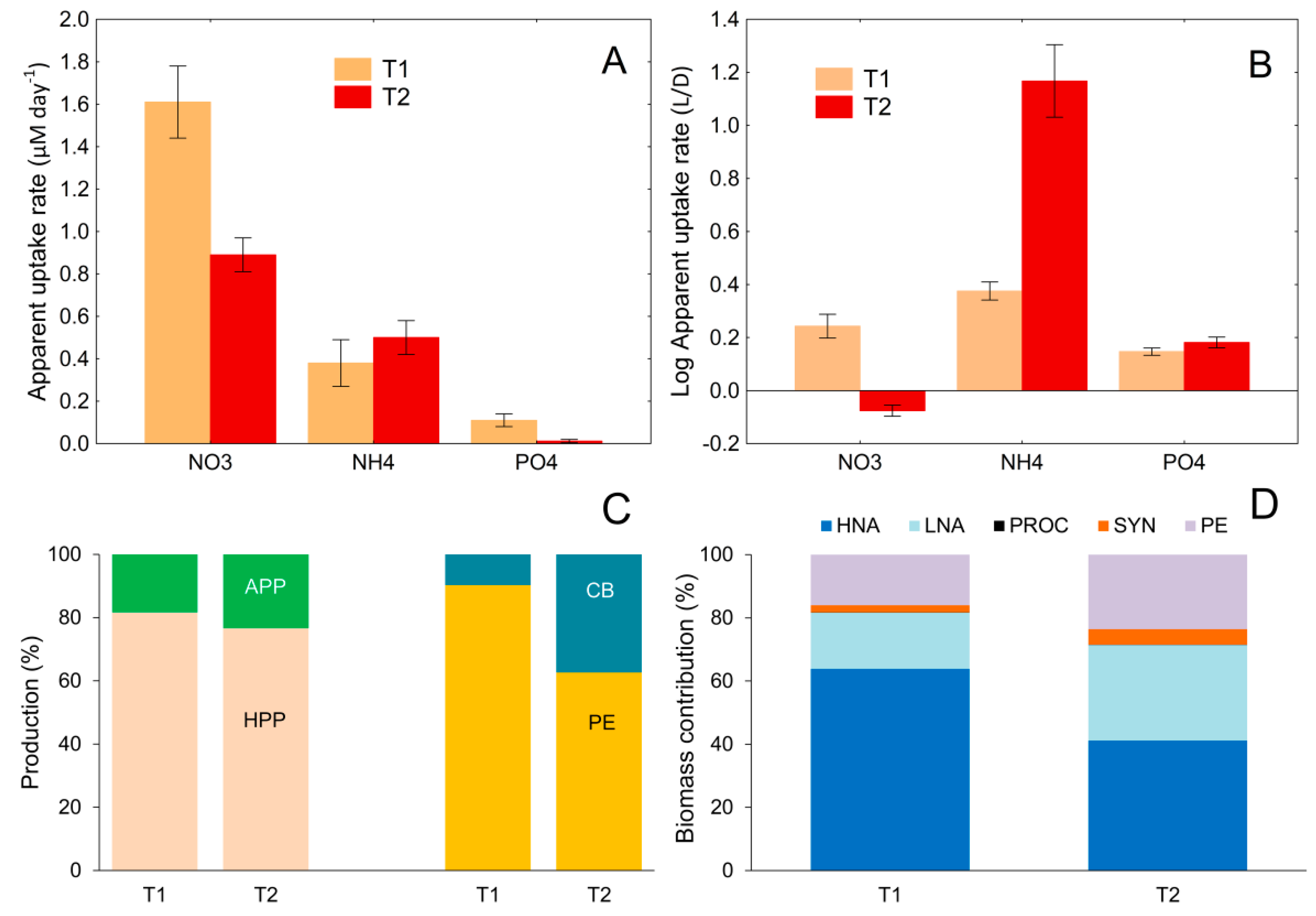
3.6. Relationship between Nutrient Uptake Rate and Changes in the Contribution of Different Microbial Groups to Total Picoplankton Biomass and Production
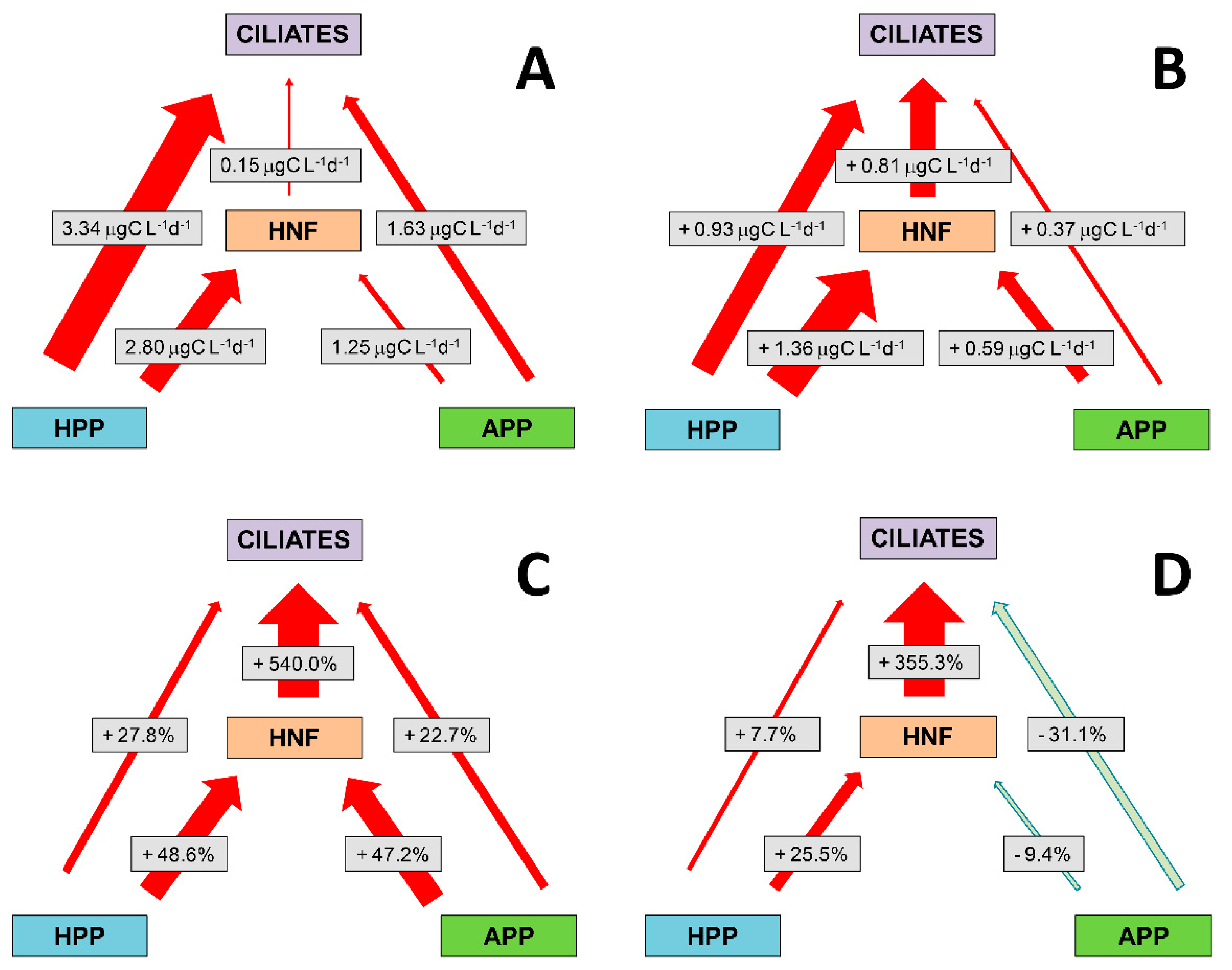
3.7. Absolute and Relative Changes in Carbon Biomass Flux in Warming Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Table of Abbreviations
| Abbreviation | Full Term |
| HPP | Heterotrophic picoplankton |
| APP | Autotrophic picoplankton |
| PROC | Prochlorococcus |
| SYN | Synechococcus |
| PE | Picoeukaryotes |
| PICO | Picoplankton |
| HB | Heterotrophic bacteria |
| MFW | Microbial Food Web |
| HNF | Heterotrophic nanoflagellates |
| CIL | Ciliates |
| HNA | High Nucleic Acid content bacterial group |
| LNA | Low Nucleic Acid content bacterial group |
| APP | Autotrophic picoplankton |
| CB | Cyanobacteria |
| SST | Sea surface temperature |
| K (cells mL−1) | Carrying capacity |
| µ (day−1) | Net growth rate |
| g (day−1) | Grazing rate |
| P (µg C L−1 day−1) | Production rate |
| G (µg C L−1 day−1) | Grazing |
| B | Cell biomass |
| I | Ingestion rate |
| BR | Bacterial carbon respiration |
| BOD | Biological Oxygen demand |
| BGE | Bacterial growth efficiency |
| BCD | Bacterial carbon demand |
| BR | Bacterial respiration |
| BP | Bacterial production |
| GGE | Gross growth efficiency |
| R | Universal gas constant (8314 J mol−1K−1) |
| SSR (%) | Standing Stock Removal |
| PPR (%) | Prey Production Removal |
| P/G | Production/Grazing ratio |
| Ea | Apparent activation energy |
| T1 (°C) | Ambient temperature |
| T2 (°C) | 3 °C elevated temperature |
| gTOT (day−1) | Total grazing rate |
| IHNF (pgC HNF−1 day−1) | HNF ingestion rate |
| ICIL (ngC CIL−1 day−1) | Ciliate ingestion rate |
| (Ea) | Apparent activation energy |
| DOC | Dissolved Organic Carbon |
| DON | Dissolved Organic Nitrogen |
References
- Magazzù, G.; Decembrini, F. Primary production, biomass and abundance of phototrophic picoplankton in the Mediterranean Sea: A review. Aquat. Microb. Ecol. 1995, 9, 97–104. [Google Scholar] [CrossRef]
- Zubkov, M.V.; Sleigh, M.A.; Burkill, P.H.; Leakey, R.J.G. Picoplankton community structure on the Atlantic Meridional Transect: A comparison between seasons. Prog. Oceanogr. 2000, 45, 369–386. [Google Scholar] [CrossRef]
- Li, W.K.W.; Harrison, W.G. Chlorophyll, bacteria and picophytoplankton in ecological provinces of the North Atlantic. Deep Sea Res. 2001, 48, 2271–2293. [Google Scholar] [CrossRef]
- Grob, C.; Ulloa, O.; Li, W.K.W.; Alarcón, G.; Fukasawa, M.; Watanabe, S. Picoplankton abundance and biomass across the eastern South Pacific Ocean along latitude 32.5°S. Mar. Ecol. Prog. Ser. 2007, 332, 53–62. [Google Scholar] [CrossRef]
- Cole, J.J.; Findlay, S.; Pace, M.L. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Mar. Ecol. Prog. Ser. 1988, 43, 1–10. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Purdie, D.A.; Williams, P.J.L.; Davis, J.M. Bacterioplankton: A sink for carbon in a coastal marine plankton community. Science 1986, 232, 865–867. [Google Scholar] [CrossRef]
- Stockner, J.G. Phototrophic picoplankton: An overview from marine and freshwater ecosystems. Limnol. Oceanogr. 1988, 33, 765–775. [Google Scholar] [CrossRef]
- Campbell, L.; Nolla, H.A.; Vaulot, D. The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol. Oceanogr. 1994, 39, 954–961. [Google Scholar] [CrossRef]
- Sarmento, H.; Montoya, J.M.; Vázquez-Dominguez, E.; Vaqué, D.; Gasol, J.M. Warming effects on marine microbial food web processes: How far can we go when it comes to predictions? Philos. Trans. R Soc. Lond. B Biol. Sci. 2010, 365, 2137–2149. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Significance of predation by protists in aquatic microbial food webs. A Van. Leeuw. J. Microb. 2002, 81, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G.; Brettar, I.; Hofle, M.G. Lysogeny and virus-induced mortality of bacterioplankton in surface, deep, and anoxic marine waters. Limnol. Oceanogr. 2003, 48, 1457–1465. [Google Scholar] [CrossRef]
- Wells, L.E.; Deming, J.W. Significance of bacterivory and viral lysis in bottom waters of Franklin Bay, Canadian Arctic, during winter. Aquat. Microb. Ecol. 2006, 43, 209–221. [Google Scholar] [CrossRef]
- Hoppe, H.G.; Breithaupt, P.; Walther, K.; Koppe, R.; Bleck, S.; Sommer, U.; Jürgens, K. Climate warming in winter affects the coupling between phytoplankton and bacteria during the spring bloom: A mesocosm study. Aquat. Microb. Ecol. 2008, 51, 105–115. [Google Scholar] [CrossRef]
- Shiah, F.; Ducklow, H.W. Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol. Oceanogr. 1994, 39, 1243–1258. [Google Scholar] [CrossRef]
- Šolić, M.; Šantić, D.; Šestanović, S.; Bojanić, N.; Ordulj, M.; Jozić, S.; Vrdoljak, A. The effect of temperature increase on microbial carbon fluxes in the Adriatic Sea: An experimental approach. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Legendre, L. Biogenic carbon cycling in the upper ocean: Effects of microbial respiration. Science 2001, 291, 2398–2400. [Google Scholar] [CrossRef]
- Vaqué, D.; Gasol, J.M.; Marrasé, C. Grazing rates on bacteria: The significance of methodology and ecological factors. Mar. Ecol. Prog. Ser. 1994, 109, 263–274. [Google Scholar] [CrossRef]
- Vázquez-Dominguez, E.; Vaqué, D.; Gasol, J.M. Temperature effects on the heterotrophic bacteria, heterotrophic nanoflagellates, and microbial top predators of the NW Mediterranean. Aquat. Microb. Ecol. 2012, 67, 107–121. [Google Scholar] [CrossRef]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Fuhrman, J.A.; Middelburg, J.J.; Noble, R.T.; Suttle, C.A. Marine viruses and global climate change. FEMS Microbiol. Rev. 2011, 35, 993–1034. [Google Scholar] [CrossRef]
- Maranger, R.; Vaqué, D.; Nguyen, D.; Hébert, M.P.; Lara, E. Pan-Arctic patterns of planktonic heterotrophic microbial abundance and processes: Controlling factors and potential impacts of warming. Prog. Oceanogr. 2015, 139, 221–232. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Shiau, W. Viral lysis and nanoflagellate grazing on prokaryotes: Effects of short-term warming in a coastal subtropical marine system. Hydrobiologia 2015, 751, 43–54. [Google Scholar] [CrossRef]
- Ordulj, M.; Krstulović, N.; Šantić, D.; Jozić, S.; Šolić, M. Viral dynamics in two trophically different areas in the Central Adriatic Sea. Helgol. Mar. Res. 2017, 71, 1–22. [Google Scholar] [CrossRef]
- López-Urrutia, Ȧ.; San Martin, E.; Harris, R.P.; Irigoien, X. Scaling the metabolic balance of the oceans. Proc. Natl. Acad. Sci. USA 2006, 103, 8739–8744. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Caffrey, J.; Cescatti, A.; Dossena, M.; del Giorgio, P.; Gasol, J.M.; Montoya, J.M.; Pumpanen, J.; Staehr, P.A.; Trimmer, M.; et al. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 2012, 487, 472–476. [Google Scholar] [CrossRef]
- Lassalle, G.; Lobry, J.; Le Loc’h, F.; Bustamante, P.; Certain, G.; Delmas, D.; Dupuy, C.; Hily, C.; Labry, C.; Le Pape, O.; et al. Lower trophic levels and detrital biomass control the Bay of Biscay continental shelf food web: Implications for ecosystem management. Prog. Oceanogr. 2011, 91, 561–575. [Google Scholar] [CrossRef]
- Edwards, M.; Richardson, A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 2004, 430, 881–884. [Google Scholar] [CrossRef]
- Wiltshire, K.H.; Malzahan, A.M.; Wirtz, K.; Greve, W.; Janish, S.; Mangelsdorf, P.; Manly, B.F.J.; Boersma, M. Resilience of North Sea Phytoplankton spring bloom dynamics: An analysis of long-term data at Helgoland Roads. Limnol. Oceanogr. 2008, 53, 1294–1302. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Chelton, D.B.; Schlax, M.G.; Samelson, R.M. Marine ecosystems’ responses to climatic and anthropogenic forcings in the Mediterranean. Prog. Oceanogr. 2011, 91, 97–166. [Google Scholar] [CrossRef]
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Šolić, M.; Grbec, B.; Matić, F.; Šantić, D.; Šestanović, S.; Ninčević Gladan, Ž.; Bojanić, N.; Ordulj, M.; Jozić, S.; Vrdoljak, A. Spatio-temporal reproducibility of the microbial food web structure associated with the change in temperature: Long-term observations in the Adriatic Sea. Prog. Oceanogr. 2018, 161, 87–101. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate; Solomon, S., Qin, D., Manning, M., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 748–845. [Google Scholar]
- Matić, F.; Grbec, B.; Morović, M. Indications of Climate Regime shifts in the Middle Adriatic Sea. Acta Adriat. 2011, 52, 235–246. [Google Scholar]
- Ninčević Gladan, Ž.; Marasović, I.; Grbec, B.; Skejić, S.; Bužančić, M.; Kušpilić, G.; Matijević, S.; Matić, F. Inter-decadal variability in phytoplankton community in the Middle Adriatic (Kaštela Bay) in relation to the North Atlantic Oscillation. Estuar. Coasts 2010, 33, 376–383. [Google Scholar] [CrossRef]
- Grbec, B.; Morović, M.; Matić, F.; Ninčević Gladan, Ž.; Marasović, I.; Vidjak, O.; Bojanić, N.; Čikeš Keč, V.; Zorica, B.; Kušpilić, G.; et al. Climate regime shifts and multi-decadal variability of the Adriatic Sea pelagic ecosystem. Acta Adriat. 2015, 56, 47–66. [Google Scholar]
- Šolić, M.; Krstulović, N.; Šantić, D.; Šestanović, S.; Kušpilić, G.; Bojanić, N.; Ordulj, M.; Jozić, S.; Vrdoljak, A. Impact of 3 °C temperature rise on bacterial growth and carbon transfer towards higher trophic levels: Empirical models for the Adriatic Sea. J. Mar. Syst. 2017, 173, 81–89. [Google Scholar] [CrossRef]
- Šolić, M.; Šantić, D.; Šestanović, S.; Bojanić, N.; Jozić, S.; Vrdoljak, A.; Ordulj, M.; Kušpilić, G. Temperature and phosphorus interacts in controlling the picoplankton carbon flux in the Adriatic Sea: An experimental vs. field study. Environ. Microbiol. 2019, 21, 2469–2484. [Google Scholar] [CrossRef]
- Wright, R.T.; Coffin, R.B. Measuring microzooplankton grazing on planktonic marine bacteria by its impact on bacterial production. Microb. Ecol. 1984, 10, 137–149. [Google Scholar] [CrossRef]
- Rassoulzadegan, F.; Sheldon, R.W. Predator-prey interactions of nanozooplankton and bacteria in an oligotrophic marine environment. Limnol. Oceanogr. 1986, 31, 1010–1021. [Google Scholar] [CrossRef]
- Stramski, D.; Morel, A. Optical properties of photosynthetic picoplankton in different physiological states as affected by growth irradiance. Deep Sea Res. 1990, 37, 245–266. [Google Scholar] [CrossRef]
- Morel, A.; Ahn, Y.H.; Partensky, F.; Vaulot, D.; Claustre, H. Prochlorococcus and Synechococcus: A comparative study of their optical properties in relation to their size and pigmentation. J. Mar. Res. 1993, 51, 617–649. [Google Scholar] [CrossRef]
- Landry, M.R.; Hassett, R.P. Estimating the grazing impact of marine microzooplankton. Mar. Biol. 1982, 67, 283–288. [Google Scholar] [CrossRef]
- James, M.; Hall, J. Microzooplankton grazing in different water masses associated with the Subtropical Convergence round the South Island, New Zealand. Deep Sea Res. Part I 1998, 45, 1689–1707. [Google Scholar] [CrossRef]
- Safi, K.A.; Griffiths, F.B.; Hall, J.A. Microzooplankton composition, biomass and grazing rates along the WOCE SR3 line between Tasmania and Antartica. Deep Sea Res. Part I 2007, 54, 1025–1041. [Google Scholar] [CrossRef]
- Manly, B.F.J. A model for certain types of selection experiments. Biometrics 1974, 30, 281–294. [Google Scholar] [CrossRef]
- Chesson, J. Measuring preference in selective predation. J. Anim. Ecol. 1978, 47, 805–816. [Google Scholar] [CrossRef]
- Chesson, J. The estimation and analysis of preference and its relationship to foraging models. Ecology 1983, 64, 1297–1304. [Google Scholar] [CrossRef]
- Smith, E.P. Niche breadth, resources availability, and inference. Ecology 1982, 63, 1675–1681. [Google Scholar] [CrossRef]
- Hurlbert, S.H. The measurement of niche overlap and some relatives. Ecology 1978, 59, 67–77. [Google Scholar] [CrossRef]
- Renkonen, O. Statisch-ökologische Untersuchungen über die terrestrische Käferwelt der finnischen Bruchmoore. Ann. Soc. Zool Bot. Fenn. Vanamo 1938, 6, 1–231. [Google Scholar]
- Horn, H. Measurements of “overlap” in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, USA, 1972; Volume 167, pp. 1–311. [Google Scholar] [CrossRef]
- Grasshoff, K. Methods of Seawater Analysis; Verlag Chemie: New York, NY, USA, 1976; pp. 1–317. [Google Scholar]
- Marie, D.; Partensky, F.; Vaulot, D.; Brussaard, C. Enumeration of phytoplankton, bacteria, and viruses in marine samples. Curr. Protoc. Cytom. 1999, 10. [Google Scholar] [CrossRef] [PubMed]
- Marie, D.; Partensky, F.; Jacquet, S.; Vaulot, D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green, I. Appl. Environ. Microb. 1997, 63, 186–193. [Google Scholar] [CrossRef]
- Christaki, U.; Courties, C.; Massana, R.; Catala, P.; Lebaron, P.; Gasol, J.M.; Zubkov, M.V. Optimized routine flow cytometric enumeration of heterotrophic flagellates using SYBR Green, I. Limnol. Oceanogr. Meth. 2011, 9, 329–339. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton—Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–37. [Google Scholar] [CrossRef]
- Lee, S.; Fuhrman, J.A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microb. 1987, 53, 1298–1303. [Google Scholar] [CrossRef]
- Kirchman, D.L.; Kell, R.G.; Simon, M.; Welschmeyer, N.A. Biomass and production of heterotrophic bacterioplankton in the oceanic subarctic Pacific. Deep Sea Res. Part I 1993, 40, 967–988. [Google Scholar] [CrossRef]
- Buitenhuis, E.T.; Li, W.K.W.; Vaulot, D.; Lomas, M.W.; Landry, M.; Partensky, F.; Karl, D.M.; Ulloa, O.; Campbell, L.; Jacquet, S.; et al. Picophytoplankton biomass distribution in the global ocean. Earth Syst. Sci. Data 2012, 4, 37–46. [Google Scholar] [CrossRef]
- Borsheim, K.Y.; Bratbak, G. Cell volume to cell carbon conversion factors for a bacteriovorous Monas sp. Enriched from seawater. Mar. Ecol. Prog. Ser. 1987, 36, 171–175. [Google Scholar] [CrossRef]
- Putt, M.; Stoecker, D.K. An experimental determined carbon: Volume ratio for marine ‘oligotrichous’ ciliates from estuarine and coastal waters. Limnol. Oceanogr. 1989, 34, 1097–1103. [Google Scholar] [CrossRef]
- Verity, P.G.; Langdon, C. Relationships between lorica volume, carbon, nitrogen and ATP content of tintinnids in Narragansett Bay. J. Plankton Res. 1984, 6, 859–868. [Google Scholar] [CrossRef]
- Agawin, N.S.; Duarte, C.M.; Agusti, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Bouvier, T.; Del Giorgio, P.A.; Gasol, J.M. A comparative study of cytometric characteristics of High and Low nucleic-acid bacterioplankton cells from different aquatic ecosystems. Environ. Microbiol. 2007, 9, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Šantić, D.; Šestanović, S.; Šolić, M.; Krstulović, N.; Kušpilić, G.; Ordulj, M.; Ninčević Gladan, Ž. Dynamics of the picoplankton community from coastal waters to the open sea in the Central Adriatic. Mediterr. Mar. Sci. 2014, 15, 179–188. [Google Scholar] [CrossRef]
- Huete-Stauffer, T.M.; Arandia-Gorostidi, N.; Diaz-Perez, L.; Morán, X.A.G. Temperature dependences of growth rates and carrying capacities of marine bacteria depart from metabolic theoretical predictions. FEMS Microb. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Schattenhofer, M.; Wulf, J.; Kostadinov, I.; Glöckner, F.O.; Zubkov, M.V.; Fuchs, B.M. Phylogenetic characterisation of pi-coplanktonic populations with high and low nucleic acid contentin the North Atlantic Ocean. Syst. Appl. Microbiol. 2011, 34, 470–475. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Gasol, J.M.; Sharma, S.; Morán, M.A. Community analysis of high- and low-nucleic acid-containingbacteria in NW Mediterranean coastal waters using 16SrDNA pyrosequencing. Environ. Microbiol. 2012, 14, 1390–1402. [Google Scholar] [CrossRef]
- Otero-Ferrer, J.L.; Cermeño, P.; Bode, A.; Fernández-Castro, B.; Gasol, J.P.; Morán, X.A.G.; Marañon, E.; Moreira-Coello, V.; Varela, M.M.; Villamaña, M.; et al. Factors controlling the community structure of picoplankton in contrasting marine environments. Biogeosciences 2018, 15, 6199–6220. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Integrating resource utilization and temperature in metabolic scaling of riverine bacterial production. Ecology 2010, 91, 1455–1465. [Google Scholar] [CrossRef]
- Savage, V.M.; Gilloly, J.F.; Brown, J.H.; Charnov, E.L. Effects of body size and temperature on population growth. Am. Nat. 2004, 163, 429–441. [Google Scholar] [CrossRef]
- Anderson-Texeira, K.J.; Vitousek, P.M. Ecosystems. In Metabolic Ecology: A Scaling Approach; Sibly, R.M., Brown, J.H., Kodric-Brown, A., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 99–111. [Google Scholar]
- Morán, X.A.G.; Ducklow, H.W.; Erickson, M. Single-cell physiological structure and growth rates of heterotrophic bacteria in a temperate estuary (Waquoit Bay, Massachusetts). Limnol. Oceanogr. 2011, 56, 37–48. [Google Scholar] [CrossRef]
- Morán, X.A.G.; Alonso-Sáez, L.; Nogueira, E.; Ducklow, H.W.; Gonzalez, N.; López-Urrutia, Á.; Díaz-Pérez, L.; Calvo-Díaz, A.; Arandia-Gorostidi, N.; Heute-Stauffer, T.M. More, smaller bacteria in response to ocean’s warming? Proc. R. Soc. B 2015, 282, 20150371. [Google Scholar] [CrossRef]
- Calvo-Díaz, A.; Franco-Vidal, L.; Morán, X.A.G. Annual cycles of bacterioplankton biomass and production suggest a general switch between temperature and resource control in temperate coastal ecosystems. J. Plankton Res. 2014, 36, 859–865. [Google Scholar] [CrossRef]
- Morán, X.A.G.; Calvo-Díaz, A.; Arandia-Gorostidi, N.; Huete-Stauffer, T.M. Temperature sensitivities of microbial plankton net growth rates are seasonally coherent and linked to nutrient availability. Environ. Microbiol. 2018, 20, 3798–3810. [Google Scholar] [CrossRef] [PubMed]
- Nedwell, D.B. Effect of low temperature on microbial growth: Lowered affinity for substrates limits growth at low temperature. FEMS Microb. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef]
- Chen, B.Z.; Laws, E.A. Is there a difference of temperature sensitivity between marine phytoplankton and heterotrophs? Limnol. Oceanogr. 2017, 62, 806–817. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellnera, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Pichler, M.F.; Leech, D.M.; Anton, A.; Bruno, J.F. Warming and resource availability shift food web structure and metabolism. PLoS Biol. 2009, 7, e1000178. [Google Scholar] [CrossRef]
- Morán, X.A.G.; Calvo-Diaz, A.; Ducklow, H.W. Total and phytoplankton mediated bottom-up control of bacterioplankton change with temperature in NE Atlantic shelf waters. Aquat. Microb. Ecol. 2010, 58, 229–239. [Google Scholar] [CrossRef]
- Van De Poll, W.H.; Kulk, G.; Timmermans, K.R.; Brussaard, C.P.D.; Van Der Woerd, H.J.; Kehoe, M.J.; Mojica, K.D.A.; Visser, R.J.W.; Rozema, P.D.; Buma, A.G.J. Phytoplankton chlorophyll a biomass, composition, and productivity along a temperature and stratification gradient in the northeast Atlantic Ocean. Biogeosciences 2013, 10, 4227–4240. [Google Scholar] [CrossRef]
- Allen, A.P.; Gillooly, J.F.; Brown, J.H. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 2005, 19, 202–213. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Rose, J.M.; Vora, N.M.; Countway, P.D.; Gast, R.J.; Caron, D.A. Effects of temperature on growth rate and gross growth efficiency of an Antarctic bacterivorous protest. ISME J. 2009, 3, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Unrein, F.; Massana, R.; Alonso-Sáez, L.; Gasol, J.M. Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol. Oceanogr. 2007, 52, 456–469. [Google Scholar] [CrossRef]
- Zingel, P.; Agasild, H.; Nõges, T.; Kisand, V. Ciliates are the dominant grazers on pico- and nanoplankton in a shallow, naturally highly eutrophic lake. Microb. Ecol. 2007, 53, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zoccarato, L.; Fonda Umani, S. Major constrains of the pelagic food web efficiency in the Mediterranean Sea. Biogeosci. Dis. 2015, 12, 4365–4403. [Google Scholar] [CrossRef]
- Calbert, A.; Landry, M.R.; Ninnery, S. Bacteria-flagellate interactions in the microbial food web of the oligotrophic subtropical North Pacific. Aquat. Microb. Ecol. 2001, 23, 283–292. [Google Scholar] [CrossRef]
- Miller, C.A.; Penry, D.L.; Gilbert, P.M. The impact of trophic interactions on the rates of nitrogen regeneration and grazing in Chesapeake Bay. Limnol. Oceanogr. 1995, 40, 1005–1011. [Google Scholar] [CrossRef]
- Bojanić, N.; Šolić, M.; Krstulović, N.; Šestanović, S.; Marasović, I.; Ninčević, Ž. Temporal variability in abundance and biomass of ciliates and copepods in the eutrophicated part of Kaštela Bay (Middle Adriatic Sea). Helgol. Mar. Res. 2005, 59, 107–120. [Google Scholar] [CrossRef][Green Version]
- Dolan, J.R. Morphology and ecology in tintinnid ciliates of the marine plankton: Correlates of lorica dimensions. Acta Protozool. 2010, 49, 235–244. [Google Scholar]
- Johansson, M.; Gorokhova, E.; Larsson, U. Annual variability in ciliate community structure, potential prey and predators on the open northern Baltic Sea proper. J. Plankton Res. 2004, 26, 67–80. [Google Scholar] [CrossRef]
- Norf, H.; Weitere, M. Resource quantity and seasonal background alter warming effects on communities of biofilm ciliates. Microb. Ecol. 2010, 74, 361–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoecker, D.K.; Capuzzo, J.M. Predation on protozoa: Its importance to zooplankton. J. Plankton Res. 1990, 12, 891–908. [Google Scholar] [CrossRef]
- Jezbera, J.; Nedoma, J.; Šimek, K. Longitudinal changes in protistan bacterivory and bacterial production in two canyon-shaped reservoirs of different trophic status. Hydrobiologia 2003, 504, 115–130. [Google Scholar] [CrossRef]
- Bojanić, N.; Šolić, M.; Krstulović, N.; Šestanović, S.; Ninčević Gladan, Ž.; Marasović, I.; Brautović, I. The role of ciliates within the microbial food web in the eutrophicated part of Kaštela Bay (middle Adriatic Sea). Sci. Mar. 2006, 70, 431–442. [Google Scholar] [CrossRef]
- Liu, H.; Dagg, M. Interactions between nutrients, phytoplankton growth, and micro- and mesozooplankton grazing in the plume of the Mississippi River. Mar. Ecol. Prog. Ser. 2003, 258, 31–42. [Google Scholar] [CrossRef]
- Legendre, L.; Rassoulzadegan, F. Plankton and nutrient dynamics in marine waters. Ophelia 1995, 41, 153–172. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Williams, P.J.L.B. Inorganic nitrogen assimilation by picoplankton and whole plankton in a coastal ecosystem. Limnol. Oceanogr. 2002, 47, 1608–1616. [Google Scholar] [CrossRef]
- Wafar, M.; L’Helguen, S.; Raikar, V.; Maguer, J.F. Nitrogen uptake by size-fractionated plankton in permaqnently well-mixed temperate coastal waters. J. Plankton Res. 2004, 26, 1207–1218. [Google Scholar] [CrossRef]
- Probyn, T.A.; Waldron, H.N.; James, A.G. Size fractionated measurements of nitrogen uptake in aged upwelled waters: Implications for pelagic food webs. Limnol. Oceanogr. 1990, 35, 202–210. [Google Scholar] [CrossRef]
- Selmer, J.S.; Ferrier-Pages, C.; Cellario, C.; Rassoulzadegan, F. New and regenerated production in relation to the microbial loop in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 1993, 100, 71–83. [Google Scholar] [CrossRef]
- Moore, L.R.; Post, A.F.; Rocap, G.; Chisholm, S.W. Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus Synechococcus. Limno Oceanogl. 2002, 47, 989–996. [Google Scholar] [CrossRef]
- Casey, J.R.; Lomas, M.; Mandechi, J.; Walker, D.E. Prochlorococcus contributes to new production in the Sargasso Sea deep chlorophyll maximum. Geophys. Res. Lett. 2007, 34, L10604. [Google Scholar] [CrossRef]
- Martiny, A.C.; Kathuria, S.; Berube, P.M. Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. Proc. Natl. Acad. Sci. USA 2009, 106, 10787–10792. [Google Scholar] [CrossRef]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]
- Mouriño-Carballido, B.; Hojas, E.; Cermeño, P.; Chouciño, P.; Fernández-Castro, B.; Latasa, M.; Marañón, E.; Morán, X.A.G.; Vidal, M. Nutrient supply controls picoplankton community structure during three contrasting seasons in the north-wastern Mediterranean Sea. Mar. Ecol. Prog. Ser. 2016, 543, 1–19. [Google Scholar] [CrossRef]
- Mary, I.; Tarran, G.A.; Warwick, P.E.; Terry, M.J.; Scanlan, D.J.; Burkill, P.H.; Zubkov, M.V. Light enhanced amino acid uptake by dominant bacterioplankton groups in surface waters of the Atlantic Ocean. FEMS Microbial. Ecol. 2008, 63, 36–45. [Google Scholar] [CrossRef]
| Experimental Settings | Size Fractions | ||
|---|---|---|---|
| 2 µm | 10 µm | 200 µm | |
| Prey organisms | PICO groups | PICO groups | PICO groups, NF |
| Predator organisms | No predators | HNF | HNF, CIL |
| Estimated growth rates | Maximal growth of PICO [µPICO(2µm)] | Net growth of PICO [µPP(10µm)] Maximal growth of HNF [µHNF(10µm)] | Net growth of PICO [µPICO(200µm)] Net growth of HNF [µHNF(200µm)] |
| Estimated grazing rates | No grazing | HNF grazing on PICO: gPICO(HNF) = µPICO(2µm) – µPICO(10µm) | Total grazing on HNF: gPICO(TOT) = µPICO(2µm) − µPICO(200µm) Ciliate grazing on HNF: gHNF(CIL) = µHNF(10µm) − µHNF(200µm) |
| Food-Niche Characteristic Title | Ambient Temperature | Ambient Temperature + 3 °C | ||
|---|---|---|---|---|
| HNF | CIL | HNF | CIL | |
| Niche breadth: Smith’s measure (95% conf. limits) | 0.943 (0.928–0.957) | 0.881 (0.860–0.901) | 0.972 (0.960–0.981) | 0.939 (0.923–0.953) |
| Hurlbert’s standardized measure (95% conf. limits) | 0.749 (0.706–0.792) | 0.642 (0.606–0.677) | 0.832 (0.784–0.880) | 0.673 (0.620–0.725) |
| Niche overlap: Percentage overlap (%) | 90.9 | 82.7 | ||
| Horn’s index | 0.984 | 0.918 | ||
| Hurlbert’s index | 1.666 | 1.423 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šolić, M.; Šantić, D.; Šestanović, S.; Bojanić, N.; Jozić, S.; Ordulj, M.; Vrdoljak Tomaš, A.; Kušpilić, G. Changes in the Trophic Pathways within the Microbial Food Web in the Global Warming Scenario: An Experimental Study in the Adriatic Sea. Microorganisms 2020, 8, 510. https://doi.org/10.3390/microorganisms8040510
Šolić M, Šantić D, Šestanović S, Bojanić N, Jozić S, Ordulj M, Vrdoljak Tomaš A, Kušpilić G. Changes in the Trophic Pathways within the Microbial Food Web in the Global Warming Scenario: An Experimental Study in the Adriatic Sea. Microorganisms. 2020; 8(4):510. https://doi.org/10.3390/microorganisms8040510
Chicago/Turabian StyleŠolić, Mladen, Danijela Šantić, Stefanija Šestanović, Natalia Bojanić, Slaven Jozić, Marin Ordulj, Ana Vrdoljak Tomaš, and Grozdan Kušpilić. 2020. "Changes in the Trophic Pathways within the Microbial Food Web in the Global Warming Scenario: An Experimental Study in the Adriatic Sea" Microorganisms 8, no. 4: 510. https://doi.org/10.3390/microorganisms8040510
APA StyleŠolić, M., Šantić, D., Šestanović, S., Bojanić, N., Jozić, S., Ordulj, M., Vrdoljak Tomaš, A., & Kušpilić, G. (2020). Changes in the Trophic Pathways within the Microbial Food Web in the Global Warming Scenario: An Experimental Study in the Adriatic Sea. Microorganisms, 8(4), 510. https://doi.org/10.3390/microorganisms8040510






