Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal, Bacterial Cultures and Nematode Inoculum
2.2. Extraction of Fungal Metabolites
2.3. Evaluation of Anti-Bacterial Activity
2.4. SEM Studies
2.5. Evaluation of Nematicidal Activity
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Effect of SMs from Trichoderma spp. Produced on Different Growth Media
3.2. SEM Observations of the Bacterial Cells
3.3. Nematicidal Effect of SMs of Trichoderma spp. against Meloidogyne Incognita
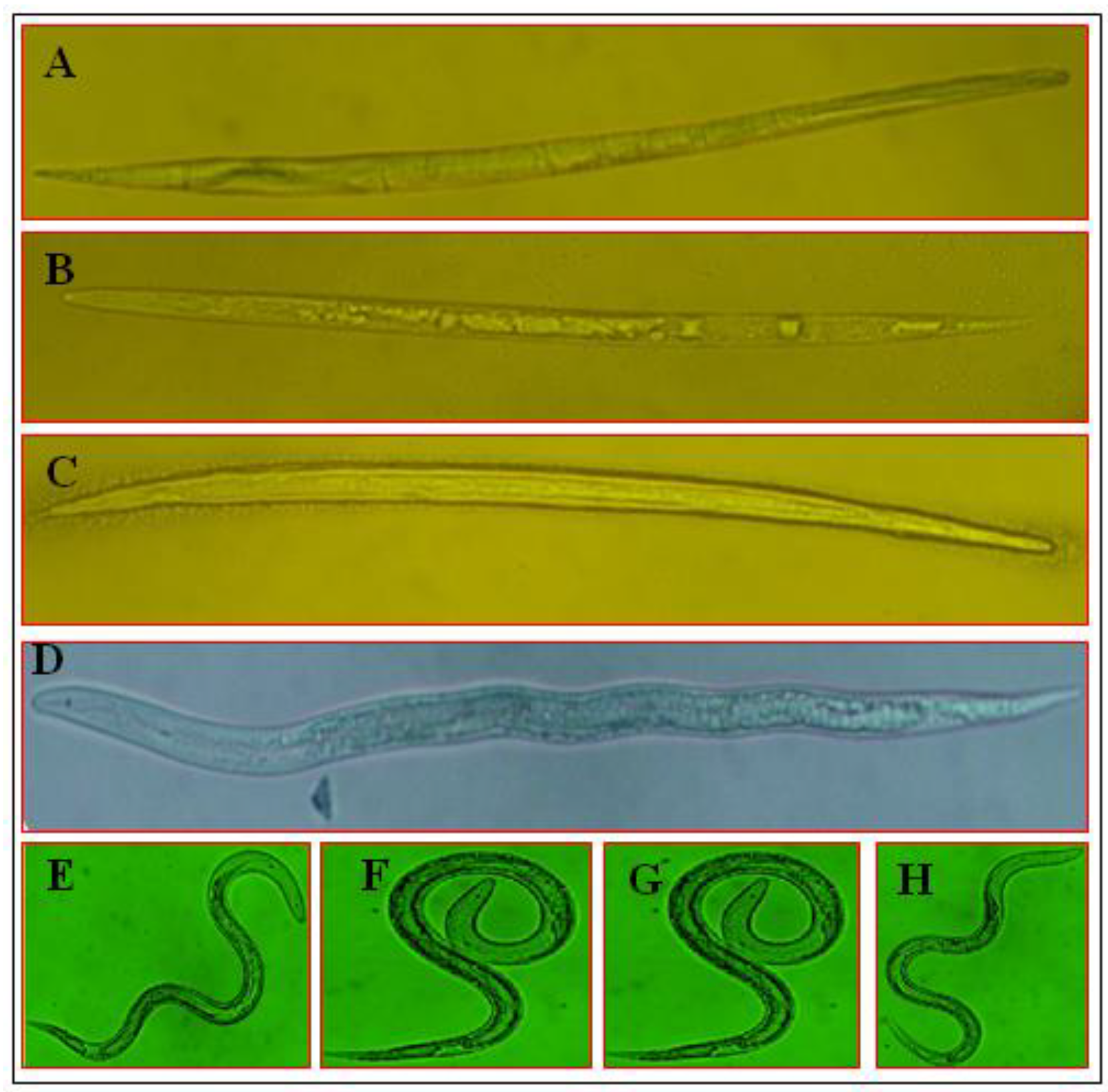
3.4. Morphological Variations of M. incognita J2 under the Influence of SMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sabat, J.; Gupta, N. Development of modified medium for the enhancement in antifungal activity of P. steckii (MF1 Mangrove Fungi) against Verticillium Wilt pathogenic fungi of rose. Braz. Arch. Boil. Technol. 2009, 52, 809–818. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Considine, D.M.; Considine, G.D. Foods and Food Production Encyclopedia; Springer: New York, NY, USA, 1995. [Google Scholar]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P. Top 10 plantpathogenic bacteria in molecular plantpathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Scherf, J.M.; Milling, A.; Allen, C. Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum race 3, biovar 2, in infected geranium, tomato, and potato plants. Appl. Environ. Microbiol. 2010, 76, 7061–7067. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Minsavage, G.V.; Stall, R.E.; Schaad, N.W. Bacterialspot–worldwide distribution, importance and review. Acta Hortic. 2005, 695, 27–34. [Google Scholar] [CrossRef]
- Camesano, T.A. Nanotechnology to Aid Chemical and Biological Defense; Springer: New York, NY, USA, 2015. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Tahna Maafi, Z. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar] [CrossRef]
- McCarter, J.P. Nematology: Terra incognita no more. Nat. Biotechnol. 2008, 26, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Choi, Y.H.; Shin, T.S.; Kim, T.H.; Shin, K.S.; Park, H.W. Biological Control of Meloidogyne incognita by Aspergillus niger F22 Producing Oxalic Acid. PLoS ONE 2016, 11, e156230. [Google Scholar] [CrossRef]
- Goode, M.J.; Sasser, M. Prevention-the key to controlling bacterial spot and bacterial speck of tomato. Plant Dis. 1980, 64, 831–834. [Google Scholar] [CrossRef]
- Mariano, R.L.R.; Silveira, N.S.S.; Michereff, S.J. Bacterial wilt in Brazil: Current status and control methods. In Bacterial Wilt Disease; Prior, P., Allen, C., Elphinstone, J., Eds.; Springer: Berlin, Germany, 1998; pp. 386–393. [Google Scholar] [CrossRef]
- Yuliar, N.Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef]
- Wang, J.F.; Olivier, J.; Thoquet, P.; Mangin, B.; Sauviac, L.; Grimsley, N.H. Resistance of tomato line Hawaii7996 to Ralstonia solanacearum Pss4 in Taiwan is controlled mainly by amajor strain-specific locus. Mol. Plant Microbe Interact. 2000, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.F.; Dittapongpitch, V. Copper-andstreptomycin-resistant strains and host differentiated races of Xanthomonas campestris pv. vesicatoria in North Carolina. Plant Dis. 1991, 75, 733–736. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Trudqill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla-Lopez, R.H.; Kenneth, E.; Bridge, J. Plant diseases caused by nematodes. In Nematology: Advanced and Perspectives. Nematode Management and Utilization; Chen, Z.X., Chen, S.Y., Dickson, D.W., Eds.; CAB International: Wallingford, UK, 2004; Volume II, pp. 637–716. [Google Scholar]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agri. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef] [PubMed]
- Fuller, V.L.; Lilley, C.J. Urwin PE. Nematode resistance. New Phytologist. 2008, 180, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Agricultural Policies in OECD Countries: Monitoring and Evaluation; OECD: Paris, France, 2001. [Google Scholar]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, e173. [Google Scholar]
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef]
- Keller, J.M.; Mcclellan-green, P.D.; Kucklick, J.R.; Keil, D.E.; Peden-adams, M.M. Effects of organochlorine contaminants on loggerhead sea turtle immunity: Comparison of a correlative field study and in vitro exposure experiments. Environ. Health Perspect. 2005, 114, 70–76. [Google Scholar] [CrossRef]
- Osbourn, A. Gene clusters for secondary metabolic pathways: An emerging theme in plant biology. Plant Physiol. 2010, 1, 154. [Google Scholar] [CrossRef]
- Daoubi, M.; Pinedo-rivilla, C.; Rubio, M.B.; Hermosa, R.; Monte, E.; Aleu, J.; Collado, I.G. Hemisynthesis and absolute configuration of novel 6-pentyl-2H-pyran-2-one derivatives from Trichoderma spp. Tetrahedron 2009, 65, 4834–4840. [Google Scholar] [CrossRef]
- Meyer, V. Genetic engineering of filamentous fungi-Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species–opportunistic avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C. Tanshinone II A and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Monte, E. Understanding Trichoderma: Between agricultural biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [PubMed]
- Vizcaino, J.A.; Sanz, L.; Cardoza, R.E.; Monte, E.; Gutierrez, S. Detection of putative peptide synthetase genes in Trichoderma species. Application of this method to the cloning of a gene from T. harzianum CECT 2413. FEMS Microbiol. Let. 2005, 244, 139–148. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2008, 8, 127–139. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Ghisalberti, E.L. Secondary metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium. Vol. 1. Basic Biology, Taxonomy and Genetics; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis: London, UK, 1998; pp. 139–191. [Google Scholar]
- Luckner, M. Secondary Metabolism in Microorganisms, Plants and Animals, 3rd ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- Carvalho Filho, M.R.; Menezes, J.E.; Mello, S.C.M.; Santos, R.P. Avaliação de Isolados de Trichoderma No Controle da Mancha Foliar do Eucalipto in Vitro e Quanto a Esporulação Em Dois Substratos Sólidos. Bol. Pesqui. Desenvolv. Brasília 2008, 225, 21. [Google Scholar]
- Khalili, E.; Sadravi, M.; Naeimi, S.; Khosravi, V. Biological control of rice brown spot with native isolates of three Trichoderma. Braz. J. Microbiol. 2012, 43, 297–305. [Google Scholar] [CrossRef]
- Jeyaseelan, E.C.; Tharmila, S.; Niranjan, K. Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato damping off. Arch. Appl. Sci. Res. 2014, 4, 1623–1627. [Google Scholar]
- Barari, H. Biocontrol of tomato Fusarium wilt by Trichoderma species under in vitro and in vivo conditions. Cercet. Agron. Mold. 2016, 49, 91–98. [Google Scholar] [CrossRef]
- Becker, J.O.; Zavaleta-Mejia, E.; Colbert, S.F.; Schroth, M.N.; Weinhold, A.R.; Hancock, J.G.; Van Gundy, S.D. Effects of rhizobacteria on root-knot nematodes and gall formation. Phytopathology 1988, 78, 1466–1469. [Google Scholar] [CrossRef]
- Vizcaino, A.; Sanz, L.; Basilio, A. Screening of antimicrobial activities in Trichoderma isolates representing three Trichoderma sections. Mycol. Res. 2006, 109, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S.; Barker, K.R. Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Tong, X.; Shen, X.Y.; Hou, C.L. Antimicrobial Activity of Fungal Endophytes from Vaccinium dunalianum Var. Urophyllum. Sains Malaysiana. 2018, 47, 1685–1692. [Google Scholar] [CrossRef]
- Hwa, S.; Ryung, Y.; Sung, S.Y.; Jae, S.S.; Jo, M.K. Detection and Antibacterial Activity of a Bacteriocin Produced by Lactobacillus plantarum. Food Sci. Biotechnol. 2001, 10, 461–467. [Google Scholar]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial Activity of Aqularia crassna Leaf Extract against Staphylococcus epidermis by Disruption of Cell Wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 20. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Verdejo-Lucas, S.; Cortada, L.; Sorribas, F.J.; Ornat, C. Selection of virulent populations of Meloidogyne javanica by repeated cultivation of Mi resistance gene tomato rootstocks under field conditions. Plant Pathol. 2009, 58, 990–998. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]
- Pramila, T.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Hara-Kishore, K.; Misra, S.; Chandra, D.R.; Prakash, K.V.V.R.; Murty, U.S. Antimicrobial efficacy of secondary metabolites from Glomerella cingulata. Braz. J. Microbiol. 2007, 38, 150–155. [Google Scholar] [CrossRef]
- Dos Santos, I.P.; Silva, L.C.N.; Silva, M.V.; Araujo, J.M.; Cavalcanti, M.S.; Lima, V.L.M. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller. (Fabaceae). Front. Microbiol. 2015, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Avis, T.J.; Gacel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Gal-hemed, I.; Atanasova, I.; Komo-zelazowsla, M.; Druzhinina, I.S.; Viterbo, A.; Yarden, O. Marine isolates of Trichoderma spp. as potential halotolerant agents of biologicla control for Arid-zone agriculture. Appl. Environ. Microbiol. 2011, 77, 5100–5109. [Google Scholar] [CrossRef]
- Xiao-yan, S.; Qing-tao, S.; Shu-tao, X.; Xiu-lan, C.; Cai-yan, S.; Yu-zhong, Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS. Microbiol. Let. 2006, 260, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Utkhede, R.; Koch, C. Biological treatments to control bacterial canker of greenhouse tomatoes. Biocontrol 2004, 49, 305–313. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Detection of viridiofungin A and another antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- Tanaka, J.C.A.; da Silva, C.C.; de Oliveira, A.J.B.; Nakamura, C.V.; Dias, B.P. Antibacterial activity of indole alkaloids from Aspidosperma ramiflorum. Braz. J. Med. Biol. Res. 2006, 39, 387–391. [Google Scholar] [CrossRef]
- Al-Obaidi, O. Studies on antibacterial and anticancer activity of Nerium oleander extracts. Eur. Chem. Bull. 2014, 3, 259–262. [Google Scholar] [CrossRef]
- Tsuchiya, H. Memberane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules 2015, 20, 18923–18966. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophy. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Sakakibara, Y.; Sakata, A.; Kurashige, R.; Murakami, D.; Kageshima, H.; Saito, A.; Miyazaki, Y. Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus. PLoS ONE 2019, 14, e0217504. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: London, UK, 1997. [Google Scholar]
- Rizk, M.; Abdel-rahman, T.; Metwally, H. Factors Affecting Growth and Antifungal Activity of Some Streptomyces Species against Candida albicans. J. Food Agric. Environ. 2007, 5, 446–449. [Google Scholar]
- El-Tayeb, O.M.; Hussein, M.M.N.; Salama, A.A.; El-Sedawy, H.F. Optimization of industrial production of rifamycin B by Amycolatopsis mediterranei. II. The role of gene amplification and physiological factors in productivity in shake flasks. Afr. J. Biotechnol. 2004, 3, 273–280. [Google Scholar] [CrossRef][Green Version]
- Slininger, P.J.; Shea-wilbur, M.A. Liquid culture pH, temperature carbon and nitrogen source regulate phenazine productivity of the take all biocontrol agent Pseudomonas fluorescence. Appl. Microbial. Biotechnol. 1995, 37, 388–392. [Google Scholar] [CrossRef]
- Ramos, H.P.; Said, S. Modulation of biological activities produced by an endophytic fungus under different culture conditions. Adv. Biosci. Biotech. 2011, 2, 443–449. [Google Scholar] [CrossRef]
- Vijaykumar, S.J.; Sasidharannair, N.K.; Nambision, B.; Mohandas, C. Optimization of Media and Temperature for Enhanced Antimicrobial Production by Bacteria Associated with Rhabitis sp. Iran. J. Microbial. 2013, 5, 136–141. [Google Scholar]
- Massoud, S.; Meyer, S.L.; Roberts, D.; Chitwood, D. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2000, 2, 871–879. [Google Scholar] [CrossRef]
- Roberts, D.P.; Lumsden, R.D. Effect of extracellular metabolites from Gliocladium virens on germination of sporangia and mycelial growth of Pythium ultimum. Phytopathology 1990, 80, 461–465. [Google Scholar] [CrossRef]
- Meyer, S.L.F.; Roberts, D.P.; Chitwood, D.J.; Carta, L.K.; Lumsden, R.D.; Mao, W. Application of Burkholderia cepacia and Trichoderma virens, alone and in Combinations, against Meloidogyne incognita on Bell Pepper. Nematropica 2001, 31, 75–86. [Google Scholar]
- Djian, C.; Pijarouvski, L.; Ponchet, M.; Arpin, N. Acetic Acid, a Selective Nematicidal Metabolite from Culture Filtrate of Paecilomyces lilacinus (Thom) Samsan and Trichoderma longibrachiatum Rifai. Nematologica 1991, 37, 101–110. [Google Scholar]
- Anitha, R.; Murugesan, K. Production of gliotoxin on natural substrates by Trichoderma virens. J. Basic Microbiol. 2005, 45, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Zhang, K.Q.; Xu, J.P.; Dong, J.Y.; Liu, Y.J. Nematicidal substances from fungi. Rec. Pat. Biotechnol. 2007, 1, 212–233. [Google Scholar] [CrossRef]
- Schuster, R.P.; Sikora, R.A.; Amin, N. Potential of endophytic fungi for the biological control of plant parasitic nematodes. Commun. Appl. Biol. Sci. 1995, 60, 1047–1052. [Google Scholar]
- Hallmann, J.; Quadt-Hallmann, A.; Miller, W.G.; Sikora, R.A.; Lindow, S.E. Endophyte colonization of plants by biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology 2001, 91, 415–422. [Google Scholar] [CrossRef]
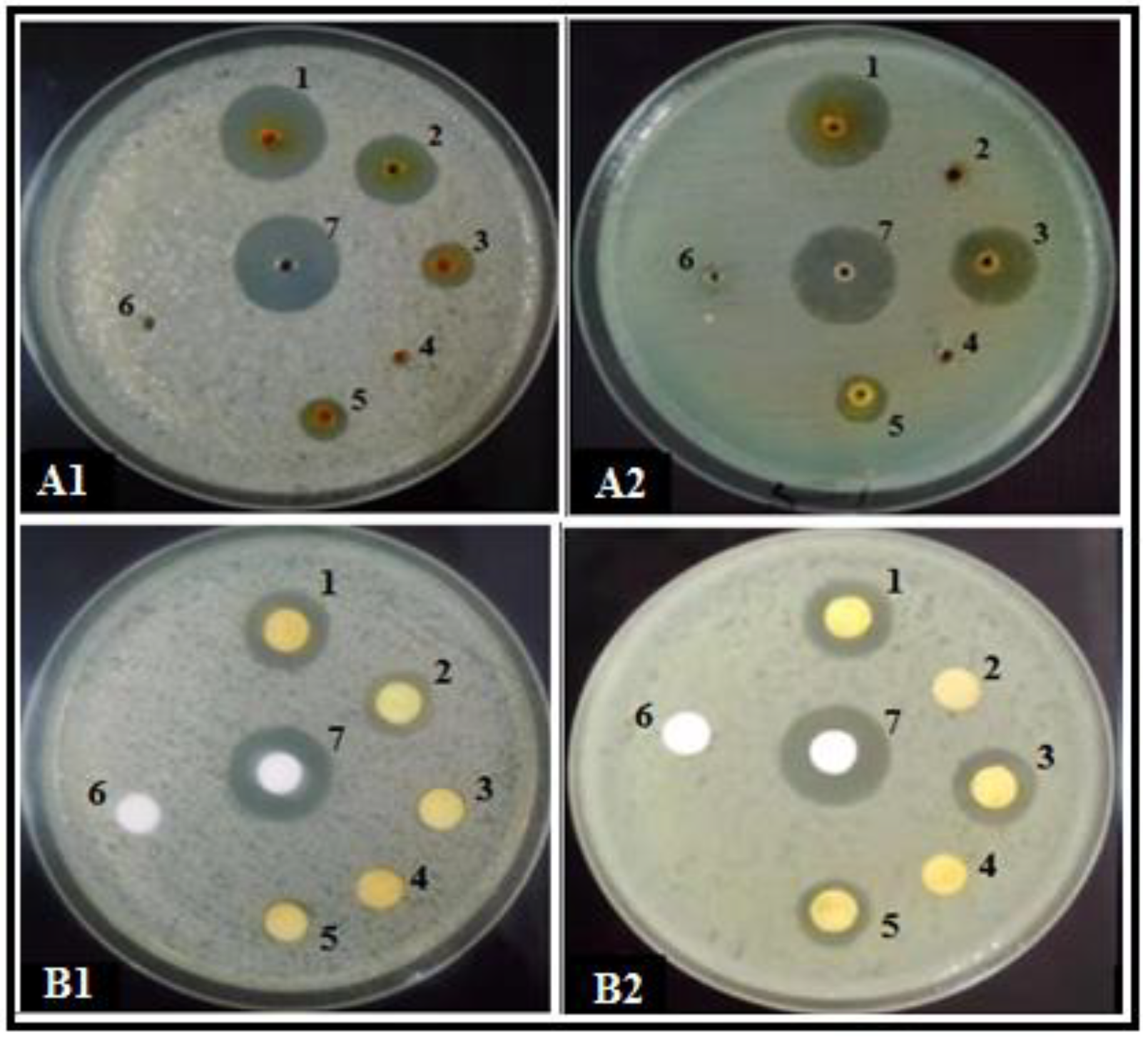
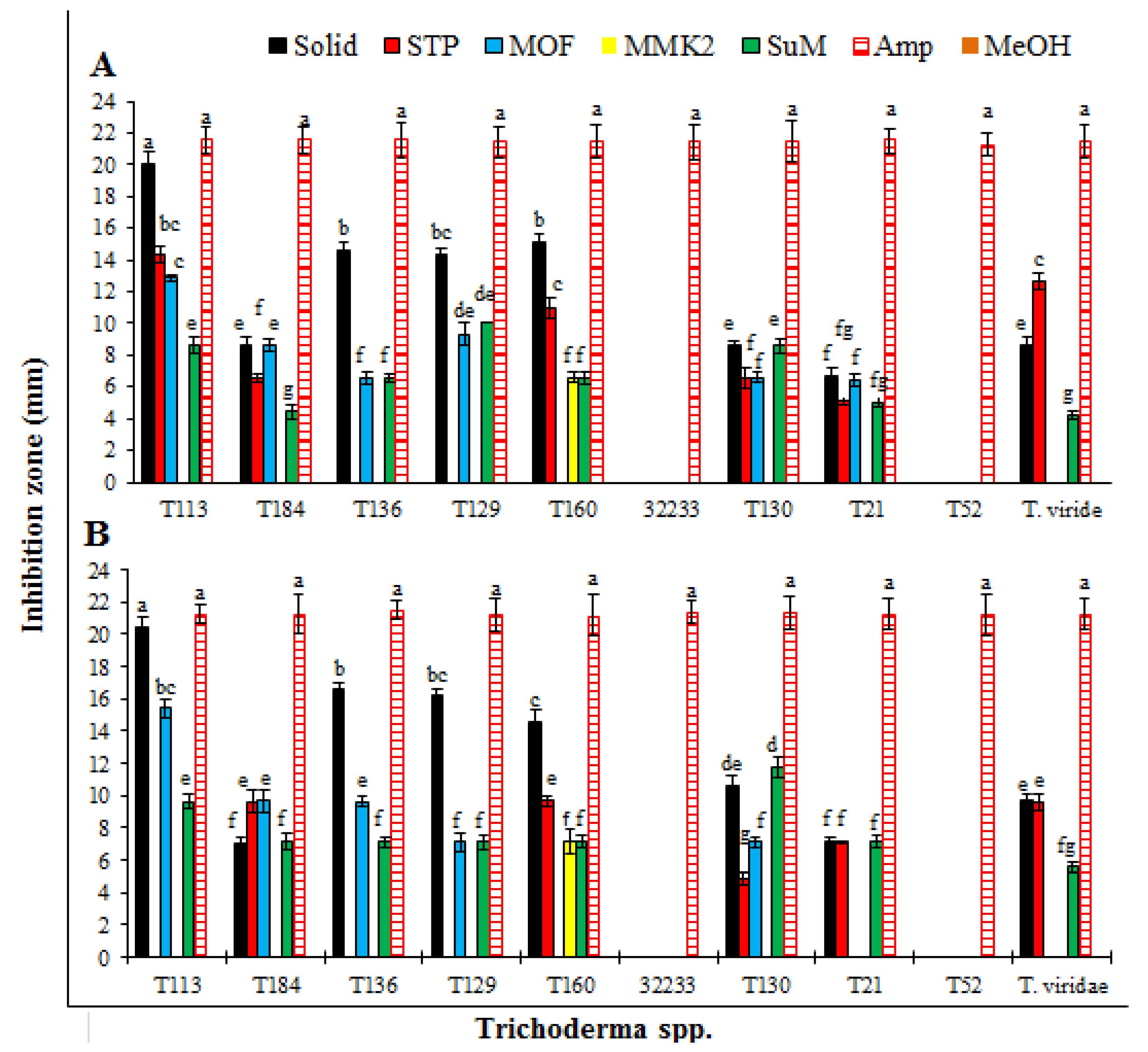

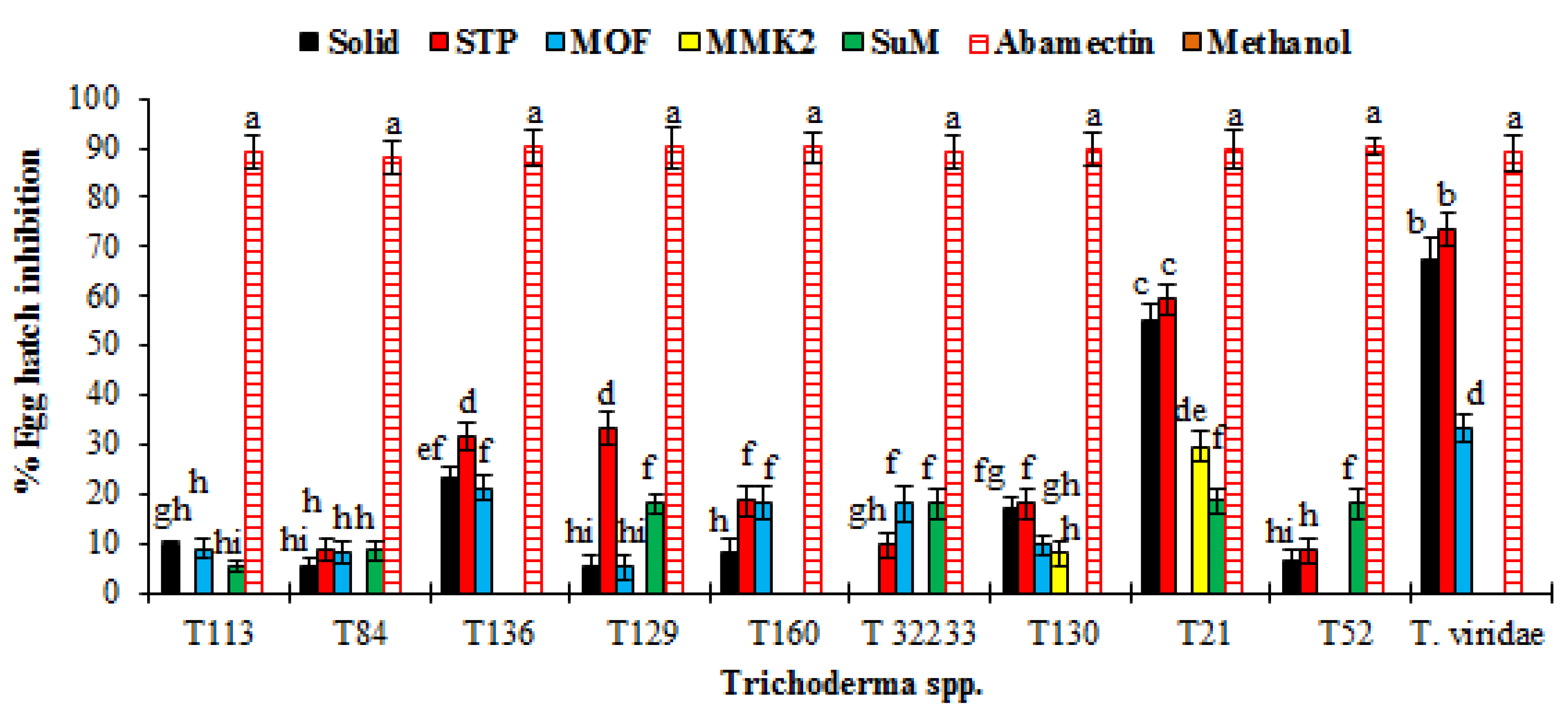
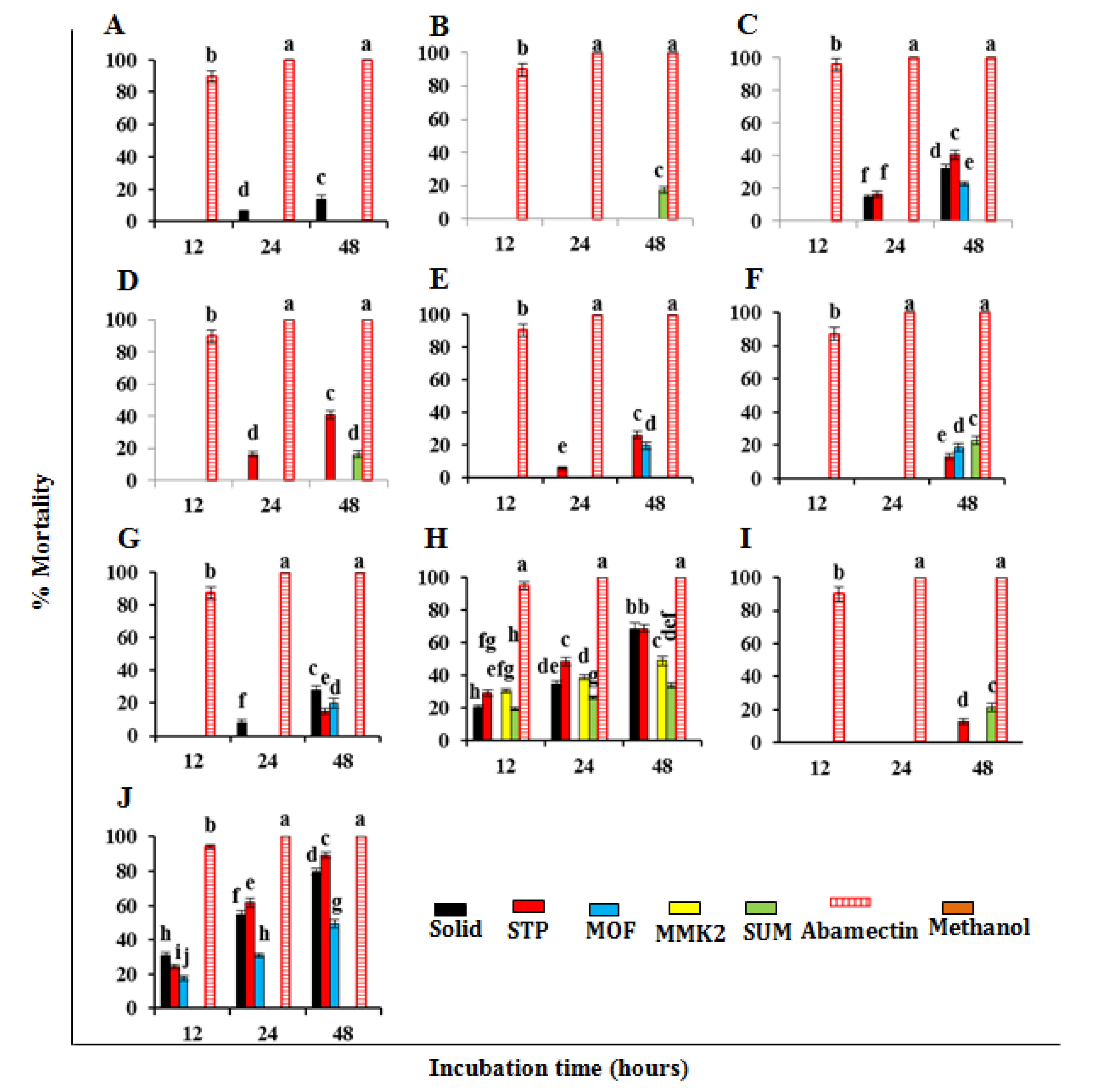
| Trichoderma spp. | Growth Media | Control | |||||
|---|---|---|---|---|---|---|---|
| Solid | STP | MOF | MMK2 | SuM | Ampicillin | Methanol | |
| T. pseudoharzianum (T113) | 21.8 ± 0.4a | 14.3 ± 0.4c | 12.2 ± 0.8d | 0.0s | 9.0 ± 0.9hij | 22.3 ± 0.5a | 0.0s |
| T. koningiopsis (T84) | 9.7 ± 0.5gh | 7.1 ± 0.3m | 8.4 ± 0.2ijk | 0.0s | 4.2 ± 0.1qr | 22.0 ± 0.5a | 0.0s |
| T. asperelloides (T136) | 15.1 ± 0.2bc | 0.0s | 7.0 ± 0.4mn | 0.0s | 7.8 ± 0.1klm | 22.2 ± 0.3a | 0.0s |
| T. pseudoharzianum (T129) | 14.2 ± 0.5c | 0.0s | 10.5 ± 0.1fg | 0.0s | 9.0 ± 0.1hij | 22.4 ± 0.2a | 0.0s |
| T. pseudoharzianum (T160) | 15.3 ± 0.7b | 11.2 ± 0.3ef | 0.0s | 6.1 ± 0.1no | 6.0 ± 0.1o | 21.6 ± 0.2a | 0.0s |
| T. afroharzianum (32233) | 0.0s | 0.0s | 0.0s | 0.0s | 0.0s | 21.8 ± 0.3a | 0.0s |
| T. acitrinoviride (T130) | 8.2 ± 0.1jkl | 5.2 ± 0.2op | 7.0 ± 0.0mn | 0.0s | 7.3 ± 0.5lm | 22.3 ± 0.2a | 0.0s |
| T. hamatum (T21) | 5.2 ± 0.2op | 4.5 ± 0.1pq | 6.1 ± 0.2no | 0.0s | 3.3 ± 0.1r | 22.5 ± 0.1a | 0.0s |
| T. afroharzianum (T52) | 0.0s | 0.0s | 0.0s | 0.0s | 0.0s | 21.7 ± 0.2a | 0.0s |
| T. viridae | 9.2 ± 0.1hi | 12.1 ± 0.1de | 0.0s | 0.0s | 4.4 ± 0.1pq | 22.2 ± 0.1a | 0.0s |
| Trichoderma spp. | Growth Media | Control | |||||
|---|---|---|---|---|---|---|---|
| Solid | STP | MOF | MMK2 | SuM | Ampicillin | Methanol | |
| T. pseudoharzianum (T113) | 21.3 ± 0.1b | 0.0n | 16.8 ± 0.2c | 0.0n | 10.3 ± 0.5e | 21.9 ± 0.5ab | 0.0n |
| T. koningiopsis (T84) | 7.8 ± 0.3gh | 7.1 ± 0.1ij | 9.5 ± 0.1f | 0.0n | 4.5 ± 0.2m | 22.2 ± 0.1a | 0.0n |
| T. asperelloides (T136) | 14.8 ± 0.3d | 0.0n | 7.1 ± 0.1ij | 0.0n | 6.2 ± 0.4k | 21.7 ± 0.4ab | 0.0n |
| T. pseudoharzianum (T129) | 15.2 ± 0.2d | 0.0n | 9.2 ± 0.1f | 0.0n | 6.4 ± 0.1k | 21.7 ± 0.2ab | 0.0n |
| T. pseudoharzianum (T160) | 15.5 ± 0.2d | 9.7 ± 0.1ef | 0.0n | 7.4 ± 0.2hi | 5.2 ± 0.2lm | 22.3 ± 0.1a | 0.0n |
| T. afroharzianum (32233) | 0.0n | 0.0n | 0.0n | 0.0n | 0.0n | 21.8 ± 0.4ab | 0.0n |
| T. acitrinoviride (T130) | 9.5 ± 0.2f | 4.9 ± 0.1lm | 6.5 ± 0.2jk | 0.0n | 9.3 ± 0.1f | 22.0 ± 0.4a | 0.0n |
| T. hamatum (T21) | 5.2 ± 0.1lm | 4.8 ± 0.1m | 5.5 ± 0.1l | 0.0n | 4.9 ± 0.3lm | 22.2± 0.1a | 0.0n |
| T. afroharzianum (T52) | 0.0n | 0.0n | 0.0n | 0.0n | 0.0n | 21.8 ± 0.4ab | 0.0n |
| T. viridae | 8.4 ± 0.2g | 9.5 ± 0.1f | 0.0n | 0.0n | 4.9 ± 0.3lm | 22.1 ± 0.2a | 0.0n |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms 2020, 8, 401. https://doi.org/10.3390/microorganisms8030401
Khan RAA, Najeeb S, Mao Z, Ling J, Yang Y, Li Y, Xie B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms. 2020; 8(3):401. https://doi.org/10.3390/microorganisms8030401
Chicago/Turabian StyleKhan, Raja Asad Ali, Saba Najeeb, Zhenchuan Mao, Jian Ling, Yuhong Yang, Yan Li, and Bingyan Xie. 2020. "Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode" Microorganisms 8, no. 3: 401. https://doi.org/10.3390/microorganisms8030401
APA StyleKhan, R. A. A., Najeeb, S., Mao, Z., Ling, J., Yang, Y., Li, Y., & Xie, B. (2020). Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms, 8(3), 401. https://doi.org/10.3390/microorganisms8030401






