Microbiota Alters Urinary Bladder Weight and Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Collection and Histology
2.3. RNA Isolation
2.4. RNA-Sequencing
2.5. RNA-Sequencing Data Analysis
2.6. Quantitative PCR
2.7. Statistical Analysis
3. Results
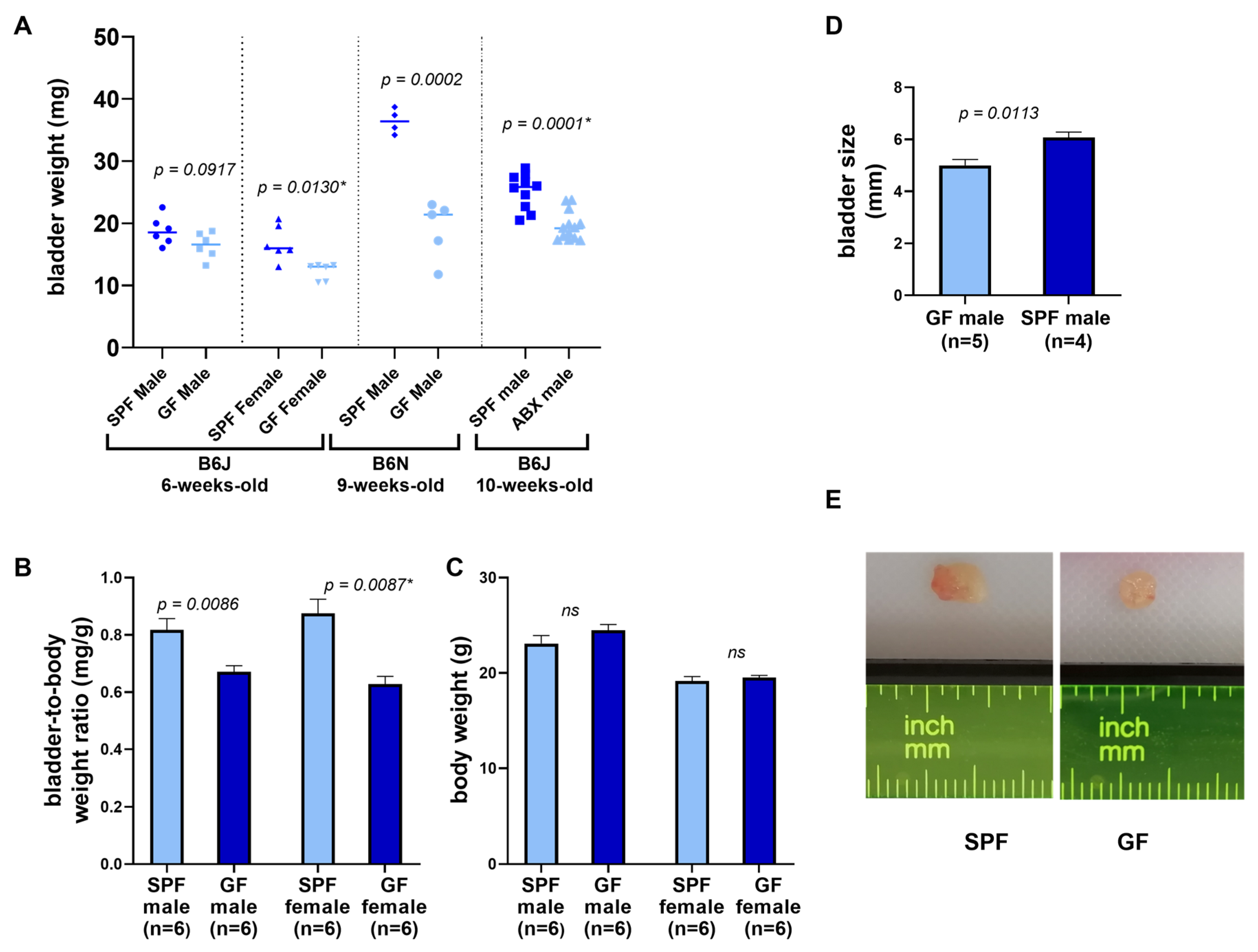
3.1. Organ and Tissue Assessment
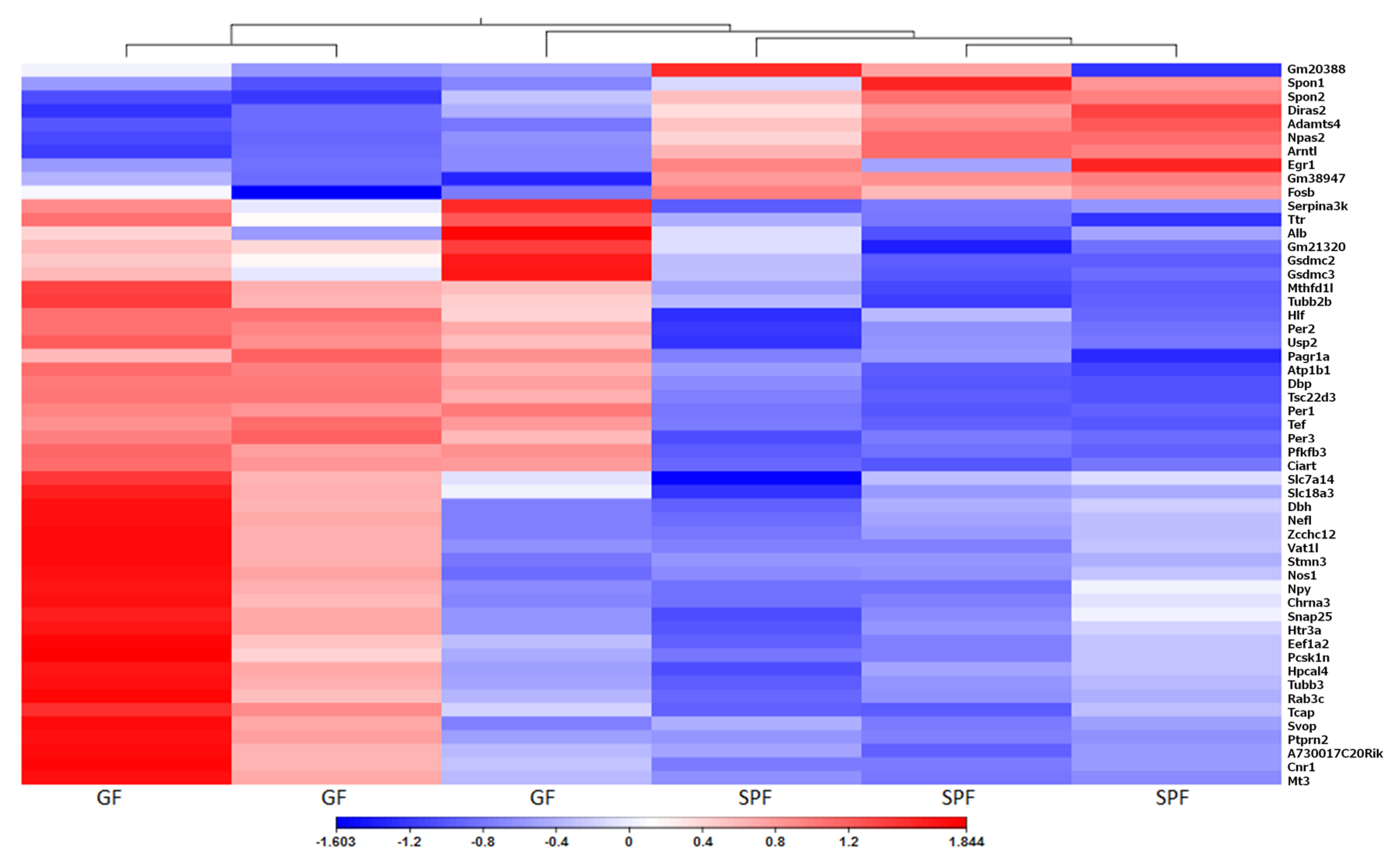
3.2. Gene Expression
3.3. Circadian Rhythm
3.4. Extracellular Matrix
3.5. Ion Homeostasis Regulation and Signalling
3.6. Neuronal Signalling
3.7. Regulation of Detoxification of Xenobiotics
3.8. Immune System
3.9. Other Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciuk, B.; Salabura, A.; Grygorcewicz, B.; Kędzierska, K.; Ciechanowski, K.; Dołęgowska, B. Urobiome: In Sickness and in Health. Microorganisms 2019, 7, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer-A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmakh, M.; Zadjali, F. Use of germ-free animal models in microbiota-related research. J. Microbiol. Biotechnol. 2015, 25, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Bleich, A.; Fox, J.G. The mammalian microbiome and its importance in laboratory animal research. ILAR J. 2015, 56, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Österreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.5.0; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 2009, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [Green Version]
- Hrdý, J.; Novotná, O.; Kocourková, I.; Prokešová, L. Cytokine expression in the colostral cells of healthy and allergic mothers. Folia Microbiol. 2012, 57, 215–219. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-W.; Li, H.; Zhang, J.; Hsu, C.-L.; Lin, E.; Zhang, N.; Guo, J.; Forbush, K.A.; Bevan, M.J. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat. Immunol. 2004, 5, 88–97. [Google Scholar] [CrossRef]
- Carrillo, G.L.; Su, J.; Monavarfeshani, A.; Fox, M.A. F-spondin is essential for maintaining circadian rhythms. Front. Neural Circuits 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Guo, R.; Wan, C.; Wu, L.; Yang, S.; Guo, D. Regulation of intestinal epithelial calcium transport proteins by stanniocalcin-1 in Caco2 cells. Int. J. Mol. Sci. 2016, 17, 1095. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, J.; Li, S.; Zhang, J.; Zheng, J.; Hou, W.; Zhao, H.; Guo, Y.; Liu, X.; Dou, K.; et al. N-myc downstream-regulated gene 2, a novel estrogen-targeted gene, is involved in the regulation of Na + /K + -ATPase. J. Biol. Chem. 2011, 286, 32289–32299. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Zhang, Q.; Wang, H.; Wang, Y.; Nakayama, M.; Ren, D. Extracellular Calcium Controls Background Current and Neuronal Excitability via an UNC79-UNC80-NALCN Cation Channel Complex. Neuron 2010, 68, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Barwick, K.E.S.; Wright, J.; Al-Turki, S.; McEntagart, M.M.; Nair, A.; Chioza, B.; Al-Memar, A.; Modarres, H.; Reilly, M.M.; Dick, K.J.; et al. Defective presynaptic choline transport underlies hereditary motor neuropathy. Am. J. Hum. Genet. 2012, 91, 1103–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Grady, G.L.; Verschuuren, C.; Yuen, M.; Webster, R.; Menezes, M.; Fock, J.M.; Pride, N.; Best, H.A.; Benavides Damm, T.; Turner, C.; et al. Variants in SLC18A3, vesicular acetylcholine transporter, cause congenital myasthenic syndrome. Neurology 2016, 87, 1442–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolleson, C.; Claassen, D. The Function of Tyrosine Hydroxylase in the Normal and Parkinsonian Brain. CNS Neurol. Disord. Drug Targets 2012, 11, 381–386. [Google Scholar] [CrossRef]
- Rush, R.A.; Geffen, L.B. Dopamine βhydroxylase in health and disease. Crit. Rev. Clin. Lab. Sci. 1980, 12, 241–277. [Google Scholar] [CrossRef]
- Mandemakers, W.; Abuhatzira, L.; Xu, H.; Caromile, L.A.; Hébert, S.S.; Snellinx, A.; Morais, V.A.; Matta, S.; Cai, T.; Notkins, A.L.; et al. Co-regulation of intragenic microRNA miR-153 and its host gene Ia-2 β: Identification of miR-153 target genes with functions related to IA-2β in pancreas and brain. Diabetologia 2013, 56, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Hamnett, R.; Crosby, P.; Chesham, J.E.; Hastings, M.H. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via ERK1/2 and DUSP4 signalling. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Bajic, P.; Wolfe, A.J.; Gupta, G.N. The Urinary Microbiome: Implications in Bladder Cancer Pathogenesis and Therapeutics. Urology 2019, 126, 10–15. [Google Scholar] [CrossRef]
- Bučević Popović, V.; Šitum, M.; Chow, C.E.T.; Chan, L.S.; Roje, B.; Terzić, J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Price, K.L.; Woolf, A.S.; Long, D.A. Unraveling the Genetic Landscape of Bladder Development in Mice. J. Urol. 2009, 181, 2366–2374. [Google Scholar] [CrossRef]
- Kloeckener-Gruissem, B.; Neidhardt, J.; Magyar, I.; Plauchu, H.; Zech, J.C.; Morlé, L.; Palmer-Smith, S.M.; MacDonald, M.J.; Nas, V.; Fry, A.E.; et al. Novel VCAN mutations and evidence for unbalanced alternative splicing in the pathogenesis of Wagner syndrome. Eur. J. Hum. Genet. 2013, 21, 352–356. [Google Scholar] [CrossRef]
- Yu, Z.; Liao, J.; Chen, Y.; Zou, C.; Zhang, H.; Cheng, J.; Liu, D.; Li, T.; Zhang, Q.; Li, J.; et al. Single-cell transcriptomic map of the human and mouse bladders. J. Am. Soc. Nephrol. 2019, 30, 2159–2176. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, S.A.J.; Schönfelder, M.; Becker, L.; Nezhad, F.Y.; de Angelis, M.H.; Wackerhage, H. Genes whose gain or loss-of-function increases skeletal muscle mass in mice: A systematic literature review. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.Y.; Xia, C.; Shen, S.; Lee, S.H.; Ratz, P.H.; Fraser, M.O.; Miner, A.; Speich, J.E.; Lysiak, J.J.; Steers, W.D. Urinary bladder organ hypertrophy is partially regulated by Akt1-mediated protein synthesis pathway. Life Sci. 2018, 201, 63–71. [Google Scholar] [CrossRef]
- Parkar, S.; Kalsbeek, A.; Cheeseman, J. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.Y.; Han, D.H.; Kim, M.H.; Ko, I.G.; Kim, S.E.; Park, N.; Choe, H.K.; Kim, K.H.; Kim, K.; Kim, C.J.; et al. Presence of multiple peripheral circadian oscillators in the tissues controlling voiding function in mice. Exp. Mol. Med. 2014, 46. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Sellers, D.J.; Chess-Williams, R. Muscarinic agonists and antagonists: Effects on the urinary bladder. Handb. Exp. Pharmacol. 2012, 208, 375–400. [Google Scholar]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Chamero, P.; Marton, T.F.; Logan, D.W.; Flanagan, K.; Cruz, J.R.; Saghatelian, A.; Cravatt, B.F.; Stowers, L. Identification of protein pheromones that promote aggressive behaviour. Nature 2007, 450, 899–902. [Google Scholar] [CrossRef]
- Weger, B.D.; Dric Gobet, C.; Yeung, J.; Chou, J.; Naef, F. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab. 2019, 29, 362–382.e8. [Google Scholar] [CrossRef] [Green Version]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Zhang, L.; Zheng, J.; Zhao, L.; Huang, J.; Shao, W.; Liao, Q.; Ma, T.; Geng, L.; Yin, C.C.; et al. Spontaneous Production of Immunoglobulin M in Human Epithelial Cancer Cells. PLoS ONE 2012, 7, e51423. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Y.; Qin, C.; Liu, Z.; Yuan, Y.; Yin, H.; Qiu, X.; Xu, T. Involvement of cancer-derived IgG in the proliferation, migration and invasion of bladder cancer cells. Oncol. Lett. 2016, 12, 5113. [Google Scholar]
- Kamei, J.; Ito, H.; Aizawa, N.; Hotta, H.; Kojima, T.; Fujita, Y.; Ito, M.; Homma, Y.; Igawa, Y. Age-related changes in function and gene expression of the male and female mouse bladder. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]


| Primer | DNA Sequence (5’–3’) |
|---|---|
| mSpon2 F | TTGCCAGGTGATGGAAAACG |
| mSpon2 R | CGGGCTGTACAAACCGATTC |
| mAdamts4 F | TGTCATGGCTCCTGTCATGG |
| mAdamts4 R | AGGCAGTGCCCATAACCATT |
| mPer1 F | CTCCTGCTCCAGTGACTTTCC |
| mPer1 R | GGCTTGGCCCGAGATTCAA |
| mArntl F | GTAGATCAGAGGGCGACAGC |
| mArntl R | CCTGTGACATTCTGCGAGGT |
| mTef F | TGTCCAGCACAGAATCGTCC |
| mTef R | GCAGGGTCAGGGTTGAAGTT |
| mPer2 F | CCATCCACAAGAAGATCCTAC |
| mPer2 R | GCTCCACGGGTTGATGAAGC |
| mRev-erba F | ACATGTATCCCCATGGACGC |
| mRev-erba R | CTGGTCGTGCTGAGAAAGGT |
| mNfil3 F | CTTTCAGGACTACCAGACATCCAA |
| mNfil3 R | GATGCAACTTCCGGCTACCA |
| mPer3 F | GAGAGGCACACTAAGCCCAG |
| mPer3 R | GCCGCGAAGGTATCTGTGTT |
| mCol2a1 F | GAGGCGATGTTGGCGAGAAA |
| mCol2a1 R | GAGGTCCGACTTCTCCCTTC |
| mLama1 F | GGTCATGCAGAGGCTGACTT |
| mLama1 R | TGCTGTCAGCTTGTTTCCGA |
| mTnc F | AACGGACTGCCCACATCTCA |
| mTnc R | TCCGGTTCAGCTTCTGTGGTAG |
| mEgfr F | TCATCTGTGCCCAGCAATGT |
| mEgfr R | TTGGCAGACCAGACAGTCAC |
| mCldn1 F | TGGGGCTGATCGCAATCTTT |
| mCldn1 R | CACTAATGTCGCCAGACCTGA |
| mActb F | CACTGTCGAGTCGCGTCC |
| mAtcb R | TCATCCATGGCGAACTGGTG |
| Microbiota Status | Facility | Strain | No. Animals in Group | Age (Weeks) | Sex | bladder Weight (mg) d | p Value e | Bladder-to-Body Weight Ratio d | p Value e |
|---|---|---|---|---|---|---|---|---|---|
| SPF | IGCa | B6J | 6 | 6 | male | 18.82 ± 2.31 | 0.09175 | 1.24 ± 0.14 | 0.0086 |
| GF | IGC | B6J | 6 | 6 | male | 16.45 ± 2.09 | 1.5 ± 0.12 | ||
| SPF | IGC | B6J | 6 | 6 | female | 16.82 ± 2.83 | 0.0130 | 1.16 ± 0.17 | 0.0087 |
| GF | IGC | B6J | 6 | 6 | female | 12.28 ± 1.35 | 1.61 ± 0.18 | ||
| SPF | HZIb | B6N | 4 | 9 | male | 36.43 ± 2.01 | 0.00023 | - | - |
| GF | HZI | B6N | 5 | 9 | male | 19.1 ± 4.65 | |||
| Control (SPF) | USSMc | B6J | 10 | 11 | male | 25.17 ± 2.85 | 0.0001 | ||
| Antibiotics reduction | USSM | B6J | 13 | 11 | male | 19.5 ± 2.33 | - | - |
| GO Biological Process | # (Total Number in Reference Genome) | # (Total Number of Genes in Our Dataset) | Fold Enrichment | p-Value | FDR p-Value |
|---|---|---|---|---|---|
| circadian rhythm | 13 | 4 | 71.35 | 7.28 × 10−7 | 5.92 × 10−5 |
| rhythmic process | 13 | 4 | 71.35 | 7.28 × 10−7 | 4.44 × 10−5 |
| neuromuscular synaptic transmission | 47 | 4 | 19.74 | 6.83 × 10−5 | 2.78 × 10−3 |
| synaptic transmission | 392 | 15 | 8.87 | 2.11 × 10−10 | 2.58 × 10−8 |
| cell-cell signaling | 588 | 18 | 7.1 | 9.30 × 10−11 | 2.27 × 10−8 |
| cell communication | 3269 | 28 | 1.99 | 2.48 × 10−4 | 6.06 × 10−3 |
| cellular process | 8762 | 58 | 1.54 | 3.64 × 10−5 | 1.78 × 10−3 |
| synaptic vesicle exocytosis | 58 | 3 | 11.99 | 2.31 × 10−3 | 3.75 × 10−2 |
| neurological system process | 1393 | 16 | 2.66 | 3.13 × 10−4 | 6.94 × 10−3 |
| system process | 1487 | 17 | 2.65 | 2.09 × 10−4 | 5.67 × 10−3 |
| single-multicellular organism process | 2258 | 23 | 2.36 | 1.08 × 10−4 | 3.77 × 10−3 |
| multicellular organismal process | 2274 | 23 | 2.35 | 1.14 × 10−4 | 3.49 × 10−3 |
| response to endogenous stimulus | 229 | 6 | 6.08 | 5.33 × 10−4 | 1.08 × 10−2 |
| biosynthetic process | 1719 | 17 | 2.29 | 1.50 × 10−3 | 2.61 × 10−2 |
| Gene Symbol | Identifier | Gene Name | Mean Total Counts a | Fold Change | FDR p-Value |
|---|---|---|---|---|---|
| Rrad | ENSMUSG00000031880 | Ras-related associated with diabetes | 788 | −2.1 | 1.2 × 10−3 |
| Stc1 | ENSMUSG00000014813 | stanniocalcin 1 | 395 | 2.0 | 1.1 × 10−3 |
| Syt4 | ENSMUSG00000024261 | synaptotagmin IV | 24 | 7.9 | 5.1 × 10−3 |
| Mmp12 | ENSMUSG00000049723 | matrix metallopeptidase 12 | 35 | 8.0 | 2.5 × 10−3 |
| Alb | ENSMUSG00000029368 | albumin | 16 | 12.4 | 8.4 × 10−4 |
| Hpcal4 | ENSMUSG00000046093 | hippocalcin-like 4 | 10 | 13.2 | 3.6 × 10−4 |
| Fstl5 | ENSMUSG00000034098 | follistatin-like 5 | 5 | 13.8 | 2.5 × 10−3 |
| Cacng5 | ENSMUSG00000040373 | calcium channel, voltage-dependent, gamma subunit 5 | 6 | 16.3 | 6.6 × 10−3 |
| Gene Symbol | Identifier | Gene Name | Mean Total Counts a | Fold Change | FDR p-Value |
|---|---|---|---|---|---|
| Tubb2b | ENSMUSG00000045136 | tubulin, beta 2B class IIB | 179 | 2.9 | 1.2 × 10−6 |
| Mapk10 | ENSMUSG00000046709 | mitogen-activated protein kinase 10 | 22 | 4.7 | 9.0 × 10−3 |
| Elavl2 | ENSMUSG00000008489 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 2 (Hu antigen B) | 38 | 5.0 | 5.3 × 10−3 |
| Ptprn | ENSMUSG00000026204 | protein tyrosine phosphatase, receptor type, N | 45 | 5.5 | 8.7 × 10−3 |
| Ngfr | ENSMUSG00000000120 | nerve growth factor receptor (TNFR superfamily, member 16) | 220 | 5.7 | 1.1 × 10−3 |
| Prph | ENSMUSG00000023484 | peripherin | 156 | 5.7 | 1.3 × 10−3 |
| Gap43 | ENSMUSG00000047261 | growth associated protein 43 | 61 | 5.9 | 1.8 × 10−3 |
| Chgb | ENSMUSG00000027350 | chromogranin B | 10 | 6.0 | 7.2 × 10−3 |
| Cnr1 | ENSMUSG00000044288 | cannabinoid receptor 1 (brain) | 18 | 7.2 | 8.3 × 10−4 |
| Nos1 | ENSMUSG00000029361 | nitric oxide synthase 1, neuronal | 55 | 7.7 | 7.1 × 10−4 |
| Syt4 | ENSMUSG00000024261 | synaptotagmin IV | 24 | 7.9 | 5.1 × 10−3 |
| Ptprn2 | ENSMUSG00000056553 | protein tyrosine phosphatase, receptor type, N polypeptide 2 | 17 | 8.0 | 7.7 × 10−4 |
| Slc5a7 | ENSMUSG00000023945 | solute carrier family 5 (choline transporter), member 7 | 19 | 8.5 | 1.8 × 10−3 |
| Nefl | ENSMUSG00000022055 | neurofilament, light polypeptide | 53 | 8.9 | 2.1 × 10−4 |
| Snap25 | ENSMUSG00000027273 | synaptosomal-associated protein 25 | 57 | 8.9 | 9.2 × 10−4 |
| Vip | ENSMUSG00000019772 | vasoactive intestinal polypeptide | 45 | 9.4 | 1.8 × 10−3 |
| Slc18a3 | ENSMUSG00000100241 | solute carrier family 18 (vesicular monoamine), member 3 | 9 | 9.4 | 7.1 × 10−4 |
| Cartpt | ENSMUSG00000021647 | CART prepropeptide | 19 | 9.7 | 2.3 × 10−3 |
| Vstm2l | ENSMUSG00000037843 | V-set and transmembrane domain containing 2-like | 14 | 10.2 | 8.7 × 10−3 |
| Cplx1 | ENSMUSG00000033615 | complexin 1 | 14 | 10.2 | 5.1 × 10−3 |
| Htr3a | ENSMUSG00000032269 | 5-hydroxytryptamine (serotonin) receptor 3A | 57 | 10.2 | 1.1 × 10−4 |
| Kcnq2 | ENSMUSG00000016346 | potassium voltage-gated channel, subfamily Q, member 2 | 10 | 10.4 | 2.8 × 10−3 |
| Htr3b | ENSMUSG00000008590 | 5-hydroxytryptamine (serotonin) receptor 3B | 8 | 10.7 | 1.5 × 10−3 |
| Vat1l | ENSMUSG00000046844 | vesicle amine transport protein 1 like | 75 | 11.2 | 2.2 × 10−5 |
| Npy | ENSMUSG00000029819 | neuropeptide Y | 27 | 11.8 | 7.4 × 10−4 |
| Zcchc12 | ENSMUSG00000036699 | zinc finger, CCHC domain containing 12 | 13 | 11.9 | 7.3 × 10−4 |
| Jph3 | ENSMUSG00000025318 | junctophilin 3 | 5 | 12.3 | 1.3 × 10−3 |
| Chrna3 | ENSMUSG00000032303 | cholinergic receptor, nicotinic, alpha polypeptide 3 | 19 | 12.6 | 7.5 × 10−4 |
| Chrnb4 | ENSMUSG00000035200 | cholinergic receptor, nicotinic, beta polypeptide 4 | 7 | 13.1 | 3.3 × 10−3 |
| Th | ENSMUSG00000000214 | tyrosine hydroxylase | 10 | 13.4 | 4.4 × 10−3 |
| Dbh | ENSMUSG00000000889 | dopamine beta hydroxylase | 20 | 14.2 | 2.2 × 10−4 |
| Ctnna2 | ENSMUSG00000063063 | catenin (cadherin associated protein), alpha 2 | 4 | 14.3 | 1.1 × 10−3 |
| Tlx2 | ENSMUSG00000068327 | T cell leukemia, homeobox 2 | 4 | 15.2 | 6.2 × 10−3 |
| Gria2 | ENSMUSG00000033981 | glutamate receptor, ionotropic, AMPA2 (alpha 2) | 2 | 25.5 | 1.4 × 10−3 |
| Svop | ENSMUSG00000042078 | SV2 related protein | 2 | 32.8 | 6.9 × 10−4 |
| Gene Symbol | Identifier | Gene Name | Mean Total Counts a | Fold Change | FDR p-Value |
|---|---|---|---|---|---|
| Iglc2 | ENSMUSG00000076937 | immunoglobulin lambda constant 2 | 4 | 14.4 | 6.0 × 10−3 |
| Igkv15-103 | ENSMUSG00000076523 | immunoglobulin kappa chain variable 15-103 | 7 | 20.7 | 2.4 × 10−3 |
| Iglv2 | ENSMUSG00000076940 | immunoglobulin lambda variable 2 | 1 | 60.2 | 1.9 × 10−3 |
| Ighv1-36 | ENSMUSG00000094051 | immunoglobulin heavy variable 1-36 | 0.3 | 159.0 | 6.3 × 10−3 |
| Igkv4-68 | ENSMUSG00000076549 | immunoglobulin kappa variable 4-68 | 0.3 | 159.5 | 3.8 × 10−3 |
| Igkv1-122 | ENSMUSG00000095497 | immunoglobulin kappa chain variable 1-122 | 0.3 | 240.3 | 1.2 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roje, B.; Elek, A.; Palada, V.; Bom, J.; Iljazović, A.; Šimić, A.; Sušak, L.; Vilović, K.; Strowig, T.; Vlahoviček, K.; et al. Microbiota Alters Urinary Bladder Weight and Gene Expression. Microorganisms 2020, 8, 421. https://doi.org/10.3390/microorganisms8030421
Roje B, Elek A, Palada V, Bom J, Iljazović A, Šimić A, Sušak L, Vilović K, Strowig T, Vlahoviček K, et al. Microbiota Alters Urinary Bladder Weight and Gene Expression. Microorganisms. 2020; 8(3):421. https://doi.org/10.3390/microorganisms8030421
Chicago/Turabian StyleRoje, Blanka, Anamaria Elek, Vinko Palada, Joana Bom, Aida Iljazović, Ana Šimić, Lana Sušak, Katarina Vilović, Till Strowig, Kristian Vlahoviček, and et al. 2020. "Microbiota Alters Urinary Bladder Weight and Gene Expression" Microorganisms 8, no. 3: 421. https://doi.org/10.3390/microorganisms8030421
APA StyleRoje, B., Elek, A., Palada, V., Bom, J., Iljazović, A., Šimić, A., Sušak, L., Vilović, K., Strowig, T., Vlahoviček, K., & Terzić, J. (2020). Microbiota Alters Urinary Bladder Weight and Gene Expression. Microorganisms, 8(3), 421. https://doi.org/10.3390/microorganisms8030421





