The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Exopolysaccharide Extraction
2.3. Cell Lines
2.4. Effect of EPS on H. pylori Adhesion to AGS Cells
2.5. Effect of EPS on AGS and THP-1 Cells Cytokines Profiles
2.6. Animals and Experimental Infection
2.7. Adhesion of H. pylori to Mouse Gastric Mucosa
2.8. Gastric and Serum Immunological Response
2.9. Statistical Analysis
3. Results
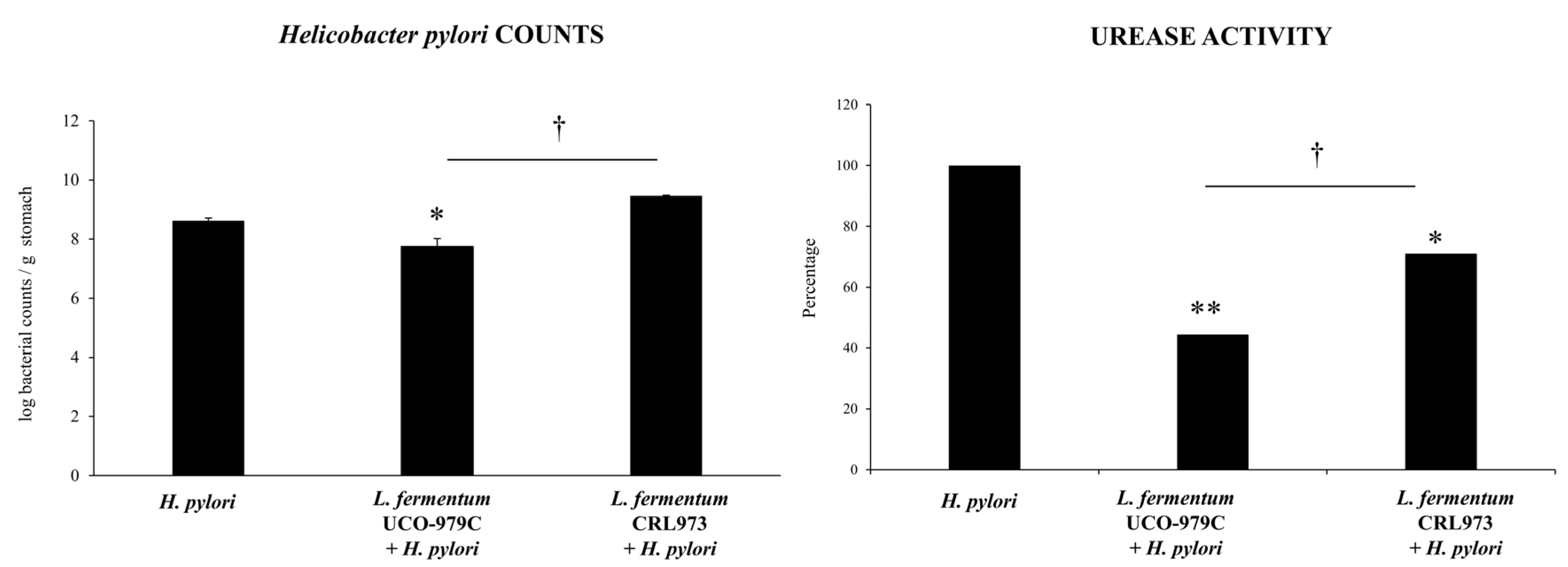
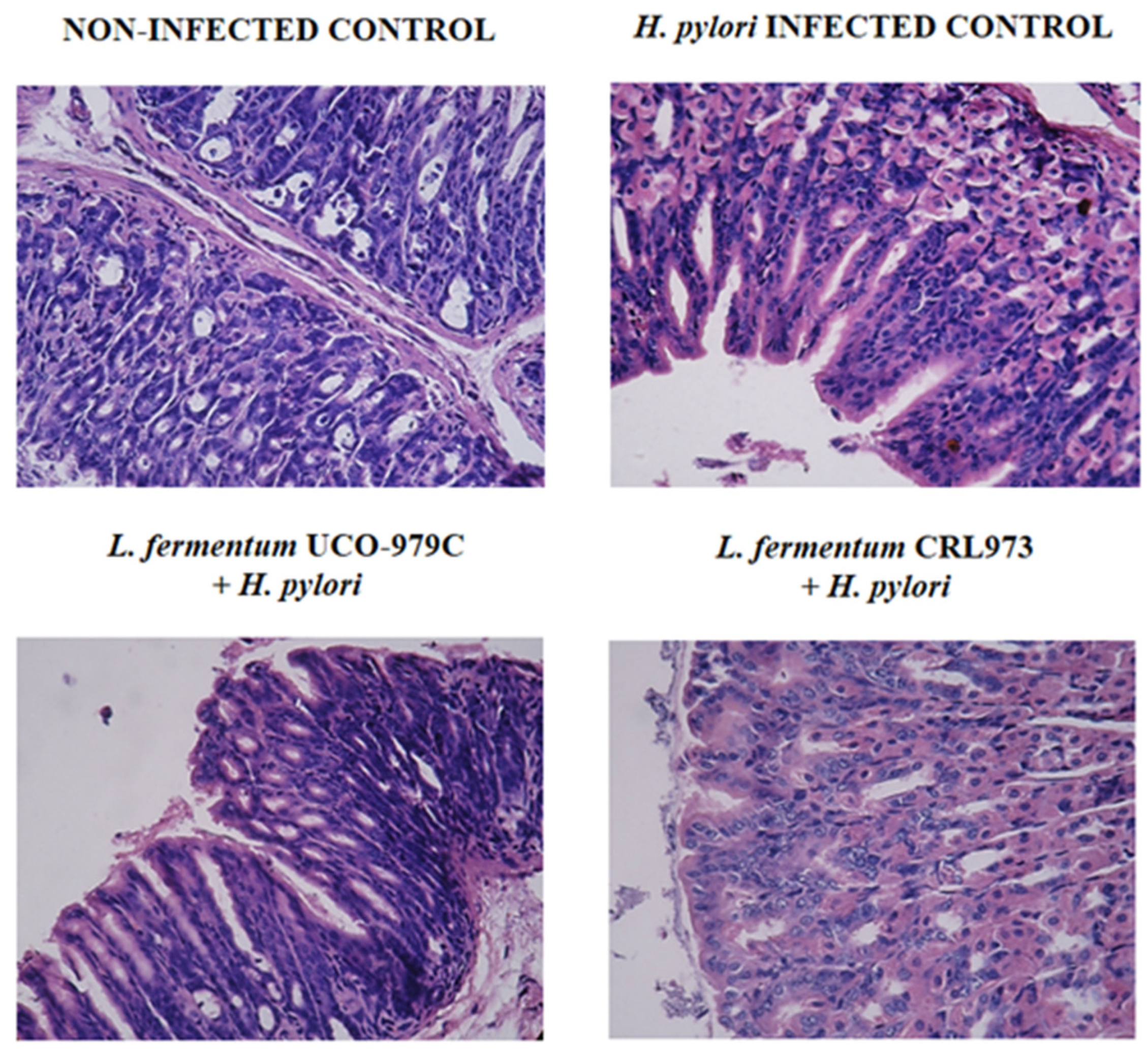
3.1. L. fermentum UCO-979C Improves the Resistance Against H. pylori Infection in Mice
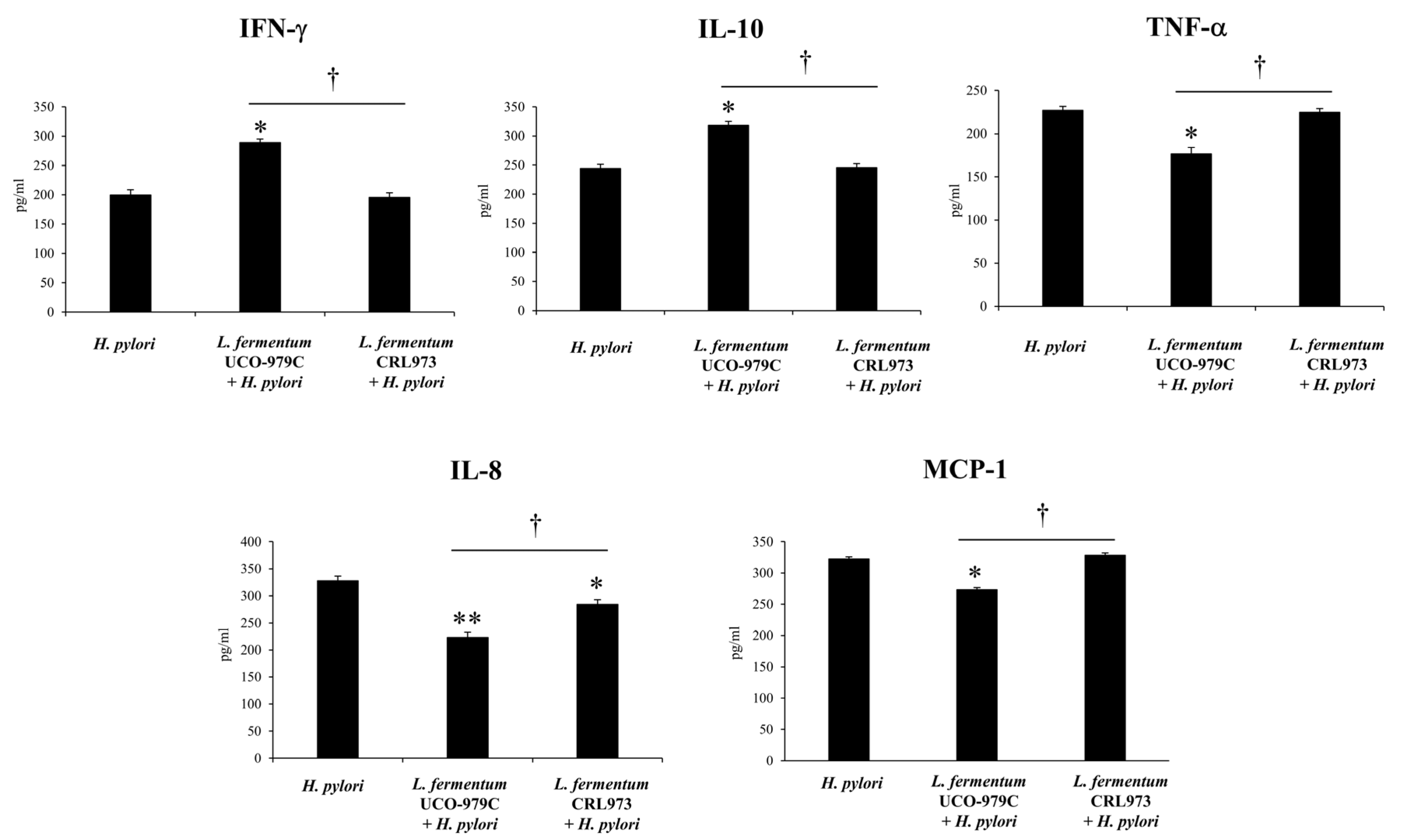
3.2. L. fermentum UCO-979C Differentially Modulates the Immune Response Against H. pylori Infection in Mice
3.3. The EPS from L. Fermentum UCO-979C Reduces H. pylori Adhesion to AGS Cells
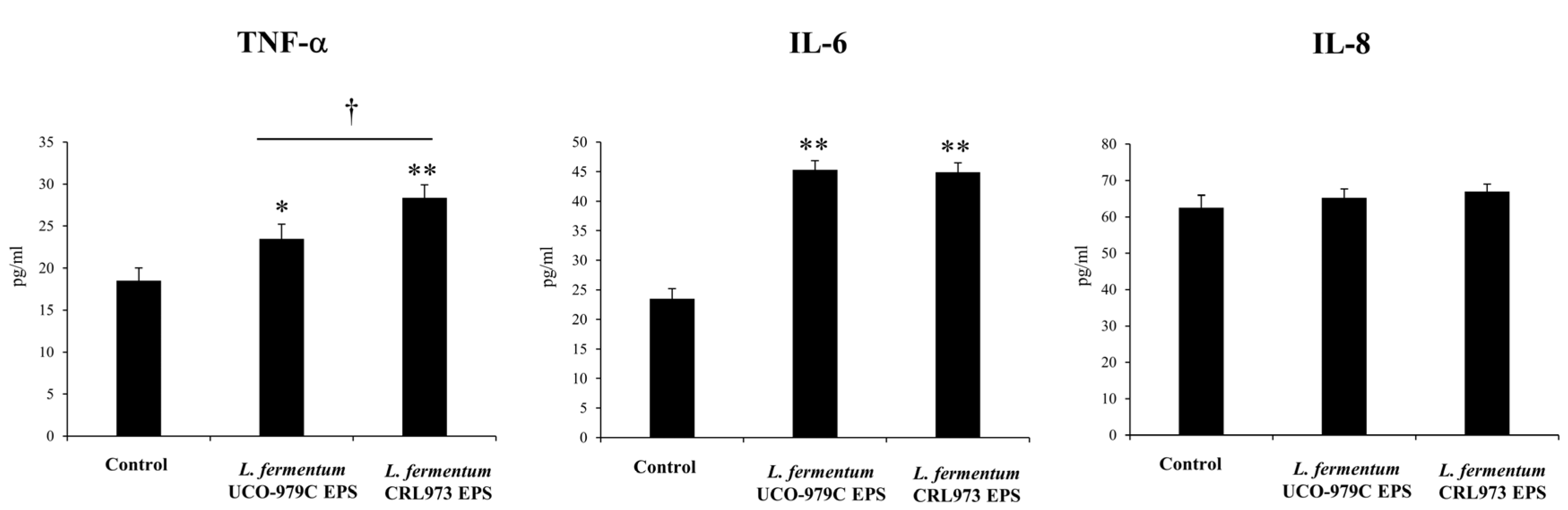
3.4. The EPS from L. fermentum UCO-979C Modulates Cytokine Profile in AGS and THP-1 Cells
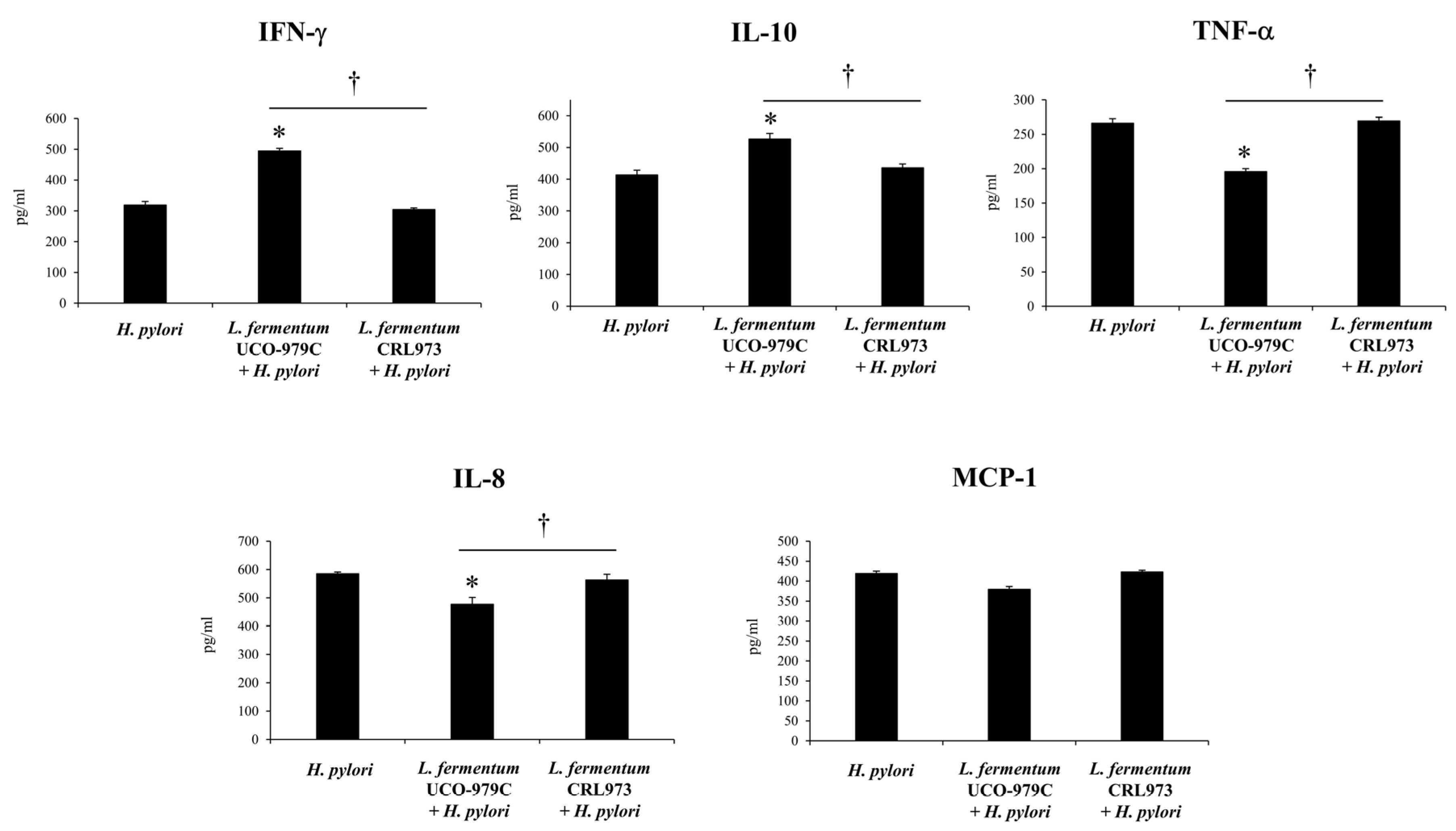
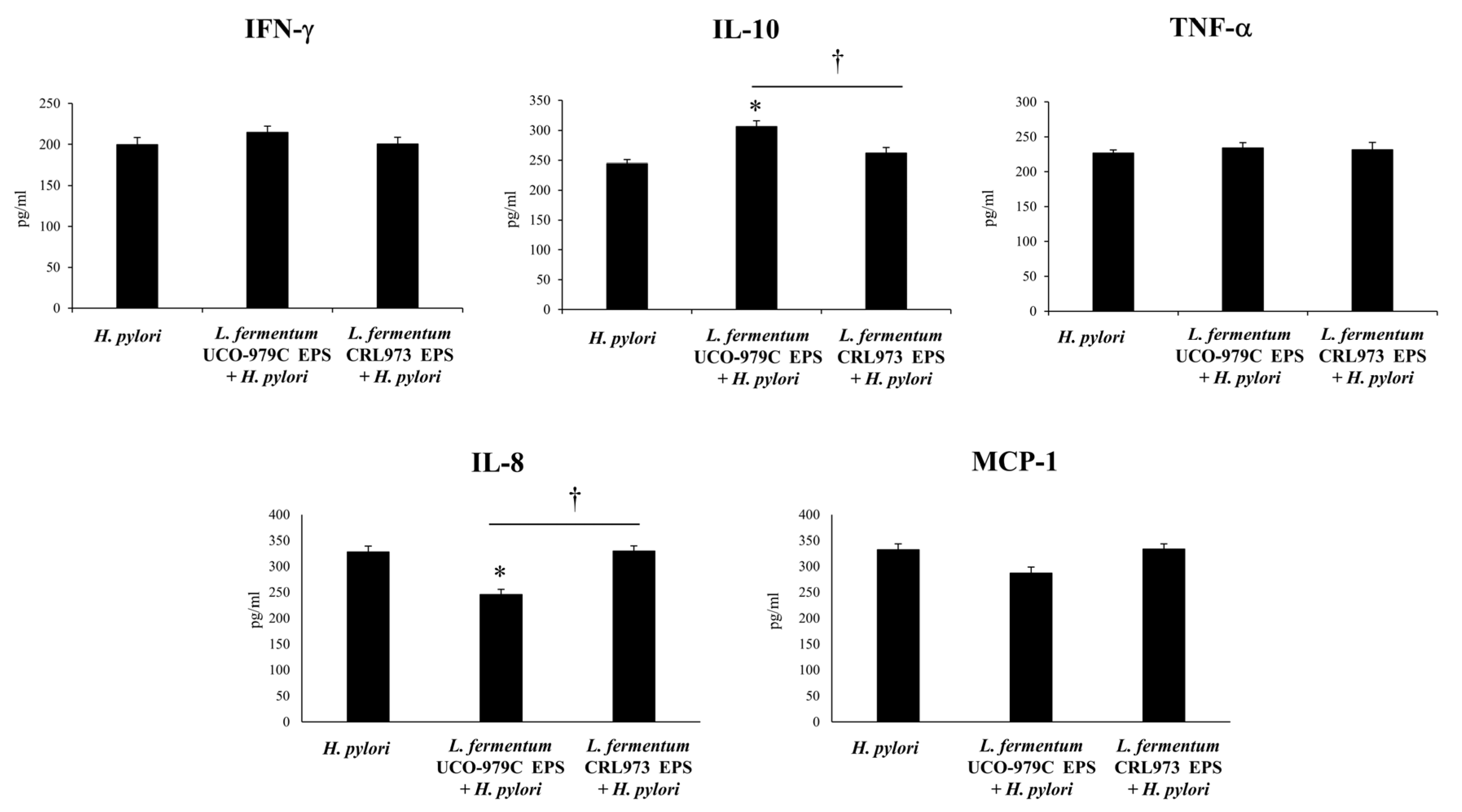
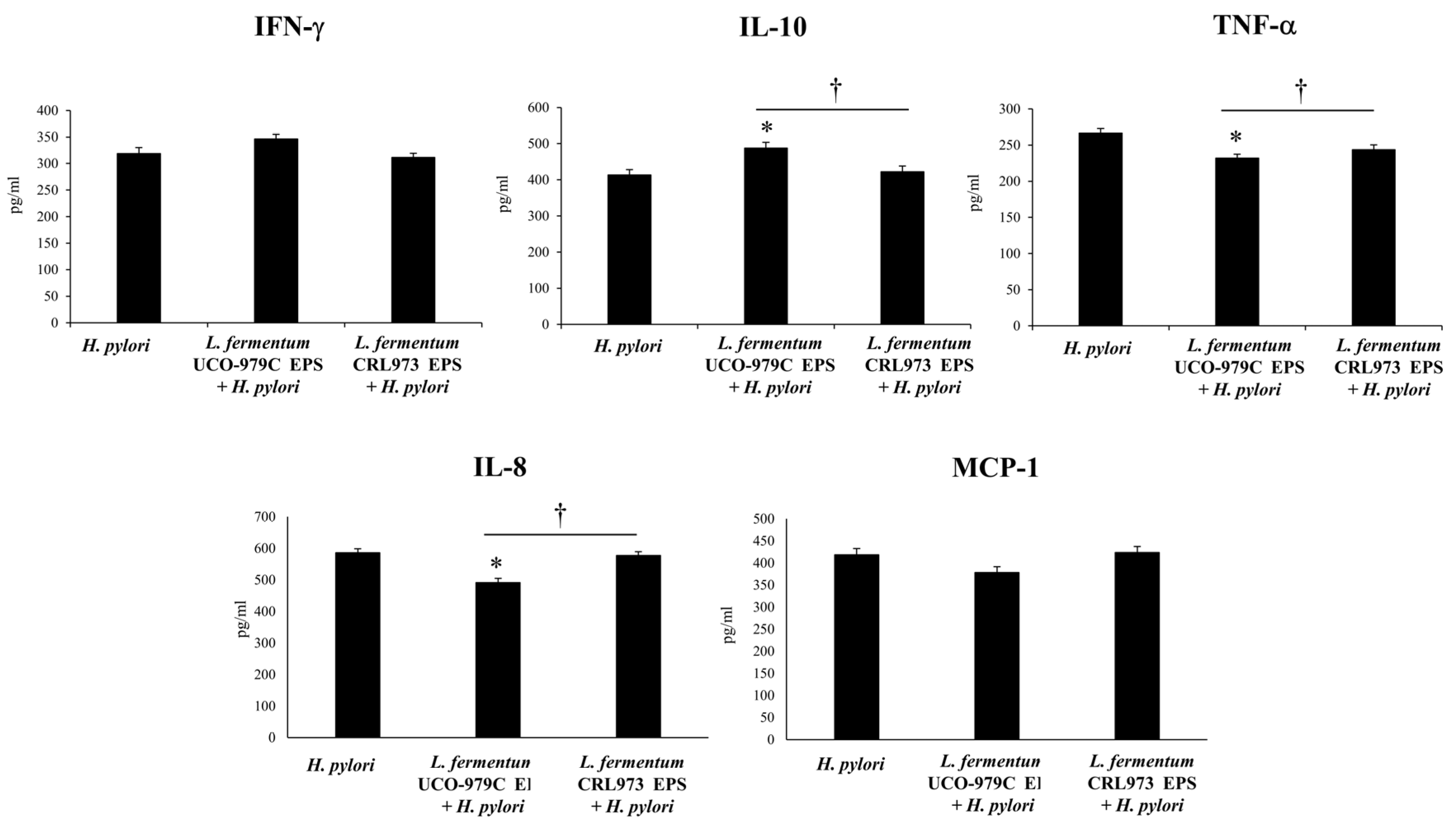
3.5. The EPS from L. fermentum UCO-979C Modulates the Inflammatory Response Triggered by H. pylori Infection in Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Martin, M.E.; Solnick, J.V. The gastric microbial community, Helicobacter pylori colonization, and disease. Gut Microbes 2014, 5, 345–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, M.E.; Bhatnagar, S.; George, M.D.; Paster, B.J.; Canfield, D.R.; Eisen, J.A.; Solnick, J.V. The Impact of Helicobacter pylori Infection on the Gastric Microbiota of the Rhesus Macaque. PLoS ONE 2013, 8, e76375. [Google Scholar] [CrossRef]
- Hunt, R.H.; Camilleri, M.; Crowe, S.E.; El-Omar, E.M.; Fox, J.G.; Kuipers, E.J.; Malfertheiner, P.; McColl, K.E.L.; Pritchard, D.M.; Rugge, M.; et al. The stomach in health and disease. Gut 2015, 64, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Minalyan, A.; Gabrielyan, L.; Scott, D.; Jacobs, J.; Pisegna, J.R. The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de la Cruz-Herrera, C.F.; Romero, I. Exploring alternative treatments for Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 1450–1469. [Google Scholar] [CrossRef]
- Isolauri, E.; Kirjavainen, P.V.; Salminen, S. Probiotics: A role in the treatment of intestinal infection and inflammation? J. Rheumatol. 2002, 50, 54–59. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H. Probiotics, infection and immunity. Curr. Opin. Infect. Dis. 2002, 15, 501–506. [Google Scholar] [CrossRef]
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory Antiviral Immunity and Immunobiotics: Beneficial Effects on Inflammation-Coagulation Interaction during Influenza Virus Infection. Front. Immunol. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal Innate Antiviral Immunity and Immunobiotics: Beneficial Effects against Rotavirus Infection. Front. Immunol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rolig, A.S.; Cech, C.; Ahler, E.; Carter, J.E.; Ottemann, K.M. The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota. Infect. Immun. 2013, 81, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Leite, A.M.O.; Ruas-Madiedo, P.; Mayo, B. Probiotic and technological properties of Lactobacillus spp. Strains from the human stomach in the search for potential candidates against gastric microbial dysbiosis. Front. Microbiol. 2014, 5, 766. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Sanhueza, E.A.; Retamal-Díaz, A.; González, C.; Urrutia, H.; García, A. Probiotic Lactobacillus fermentum UCO-979C biofilm formation on AGS and Caco-2 cells and Helicobacter pylori inhibition. Biofouling 2016, 32, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a probiotic strain with a potent anti-Helicobacter pylori activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Zelaya, H.; Ilabaca, A.; Espinoza-Monje, M.; Komatsu, R.; Albarracin, L.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. Lactobacillus fermentum UCO-979C beneficially modulates the innate immune response triggered by Helicobacter pylori infection in vitro. Benef. Microbes 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Komatsu, R.; Clua, P.; Indo, Y.; Takagi, M.; Salva, S.; Islam, M.A.; Alvarez, S.; Takahashi, H.; Garcia-Cancino, A.; et al. Evaluation of the Immunomodulatory Activities of the Probiotic Strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Ferrer, J.; Pinuer, L.; García, A.; Bórquez, R. Effect of pH and dilution rate on specific production rate of extra cellular metabolites by Lactobacillus salivarius UCO_979C in continuous culture. Appl. Microbiol. Biotechnol. 2015, 99, 6417–6429. [Google Scholar]
- Sgouras, D.; Maragkoudakis, P.; Petraki, K.; Eriotou, E.; Michopoulos, S.; Tsakalidou, E.; Mentis, A. In Vitro and In Vivo Inhibition of Helicobacter pylori by Lactobacillus casei Strain Shirota. Society 2004, 70, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.A. Methods for Enumerating Microorganisms and Determining Biomass in Natural Environments. Methods Microbiol. 1990, 22, 1–39. [Google Scholar]
- Coconnier, M.H.; Lievin, V.; Hemery, E.; Servin, A.L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 1998, 64, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Hazell, S.L.; Borody, T.J.; Gal, A.; Lee, A. Campylobacter pyloridis Gastritis I: Detection of Urease as a Marker of Bacterial Colonization and Gastritis. Am. J. Gastroenterol. 1987, 82, 292–296. [Google Scholar]
- Zhang, L.; Su, P.; Henriksson, A.; O’Rourke, J.; Mitchell, H. Investigation of the immunomodulatory effects of Lactobacillus casei and Bifidobacterium lactis on Helicobacter pylori infection. Helicobacter 2008, 13, 183–190. [Google Scholar] [CrossRef]
- Ghosh, N.; Ghosh, P.; Kesh, K.; Mukhopadhyay, A.K.; Swarnakar, S. Attenuation of Helicobacter pylori-induced gastric inflammation by prior cag—Strain (AM1) infection in C57BL/6 mice. Gut Pathog. 2017, 9, 14. [Google Scholar] [CrossRef]
- Li, B.; Chen, L.; Sun, H.; Yang, W.; Hu, J.; He, Y.; Wei, S.; Zhao, Z.; Zhang, J.; Li, H.; et al. Immunodominant epitope-specific Th1 but not Th17 responses mediate protection against Helicobacter pylori infection following UreB vaccination of BALB/c mice. Sci. Rep. 2015, 5, 14793. [Google Scholar] [CrossRef]
- Kienesberger, S.; Cox, L.M.; Livanos, A.; Zhang, X.S.; Chung, J.; Perez-Perez, G.I.; Gorkiewicz, G.; Zechner, E.L.; Blaser, M.J. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016, 14, 1395–1407. [Google Scholar] [CrossRef]
- Kao, J.Y.; Rathinavelu, S.; Eaton, K.A.; Bai, L.; Zavros, Y.; Takami, M.; Pierzchala, A.; Merchant, J.L. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: A mechanism of ineffective host defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G73–G81. [Google Scholar] [CrossRef]
- Walduck, A.; Andersen, L.P.; Raghavan, S. Inflammation, Immunity, and Vaccines for Helicobacter pylori Infection. Helicobacter 2015, 20, 17–25. [Google Scholar] [CrossRef]
- Niwa, T.; Tsukamoto, T.; Toyoda, T.; Mori, A.; Tanaka, H.; Maekita, T.; Ichinose, M.; Tatematsu, M.; Ushijima, T. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010, 70, 1430–1440. [Google Scholar] [CrossRef]
- Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi, L.; Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; Scudiero, O. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, K.; Gaur, S. Role of Probiotics in Prophylaxis of Helicobacter pylori Infection. Curr. Pharm. Biotechnol. 2019, 20, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.M.; Aiba, Y.; Takagi, A.; Kamiya, S.; Miwa, T.; Koga, Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut 1997, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.C.; Mitchell, D.J.; Avitzur, Y.; Galindo-Mata, E.; Jones, N.L.; Sherman, P.M. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 2004, 49, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, F.Z.; Deng, X.J.; Yuan, H.; Ma, H.S. Lactobacilli inhibit interleukin-8 production induced by Helicobacter pylori lipopolysaccharide-activated Toll-like receptor 4. World J. Gastroenterol. 2008, 14, 5090–5095. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Marín-Vega, A.M.; Ilabaca, A.; Albarracín, L.; Marcial, G.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. Characterization of the immunomodulatory and anti-Helicobacter pylori properties of the human gastric isolate Lactobacillus rhamnosus UCO-25A. Biofouling 2019, 35, 922–937. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Chuang, C.-C.; Yang, H.-B.; Lu, C.-C.; Sheu, B.-S. Lactobacillus acidophilus ameliorates H. pylori-induced gastric inflammation by inactivating the Smad7 and NFκB pathways. BMC Microbiol. 2012, 12, 38. [Google Scholar] [CrossRef]
- Lee, J.S.; Paek, N.S.; Kwon, O.S.; Hahm, K.B. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: A novel mechanism. J. Gastroenterol. Hepatol. 2010, 25, 194–202. [Google Scholar] [CrossRef]
- Garcia, C.A.; Henriquez, A.P.; Retamal, R.C.; Pineda, C.S.; Delgado Sch, C.; Gonzalez, C.C. Probiotic properties of Lactobacillus spp isolated from gastric biopsies of Helicobacter pylori infected and non-infected individuals. Rev. Med. Chile 2009, 137, 369–376. [Google Scholar]
- Merino, J.S.; García, A.; Pastene, E.; Salas, A.; Saez, K.; González, C.L. Lactobacillus fermentum UCO-979C strongly inhibited Helicobacter pylori SS1 in Meriones unguiculatus. Benef. Microbes 2018, 9, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Bockerstett, K.A.; DiPaolo, R.J. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. CMGH 2017, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Kwok, T.; Hartig, R.; Konig, W.; Backert, S. NF- B activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kato, S.; Matsuhisa, T.; Makonkawkeyoon, L.; Yoshida, M.; Chakrabandhu, T.; Lertprasertsuk, N.; Suttharat, P.; Chakrabandhu, B.; Nishiumi, S.; et al. Predominant mucosal IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World J. Gastroenterol. 2013, 19, 2941–2949. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Devi, S.; Tenguria, S.; Kumar, A.; Ahmed, N. Helicobacter pylori protein HP0986 (TieA) interacts with mouse TNFR1 and triggers proinflammatory and proapoptotic signaling pathways in cultured macrophage cells (RAW 264.7). Cytokine 2014, 68, 110–117. [Google Scholar] [CrossRef]
- Wang, F.; Mao, Z.; Liu, D.; Yu, J.; Wang, Y.; Ye, W.; Lin, D.; Zhou, N.; Xie, Y. Overexpression of Tim-3 reduces Helicobacter pylori-associated inflammation through TLR4/NFκB signaling in vitro. Mol. Med. Rep. 2017, 15, 3252–3258. [Google Scholar] [CrossRef]
- Bauditz, J.; Ortner, M.; Bierbaum, M.; Niedobitek, G.; Lochs, H.; Schreiber, S. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin. Exp. Immunol. 1999, 117, 316–323. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Li, J.; Rong, Q.; Ji, X.; Li, B. The leukocyte-associated immunoglobulin (Ig)–like receptor-1 modulating cell apoptosis and inflammatory cytokines secretion in THP-1 cells after Helicobacter pylori infection. Microb. Pathog. 2017, 109, 292–299. [Google Scholar] [CrossRef]
- Viladomiu, M.; Bassaganya-Riera, J.; Tubau-Juni, N.; Kronsteiner, B.; Leber, A.; Philipson, C.W.; Zoccoli-Rodriguez, V.; Hontecillas, R. Cooperation of Gastric Mononuclear Phagocytes with Helicobacter pylori during Colonization. J. Immunol. 2017, 198, 3195–3204. [Google Scholar] [CrossRef]
- Serrano, C.; Diaz, M.I.; Valdivia, A.; Godoy, A.; Peña, A.; Rollan, A.; Kirberg, A.; Hebel, E.; Fierro, J.; Klapp, G.; et al. Relationship between Helicobacter pylori virulence factors and regulatory cytokines as predictors of clinical outcome. Microbes Infect. 2007, 9, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Straubinger, R.K.; Greiter, A.; McDonough, S.P.; Gerold, A.; Scanziani, E.; Soldati, S.; Dailidiene, D.; Dailide, G.; Berg, D.E.; Simpson, K.W. Quantitative evaluation of inflammatory and immune responses in the early stages of chronic Helicobacter pylori infection. Infect. Immun. 2003, 71, 2693–2703. [Google Scholar] [CrossRef][Green Version]
- Peek, R.M.; Fiske, C.; Wilson, K.T. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol. Rev. 2010, 90, 831–858. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yoshida, M.; Nishiumi, S.; Ohnishi, N.; Kobayashi, K.; Yamamoto, K.; Fujita, T.; Hatakeyama, M.; Azuma, T. The CagA protein of Helicobacter pylori suppresses the functions of dendritic cell in mice. Arch. Biochem. Biophys. 2010, 498, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Eskandari-Nasab, E.; Sepanjnia, A.; Moghadampour, M.; Hadadi-Fishani, M.; Rezaeifar, A.; Asadi-Saghandi, A.; Sadeghi-Kalani, B.; Manshadi, M.D.; Pourrajab, F.; Pourmasoumi, H. Circulating levels of interleukin (IL)-12 and IL-13 in Helicobacter pylori-infected patients, and their associations with bacterial CagA and VacA virulence factors. Scand. J. Infect. Dis. 2013, 45, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Indira, M.; Venkateswarulu, T.C.; Abraham Peele, K.; Nazneen Bobby, M.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 2019, 9, 306. [Google Scholar] [CrossRef]
- Peña, J.A.; Versalovic, J. Lactobacillus rhamnosus GG decreases TNF-a production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell. Microbiol. 2003, 5, 277–285. [Google Scholar] [CrossRef]
- Sunanliganon, C.; Thong-Ngam, D.; Tumwasorn, S.; Klaikeaw, N. Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J. Gastroenterol. 2012, 18, 2472–2480. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from bifidobacteriumand lactobacilluswithin the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef]
- Panpetch, W.; Spinler, J.K.; Versalovic, J.; Tumwasorn, S. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori—Stimulated gastric epithelial cells. BMC Microbiol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thiraworawong, T.; Spinler, J.K.; Werawatganon, D.; Klaikeaw, N.; Venable, S.F.; Versalovic, J.; Tumwasorn, S. Anti-inflammatory Properties of Gastric-derived Lactobacillus plantarum XB7 in the Context of Helicobacter pylori Infection. Helicobacter 2014, 19, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Coretti, L.; Nigro, E.; Lembo, F.; Laneri, S.; Lombardo, B.; Daniele, A.; Scudiero, O. β-defensins in the fight against Helicobacter pylori. Molecules 2017, 22, 424. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Laneri, S.; De Biasi, M.G.; Lombardo, B.; Scudiero, O. A novel view of human Helicobacter pylori infections: Interplay between microbiota and beta-defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Castillo, V.; Marcial, G.; Albarracín, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. https://doi.org/10.3390/microorganisms8040479
Garcia-Castillo V, Marcial G, Albarracín L, Tomokiyo M, Clua P, Takahashi H, Kitazawa H, Garcia-Cancino A, Villena J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms. 2020; 8(4):479. https://doi.org/10.3390/microorganisms8040479
Chicago/Turabian StyleGarcia-Castillo, Valeria, Guillermo Marcial, Leonardo Albarracín, Mikado Tomokiyo, Patricia Clua, Hideki Takahashi, Haruki Kitazawa, Apolinaria Garcia-Cancino, and Julio Villena. 2020. "The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection" Microorganisms 8, no. 4: 479. https://doi.org/10.3390/microorganisms8040479
APA StyleGarcia-Castillo, V., Marcial, G., Albarracín, L., Tomokiyo, M., Clua, P., Takahashi, H., Kitazawa, H., Garcia-Cancino, A., & Villena, J. (2020). The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms, 8(4), 479. https://doi.org/10.3390/microorganisms8040479






