High-Throughput Screening of the ReFRAME Library Identifies Potential Drug Repurposing Candidates for Trypanosoma cruzi
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
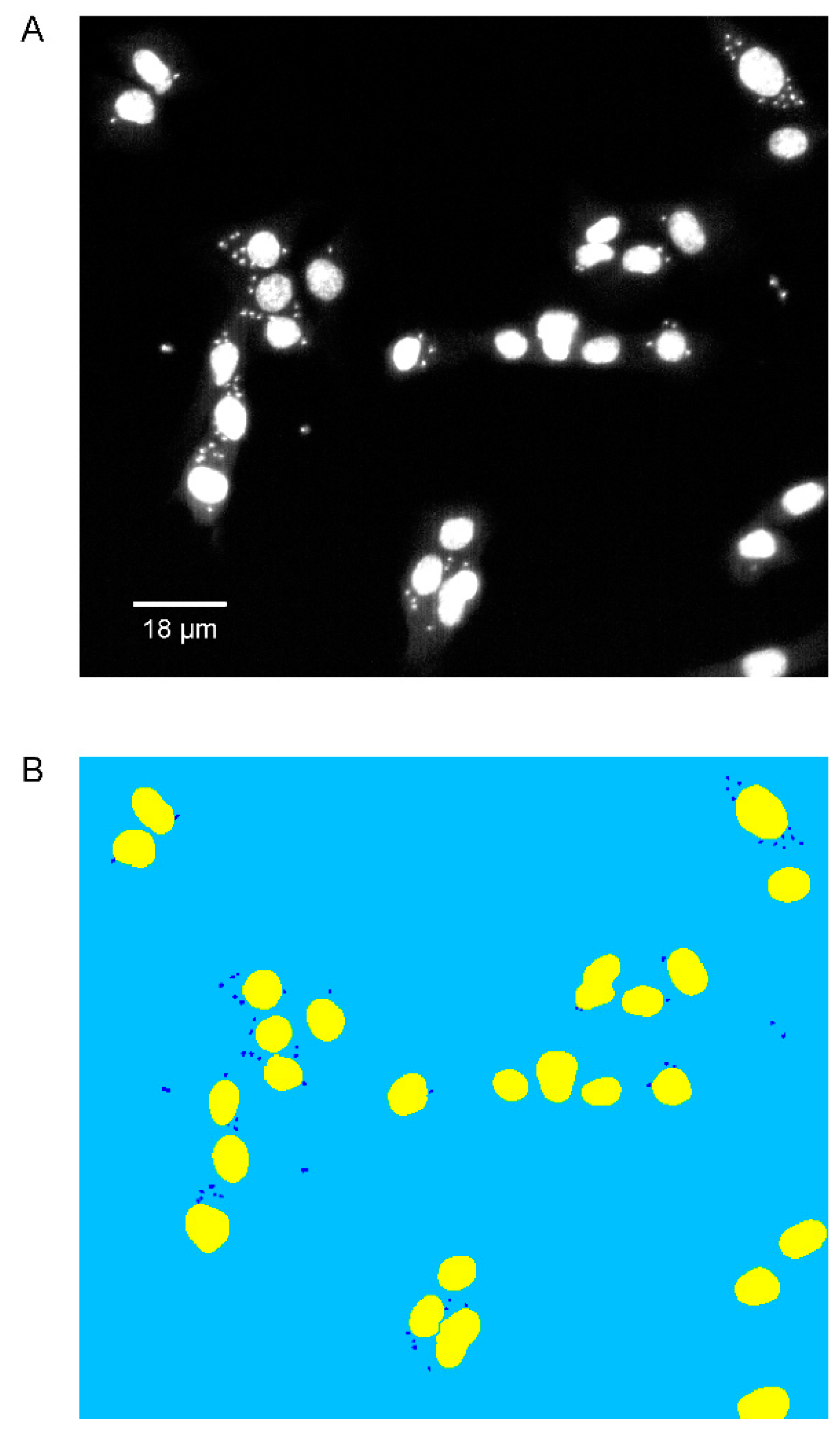
2.2. Phenotypic Imaging Assay
2.3. Software
3. Results
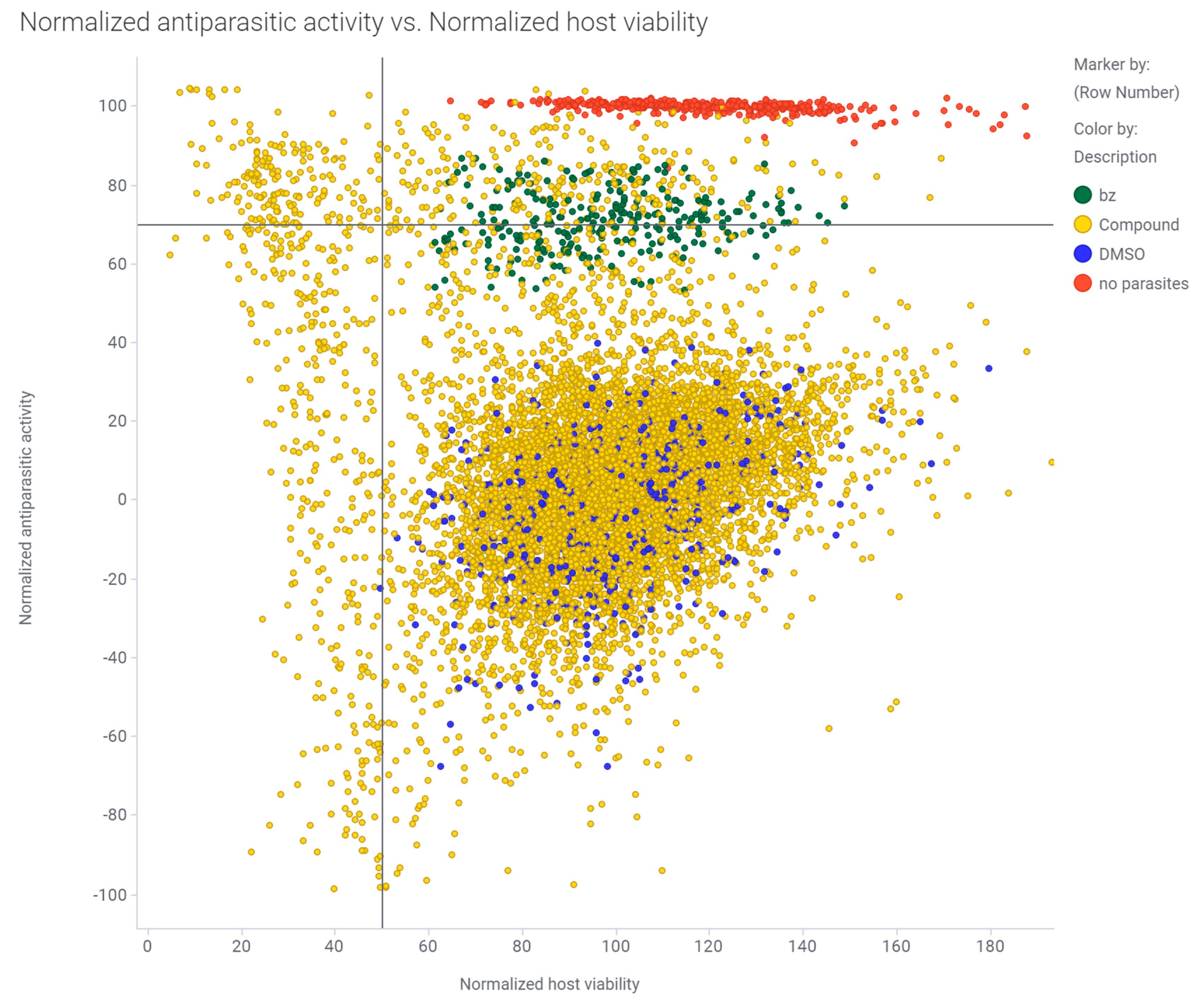
3.1. Primary Screening of the ReFRAME Library against T. cruzi Using a High-Content Imaging Assay
3.2. Counter-Screen of 238 Hits in Dose Response
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef]
- Caldas, I.S.; Santos, E.G.; Novaes, R.D. An evaluation of benznidazole as a Chagas disease therapeutic. Expert Opin. Pharmacother. 2019, 20, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. BENEFIT Investigators Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Marin, J.A.; Rassi, A. Chronic Chagas cardiomyopathy: A review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) trial. Memórias Inst. Oswaldo Cruz 2017, 112, 224–235. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Janes, J.; Young, M.E.; Chen, E.; Rogers, N.H.; Burgstaller-Muehlbacher, S.; Hughes, L.D.; Love, M.S.; Hull, M.V.; Kuhen, K.L.; Woods, A.K.; et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl. Acad. Sci. USA 2018, 115, 10750–10755. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cubitt, B.; Chen, E.; Hull, M.V.; Chatterjee, A.K.; Cai, Y.; Kuhn, J.H.; de la Torre, J.C. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antivir. Res. 2019, 169, 104558. [Google Scholar] [CrossRef]
- Moon, S.; Siqueira-Neto, J.L.; Moraes, C.B.; Yang, G.; Kang, M.; Freitas-Junior, L.H.; Hansen, M.A.E. An image-based algorithm for precise and accurate high throughput assessment of drug activity against the human parasite Trypanosoma cruzi. PLoS ONE 2014, 9, e87188. [Google Scholar] [CrossRef]
- Boudreau, P.D.; Miller, B.W.; McCall, L.-I.; Almaliti, J.; Reher, R.; Hirata, K.; Le, T.; Siqueira-Neto, J.L.; Hook, V.; Gerwick, W.H. Design of Gallinamide A Analogs as Potent Inhibitors of the Cysteine Proteases Human Cathepsin L and Trypanosoma cruzi Cruzain. J. Med. Chem. 2019, 62, 9026–9044. [Google Scholar] [CrossRef]
- Ekins, S.; de Siqueira-Neto, J.L.; McCall, L.-I.; Sarker, M.; Yadav, M.; Ponder, E.L.; Kallel, E.A.; Kellar, D.; Chen, S.; Arkin, M.; et al. Machine Learning Models and Pathway Genome Data Base for Trypanosoma cruzi Drug Discovery. PLoS Negl. Trop. Dis. 2015, 9, e0003878. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.M.; Verlinde, C.L.M.J.; Karimi, M.; Lepesheva, G.I.; Gelb, M.H.; Buckner, F.S. Rational modification of a candidate cancer drug for use against Chagas disease. J. Med. Chem. 2009, 52, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Kraus, J.M.; Tatipaka, H.B.; McGuffin, S.A.; Chennamaneni, N.K.; Karimi, M.; Arif, J.; Verlinde, C.L.M.J.; Buckner, F.S.; Gelb, M.H. Second generation analogues of the cancer drug clinical candidate tipifarnib for anti-Chagas disease drug discovery. J. Med. Chem. 2010, 53, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Rodrígues-Poveda, C.A.; González-Pacanowska, D.; Szajnman, S.H.; Rodríguez, J.B. 2-alkylaminoethyl-1,1-bisphosphonic acids are potent inhibitors of the enzymatic activity of Trypanosoma cruzi squalene synthase. Antimicrob. Agents Chemother. 2012, 56, 4483–4486. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Li, Q.; Ko, T.-P.; Chan, H.-C.; Li, J.; Zheng, Y.; Huang, C.-H.; Ren, F.; Chen, C.-C.; Zhu, Z.; et al. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog. 2014, 10, e1004114. [Google Scholar] [CrossRef] [PubMed]
- Bosc, D.; Mouray, E.; Cojean, S.; Franco, C.H.; Loiseau, P.M.; Freitas-Junior, L.H.; Moraes, C.B.; Grellier, P.; Dubois, J. Highly improved antiparasitic activity after introduction of an N-benzylimidazole moiety on protein farnesyltransferase inhibitors. Eur. J. Med. Chem. 2016, 109, 173–186. [Google Scholar] [CrossRef]
- Douc-Rasy, S.; Kayser, A.; Riou, G. A specific inhibitor of type I DNA-topoisomerase of Trypanosoma cruzi: Dimethyl-hydroxy-ellipticinium. Biochem. Biophys. Res. Commun. 1983, 117, 1–5. [Google Scholar] [CrossRef]
- Kerschmann, R.L.; Wolfson, J.S.; McHugh, G.L.; Dickersin, G.R.; Hooper, D.C.; Swartz, M.N. Novobiocin-induced ultrastructural changes and antagonism of DNA synthesis in Trypanosoma cruzi amastigotes growing in cell-free medium. J. Protozool. 1989, 36, 14–20. [Google Scholar] [CrossRef]
- Gonzales-Perdomo, M.; de Castro, S.L.; Meirelles, M.N.; Goldenberg, S. Trypanosoma cruzi proliferation and differentiation are blocked by topoisomerase II inhibitors. Antimicrob. Agents Chemother. 1990, 34, 1707–1714. [Google Scholar] [CrossRef]
- Zuma, A.A.; Cavalcanti, D.P.; Maia, M.C.P.; de Souza, W.; Motta, M.C.M. Effect of topoisomerase inhibitors and DNA-binding drugs on the cell proliferation and ultrastructure of Trypanosoma cruzi. Int. J. Antimicrob. Agents 2011, 37, 449–456. [Google Scholar] [CrossRef]
- Yu, H.A.; Spira, A.; Horn, L.; Weiss, J.; West, H.; Giaccone, G.; Evans, T.; Kelly, R.J.; Desai, B.; Krivoshik, A.; et al. A Phase I, Dose Escalation Study of Oral ASP8273 in Patients with Non-small Cell Lung Cancers with Epidermal Growth Factor Receptor Mutations. Clin. Cancer Res. 2017, 23, 7467–7473. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasuda, H.; Hamamoto, J.; Nukaga, S.; Masuzawa, K.; Kawada, I.; Naoki, K.; Niimi, T.; Mimasu, S.; Sakagami, H.; et al. Pharmacological and Structural Characterizations of Naquotinib, a Novel Third-Generation EGFR Tyrosine Kinase Inhibitor, in EGFR-Mutated Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2018, 17, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakagami, H.; Kaneko, N.; Konagai, S.; Yamamoto, H.; Matsuya, T.; Yuri, M.; Yamanaka, Y.; Mori, M.; Takeuchi, M.; et al. Mutant-Selective Irreversible EGFR Inhibitor, Naquotinib, Inhibits Tumor Growth in NSCLC Models with EGFR-Activating Mutations, T790M Mutation, and AXL Overexpression. Mol. Cancer Ther. 2019, 18, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Klug, D.M.; Tschiegg, L.; Díaz González, R.; Rojas-Barros, D.I.; Perez-Moreno, G.; Ceballos-Perez, G.; García-Hernández, R.; Martínez-Martínez, M.S.; Manzano, P.; Ruiz-Perez, L.M.; et al. Hit-to-lead optimization of benzoxazepinoindazoles as human African trypanosomiasis therapeutics. J. Med. Chem. 2019, 63, 2527–2546. [Google Scholar] [CrossRef]
- Simões-Silva, M.R.; De Araújo, J.S.; Peres, R.B.; Da Silva, P.B.; Batista, M.M.; De Azevedo, L.D.; Bastos, M.M.; Bahia, M.T.; Boechat, N.; Soeiro, M.N.C. Repurposing strategies for Chagas disease therapy: The effect of imatinib and derivatives against Trypanosoma cruzi. Parasitology 2019, 146, 1006–1012. [Google Scholar] [CrossRef]
- Wyllie, S.; Thomas, M.; Patterson, S.; Crouch, S.; De Rycker, M.; Lowe, R.; Gresham, S.; Urbaniak, M.D.; Otto, T.D.; Stojanovski, L.; et al. Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature 2018, 560, 192–197. [Google Scholar] [CrossRef]
- Diouf, O.; Carato, P.; Lesieur, I.; Rettori, M.; Caignard, D. Synthesis and pharmacological evaluation of novel 4-(4-fluorobenzoyl)piperidine derivatives as mixed 5-HT1A/5-HT2A/D2 receptor ligands. Eur. J. Med. Chem. 1999, 34, 69–73. [Google Scholar] [CrossRef]
- Spector, T.; Harrington, J.A.; Porter, D.J. Herpes and human ribonucleotide reductases. Inhibition by 2-acetylpyridine 5-[(2-chloroanilino)-thiocarbonyl]-thiocarbonohydrazone (348U87). Biochem. Pharmacol. 1991, 42, 91–96. [Google Scholar] [CrossRef]
- Spector, T.; Lobe, D.C.; Ellis, M.N.; Blumenkopf, T.A.; Szczech, G.M. Inactivators of herpes simplex virus ribonucleotide reductase: Hematological profiles and in vivo potentiation of the antiviral activity of acyclovir. Antimicrob. Agents Chemother. 1992, 36, 934–937. [Google Scholar] [CrossRef]
- Safrin, S.; Schacker, T.; Delehanty, J.; Hill, E.; Corey, L. Potential for combined therapy with 348U87, a ribonucleotide reductase inhibitor, and acyclovir as treatment for acyclovir-resistant herpes simplex virus infection. J. Med. Virol. 1993, 1, 146–149. [Google Scholar] [CrossRef]
- Safrin, S.; Schacker, T.; Delehanty, J.; Hill, E.; Corey, L. Topical treatment of infection with acyclovir-resistant mucocutaneous herpes simplex virus with the ribonucleotide reductase inhibitor 348U87 in combination with acyclovir. Antimicrob. Agents Chemother. 1993, 37, 975–979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellis, S.; Sexton, D.W.; Steverding, D. Trypanotoxic activity of thiosemicarbazone iron chelators. Exp. Parasitol. 2015, 150, 7–12. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | EC50 CA-I/72 T. cruzi (nM) | CC50 C2C12 (nM) | Selectivity Index (SI) |
|---|---|---|---|
| NSC-706744 | 0.44 +/− 0.08 | 94 +/− 56 | 214 |
| 348U87 | 0.63 +/− 0.45 | 815 +/− 215 | 1294 |
| ASP-8273 | 2.7 +/− 1.9 | 515 +/− 280 | 191 |
| XR 5944 | 3.5 +/− 6.8 | 46 +/− 21 | 13 |
| Prenyl-IN-1 | 18 +/− 12 | 182 +/− 90 | 10 |
| 3-[4-[4-(2-Methoxyphenyl)piperazine-1-yl]butyl]-6-[2-[4-(4-fluorobenzoyl)piperidine-1-yl]ethyl]benzothiazole-2(3H)-one | 22 +/− 23 | 3190 +/− 1202 | 145 |
| Incadronate Disodium | 480 +/− 385 | >10,000 | >20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatchez, J.A.; Chen, E.; Hull, M.V.; McNamara, C.W.; McKerrow, J.H.; Siqueira-Neto, J.L. High-Throughput Screening of the ReFRAME Library Identifies Potential Drug Repurposing Candidates for Trypanosoma cruzi. Microorganisms 2020, 8, 472. https://doi.org/10.3390/microorganisms8040472
Bernatchez JA, Chen E, Hull MV, McNamara CW, McKerrow JH, Siqueira-Neto JL. High-Throughput Screening of the ReFRAME Library Identifies Potential Drug Repurposing Candidates for Trypanosoma cruzi. Microorganisms. 2020; 8(4):472. https://doi.org/10.3390/microorganisms8040472
Chicago/Turabian StyleBernatchez, Jean A., Emily Chen, Mitchell V. Hull, Case W. McNamara, James H. McKerrow, and Jair L. Siqueira-Neto. 2020. "High-Throughput Screening of the ReFRAME Library Identifies Potential Drug Repurposing Candidates for Trypanosoma cruzi" Microorganisms 8, no. 4: 472. https://doi.org/10.3390/microorganisms8040472
APA StyleBernatchez, J. A., Chen, E., Hull, M. V., McNamara, C. W., McKerrow, J. H., & Siqueira-Neto, J. L. (2020). High-Throughput Screening of the ReFRAME Library Identifies Potential Drug Repurposing Candidates for Trypanosoma cruzi. Microorganisms, 8(4), 472. https://doi.org/10.3390/microorganisms8040472






