The Effect of Posaconazole, Itraconazole and Voriconazole in the Culture Medium on Aspergillus fumigatus Triazole Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. A. fumigatus Isolates
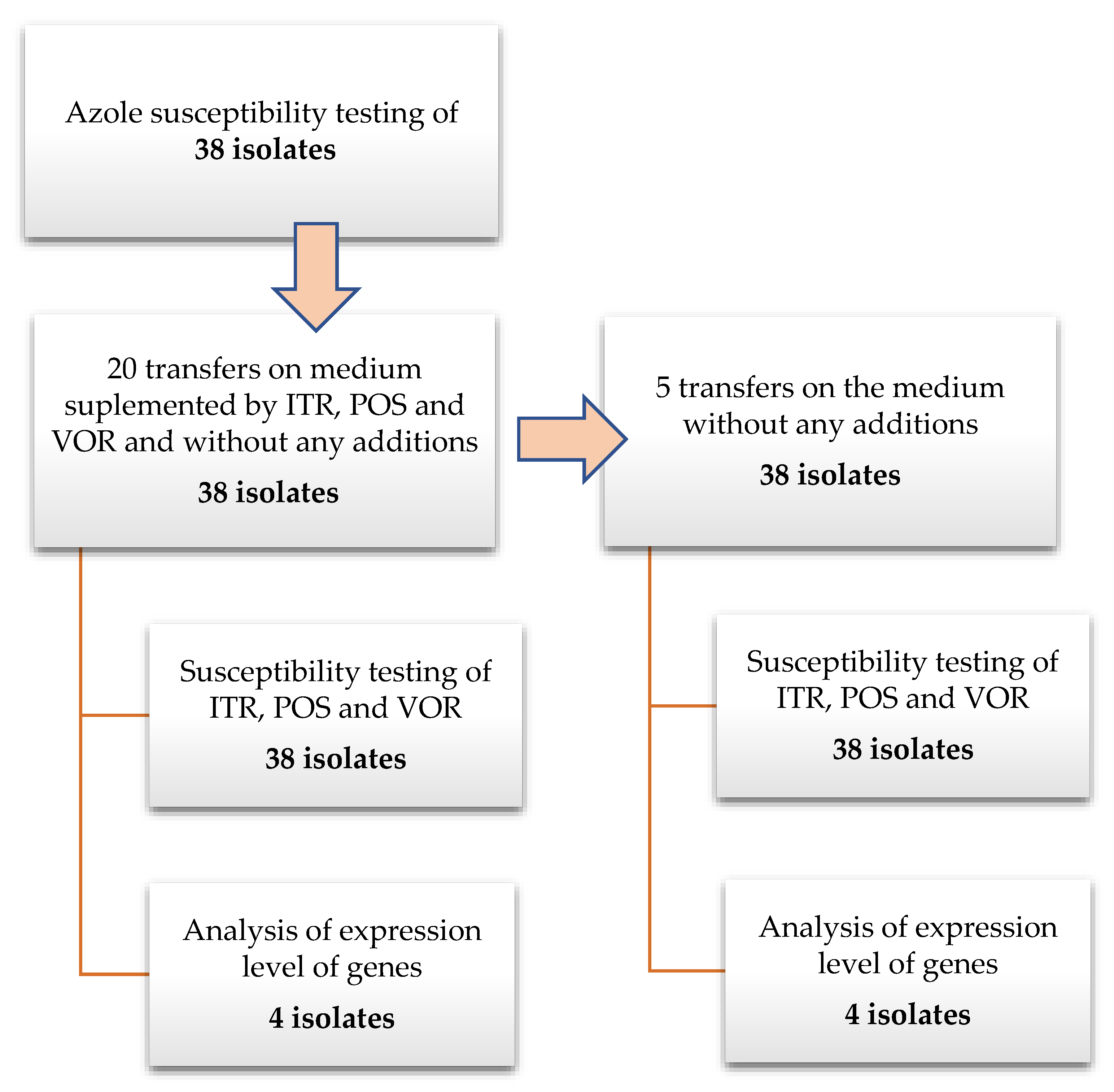
2.2. Azole Exposure
2.3. Susceptibility Testing
2.4. Gene Expression Analysis
3. Results
3.1. Exposure of A. fumigatus Cultures to Azoles
3.2. Azoles Susceptibility Testing
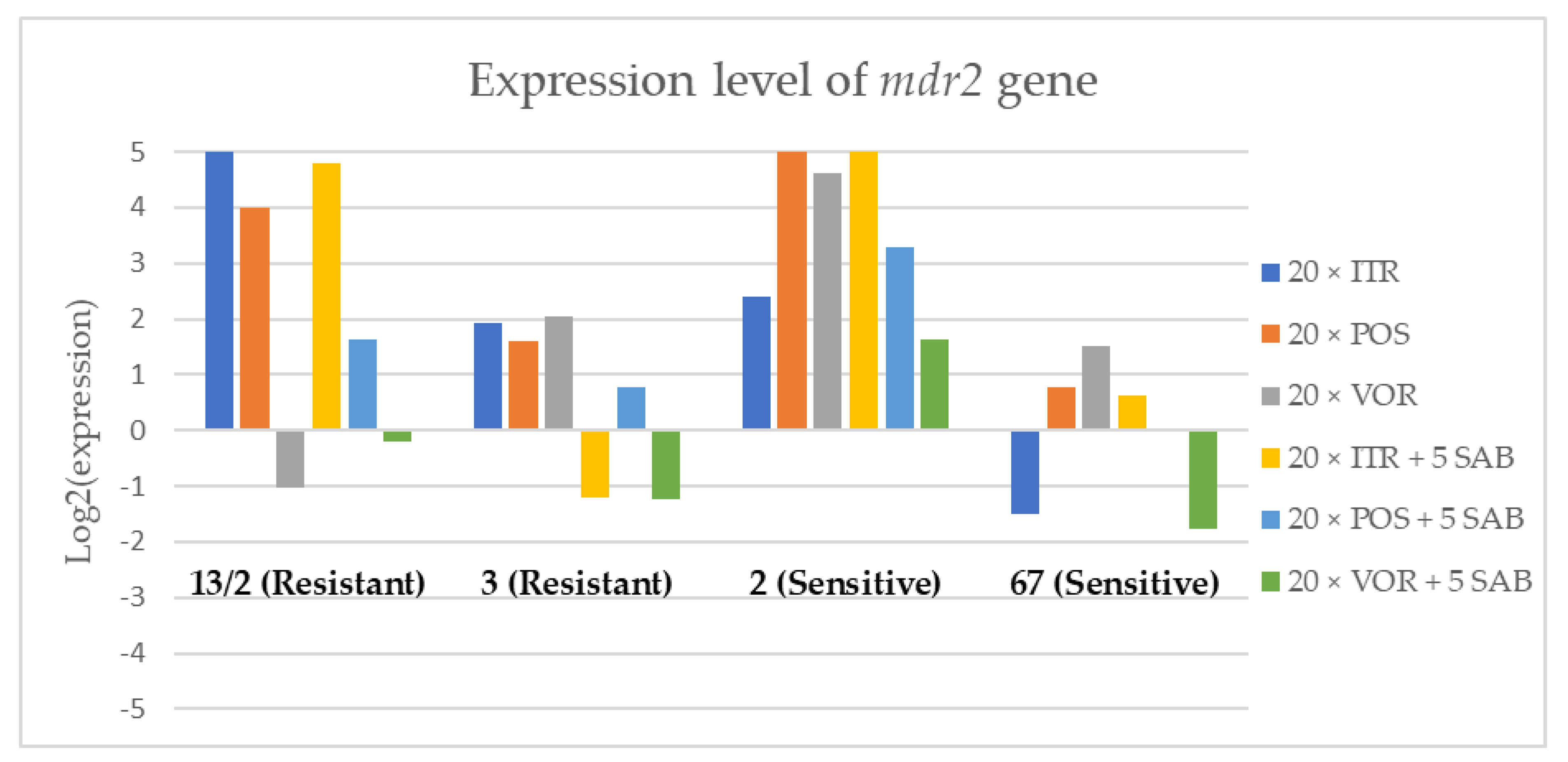
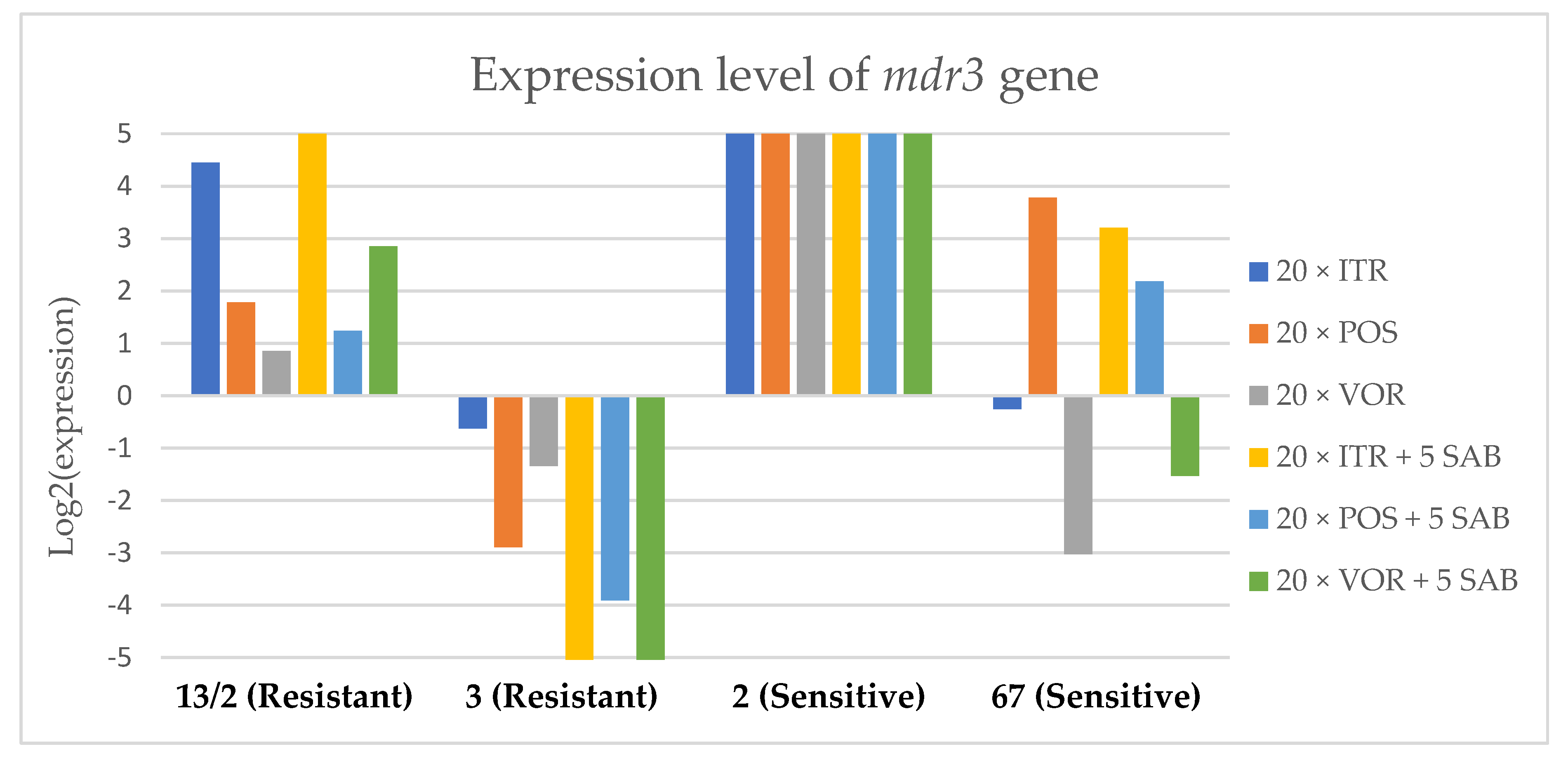
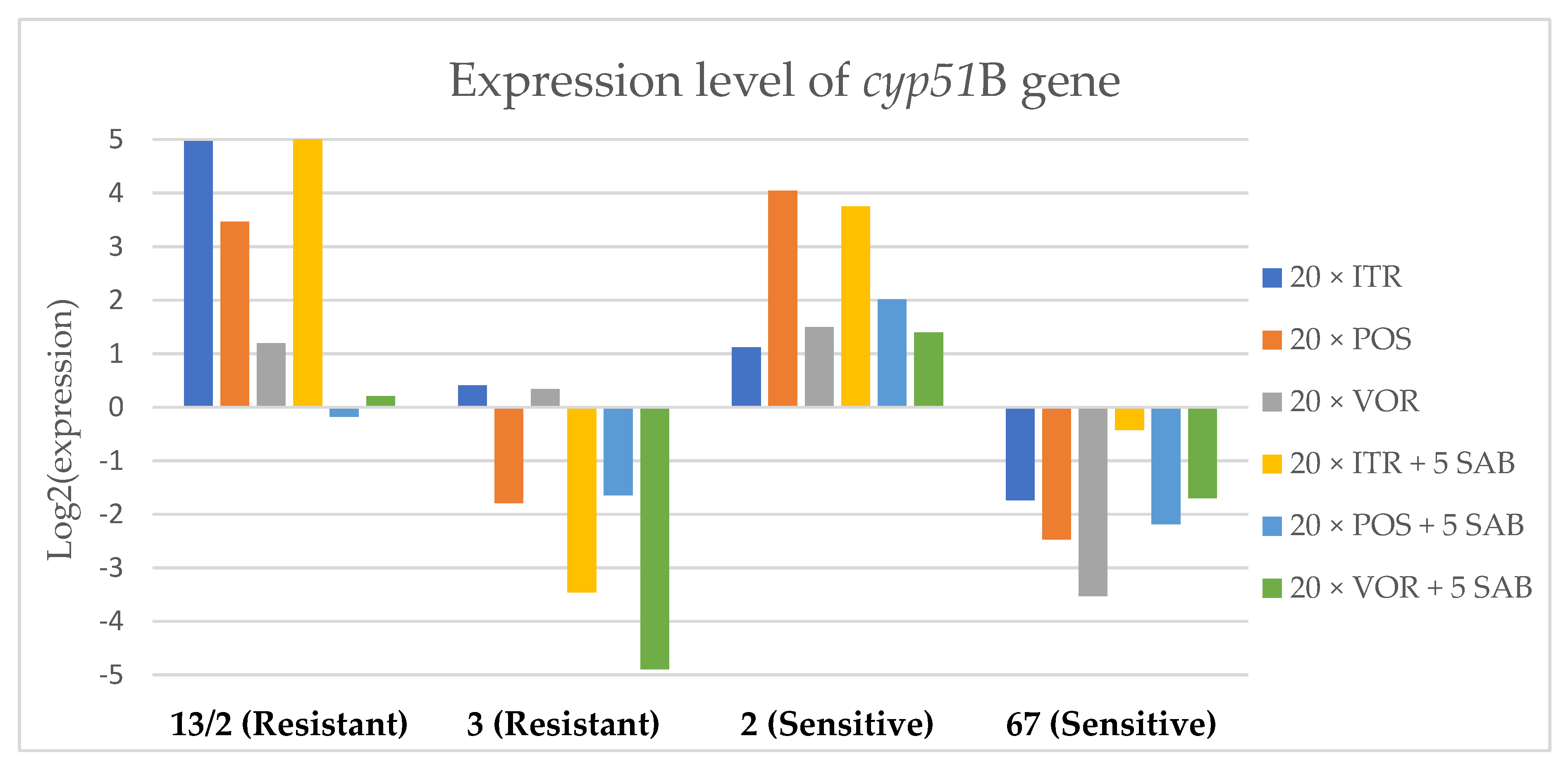
3.3. Changes in Gene Expression in Response to Azole Exposure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Larone, D.H. Medically Important Fungi, 5th ed.; ASM Press: Washington, DC, USA, 2011; ISBN 978-1-55581-660-5. [Google Scholar]
- Ramakrishnan, J.; Rathore, S.S.; Raman, T. Review on fungal enzyme inhibitors—Potential drug targets to manage human fungal infections. RSC Adv. 2016, 6, 42387–42401. [Google Scholar] [CrossRef]
- Gajdács, M.; Dóczi, I.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: Results from a 10-year retrospective survey. Cent. Eur. J. Urol. 2019, 72, 209–214. [Google Scholar]
- Denning, D.W.; Perlin, D.S. Azole resistance in Aspergillus: A growing public health menace. Future Microbiol. 2011, 6, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.-A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G.; et al. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care Lond. Engl. 2015, 19, 7. [Google Scholar] [CrossRef]
- van der Linden, J.W.M.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Huis In’t Veld, R.A.G.; Rijs, A.J.M.M.; Kema, G.H.J.; Melchers, W.J.G.; Verweij, P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 2009, 75, 4053–4057. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Jørgensen, L.N.; Arendrup, M.C. Azole-Resistant Invasive Aspergillosis: Relationship to Agriculture. Curr. Fungal Infect. Rep. 2012, 6, 178–191. [Google Scholar] [CrossRef]
- Azevedo, M.-M.; Faria-Ramos, I.; Cruz, L.C.; Pina-Vaz, C.; Rodrigues, A.G. Genesis of Azole Antifungal Resistance from Agriculture to Clinical Settings. J. Agric. Food Chem. 2015, 63, 7463–7468. [Google Scholar] [CrossRef] [PubMed]
- García-Valcárcel, A.I.; Tadeo, J.L. Determination of azoles in sewage sludge from Spanish wastewater treatment plants by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2011, 34, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Müller, M.D.; Poiger, T. Azole Fungicides: Occurrence and Fate in Wastewater and Surface Waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. Critical Annotations to the Use of Azole Antifungals for Plant Protection. Antimicrob. Agents Chemother. 2001, 45, 2987–2990. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Snelders, E.; Kema, G.H.J.; Mellado, E.; Melchers, W.J.G. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Snelders, E.; van der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.-A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef]
- Slaven, J.W.; Anderson, M.J.; Sanglard, D.; Dixon, G.K.; Bille, J.; Roberts, I.S.; Denning, D.W. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet. Biol. 2002, 36, 199–206. [Google Scholar] [CrossRef]
- Spengler, G.; Kincses, A.; Gajdács, M.; Amaral, L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules 2017, 22, 468. [Google Scholar] [CrossRef]
- Brillowska-Dąbrowska, A.; Mroczyńska, M.; Nawrot, U.; Włodarczyk, K.; Kurzyk, E. Examination of cyp51A and cyp51B expression level of the first Polish azole resistant clinical Aspergillus fumigatus isolate. Acta Biochim. Pol. 2015, 62, 837–839. [Google Scholar] [CrossRef]
- Nawrot, U.; Kurzyk, E.; Arendrup, M.C.; Mroczyńska, M.; Włodarczyk, K.; Sulik-Tyszka, B.; Wróblewska, M.; Ussowicz, M.; Zdziarski, P.; Niewińska, K.; et al. Detection of Polish clinical Aspergillus fumigatus isolates resistant to triazoles. Med. Mycol. 2018, 56, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Albarrag, A.M.; Anderson, M.J.; Howard, S.J.; Robson, G.D.; Warn, P.A.; Sanglard, D.; Denning, D.W. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob. Agents Chemother. 2011, 55, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Buied, A.; Moore, C.B.; Denning, D.W.; Bowyer, P. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J. Antimicrob. Chemother. 2013, 68, 512–514. [Google Scholar] [CrossRef]
- Ren, J.; Jin, X.; Zhang, Q.; Zheng, Y.; Lin, D.; Yu, Y. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J. Hazard. Mater. 2017, 326, 54–60. [Google Scholar] [CrossRef]
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496. [Google Scholar] [CrossRef]
- Diaz-Guerra, T.M.; Mellado, E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2003, 47, 1120–1124. [Google Scholar] [CrossRef]
- Howard, S.J.; Arendrup, M.C. Acquired antifungal drug resistance in Aspergillus fumigatus: Epidemiology and detection. Med. Mycol. 2011, 49 (Suppl. 1), S90–S95. [Google Scholar] [CrossRef]
- Mellado, E.; Garcia-Effron, G.; Alcázar-Fuoli, L.; Melchers, W.J.G.; Verweij, P.E.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L. A New Aspergillus fumigatus Resistance Mechanism Conferring In Vitro Cross-Resistance to Azole Antifungals Involves a Combination of cyp51A Alterations. Antimicrob. Agents Chemother. 2007, 51, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Karawajczyk, A.; Verhoeven, R.J.A.; Venselaar, H.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J.G. The structure-function relationship of the Aspergillus fumigatuscyp51A L98H conversion by site-directed mutagenesis: The mechanism of L98H azole resistance. Fungal Genet. Biol. 2011, 48, 1062–1070. [Google Scholar] [CrossRef] [PubMed]




| No. of Isolate | Place of Isolates Origin | Mutations at Sequence of: | TR34 | Resistance | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Nucleotide | Amino Acid | ITR | POS | VOR | ||||
| 2 | Clinical isolates from Wroclaw | - | - | - | - | - | - | [22,23] |
| 13/2 | T293A | Leu-His | + | + | + | + | [22,23] | |
| 25 | - | - | - | - | - | - | [23] | |
| 29 | G784T | Asp-Tyr | - | - | - | - | [23] | |
| 55 | T293A | Leu-His | + | + | + | + | [22,23] | |
| 72 | - | - | - | - | - | - | [23] | |
| 73 | - | - | - | - | - | - | [23] | |
| 79 | - | - | - | - | - | - | [23] | |
| 1 | Clinical isolates from Warsaw | T229G G393A G433A | Leu-Val Gln-Gln Glu-Lis | - | - | - | - | [23] |
| 5 | T229G | Leu-Val | - | - | - | - | [23] | |
| 12 | T229G G333C | Leu-Val Thr-Thr | - | - | - | - | [23] | |
| 13/3 | T229G G313A | Leu-Val Glu-Lys | - | - | - | - | [23] | |
| 14 | G247T | Val-Phe | - | - | - | - | [23] | |
| 15 | T293A | Leu-His | + | + | + | - | [23] | |
| 16 | A217T T229G | Thr-Pro Leu-Val | - | - | - | - | [23] | |
| 20 | T441G | His-Gln | - | - | - | - | [23] | |
| 21 | T293A | Leu-His | + | + | + | + | [23] | |
| 22 | - | - | - | - | - | - | [23] | |
| 24 | T293A | Leu-His | + | + | + | - | [23] | |
| 37A | G393A G433A | Gln-Gln Glu-Lys | - | - | - | - | [23] | |
| 132H | T744A T912G | Asp-Lys Trp-Cys | - | - | - | - | [23] | |
| 1G | Clinical isolates from Gdansk | A81C | Leu-Phe | - | - | - | - | - |
| 64 | Goose breeding | G777C | Gln-His | - | + | - | - | - |
| 67 | - | - | - | - | - | - | - | |
| 83 | T667G C672T | Trp-Gly Ala-Ala | - | + | - | - | - | |
| 84 | G423A | Gln-Gln | - | + | - | - | - | |
| 85 | G885T | Gln-His | - | + | - | - | - | |
| 104 | C672T | Ala-Ala | - | + | - | - | - | |
| 103 | - | - | - | + | - | - | - | |
| 106 | - | - | - | - | - | - | - | |
| 113 | G423A G713A G905A | Gln-Gln Arg-His Ser-Asn | - | + | - | - | - | |
| 121 | G423A | Gln-Gln | - | + | - | - | - | |
| 140 | G423A G777C | Gln-Gln Gln-His | - | + | - | - | - | |
| 141 | G423A | Gln-Gln | - | + | - | - | - | |
| 143 | - | - | - | + | - | - | - | |
| 3 | Environmental isolates from Gdansk and surroundings | T293A | Leu-His | + | + | + | + | - |
| 5 | T229G | Leu-Val | - | - | - | - | - | |
| 8 | G393A | Gln-Gln | - | - | - | - | - | |
| Gene | Primer sequence (5′ –> 3′) | Reference |
|---|---|---|
| mdr1 | GCTCTTCCCTTGTTCACAATTC | This study |
| CGGCAATACCGAGATACACA | ||
| mdr2 | TTTAGCTCCACCGGGTTTG | [25] |
| TCGAAAGACCGAACATGCTTGA | ||
| mdr3 | TCTGATGGCGGTCATCACT | [25] |
| ATATCCATCCCCCAGGC | ||
| atrf | AGAGAAATCGGACAACTGCTGA | [25] |
| CCTCGTCGCAGATAGTCTTGTA | ||
| cyp51A | TGCAGAGAAAAGTATGGCGA | [25] |
| CGCATTGACATCCTTGAGC | ||
| cyp51B | AGCAGAAGAAGTTCGTCAAATAC | [25] |
| TCGAAGACGCCCTTGTG | ||
| β-tubulin | TTCCCCCGTCTCCACTTCTTCATG | [26] |
| GACGAGATCGTTCATGTTGAACTC |
| Steps of Real-Time PCR | Temperature (°C) | Time (s) | Ramp (°C/s) |
|---|---|---|---|
| Initial denaturation | 95 | 300 | 4.4 |
| Denaturation | 95 | 30 | 4.4 |
| cyp51A, cyp51B | 95 | 15 | 4.4 |
| mdr1, mdr2, mdr3, atrF, β-tubulin | 95 | 30 | 4.4 |
| Annealing | |||
| cyp51A | 60 | 15 | 2.2 |
| cyp51B | 52 | 15 | 2.2 |
| mdr1 | 59 | 60 | 2.2 |
| mdr2, mdr3 | 62 | 60 | 2.2 |
| atrF, β-tubulin | 60 | 60 | 2.2 |
| Extension | |||
| cyp51A, cyp51B | 72 | 20 | 4.4 |
| mdr1, mdr2, mdr3, atrF, β-tubulin | 72 | 60 | 4.4 |
| Denaturation | 95 | 10 | 4.4 |
| Melting of PCR products | 60 | 60 | 2.2 |
| 97 | 60 | 0.1 | |
| Cooling | 40 | 300 | 2.2 |
| MIC Value (mg/L) | Number of Isolates with Defined ITR MIC Values after Exposure Isolates on Respective Agents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 20 SAB * | 20 SAB + 5 SAB ** | 20 ITR | 20 ITR + 5 SAB | 20 VOR | 20 VOR + 5 SAB | 20 POS | 20 POS + 5 SAB | |
| >8 | 3 | 1 | 1 | 38 | 3 | 27 | 3 | 38 | 3 |
| 8 | 8 | 4 | 4 | 0 | 2 | 8 | 3 | 0 | 2 |
| 4 | 5 | 0 | 0 | 0 | 0 | 3 | 7 | 0 | 0 |
| 2 | 1 | 1 | 1 | 0 | 6 | 0 | 4 | 0 | 5 |
| 1 | 1 | 1 | 1 | 0 | 6 | 0 | 1 | 0 | 7 |
| 0.5 | 12 | 16 | 16 | 0 | 11 | 0 | 12 | 0 | 12 |
| 0.25 | 7 | 12 | 12 | 0 | 9 | 0 | 7 | 0 | 8 |
| 0.125 | 1 | 3 | 3 | 0 | 1 | 0 | 1 | 0 | 1 |
| 0.0625 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.032 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MIC Value (mg/L) | Number of Isolates with Defined POS MIC Values after Exposure Isolates on Respective Agents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 20 SAB | 20 SAB + 5 SAB | 20 ITR | 20 ITR + 5 SAB | 20 VOR | 20 VOR + 5 SAB | 20 POS | 20 POS + 5 SAB | |
| >8 | 0 | 0 | 0 | 38 | 0 | 33 | 0 | 38 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 0.5 | 3 | 3 | 3 | 0 | 8 | 0 | 7 | 0 | 8 |
| 0.25 | 3 | 3 | 3 | 0 | 5 | 0 | 5 | 0 | 4 |
| 0.125 | 5 | 5 | 5 | 0 | 6 | 0 | 2 | 0 | 5 |
| 0.0625 | 13 | 13 | 13 | 0 | 9 | 0 | 9 | 0 | 11 |
| 0.032 | 12 | 12 | 12 | 0 | 8 | 0 | 8 | 0 | 8 |
| 0.016 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| MIC Value (mg/L) | Number of Isolates with Defined VOR MIC Values after Exposure Isolates on Respective Agents | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 20 SAB | 20 SAB + 5 SAB | 20 ITR | 20 ITR + 5 SAB | 20 VOR | 20 VOR + 5 SAB | 20 POS | 20 POS + 5 SAB | |
| >16 | 0 | 0 | 0 | 38 | 0 | 35 | 0 | 38 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 4 | 4 | 4 | 0 | 5 | 0 | 4 | 0 | 4 |
| 1 | 2 | 2 | 2 | 0 | 7 | 0 | 8 | 0 | 4 |
| 0.5 | 5 | 5 | 5 | 0 | 6 | 0 | 6 | 0 | 9 |
| 0.25 | 22 | 22 | 22 | 0 | 19 | 0 | 17 | 0 | 20 |
| 0.125 | 5 | 5 | 5 | 0 | 1 | 0 | 3 | 0 | 1 |
| 0.0625 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.032 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mroczyńska, M.; Kurzyk, E.; Śliwka-Kaszyńska, M.; Nawrot, U.; Adamik, M.; Brillowska-Dąbrowska, A. The Effect of Posaconazole, Itraconazole and Voriconazole in the Culture Medium on Aspergillus fumigatus Triazole Resistance. Microorganisms 2020, 8, 285. https://doi.org/10.3390/microorganisms8020285
Mroczyńska M, Kurzyk E, Śliwka-Kaszyńska M, Nawrot U, Adamik M, Brillowska-Dąbrowska A. The Effect of Posaconazole, Itraconazole and Voriconazole in the Culture Medium on Aspergillus fumigatus Triazole Resistance. Microorganisms. 2020; 8(2):285. https://doi.org/10.3390/microorganisms8020285
Chicago/Turabian StyleMroczyńska, Martyna, Ewelina Kurzyk, Magdalena Śliwka-Kaszyńska, Urszula Nawrot, Marta Adamik, and Anna Brillowska-Dąbrowska. 2020. "The Effect of Posaconazole, Itraconazole and Voriconazole in the Culture Medium on Aspergillus fumigatus Triazole Resistance" Microorganisms 8, no. 2: 285. https://doi.org/10.3390/microorganisms8020285
APA StyleMroczyńska, M., Kurzyk, E., Śliwka-Kaszyńska, M., Nawrot, U., Adamik, M., & Brillowska-Dąbrowska, A. (2020). The Effect of Posaconazole, Itraconazole and Voriconazole in the Culture Medium on Aspergillus fumigatus Triazole Resistance. Microorganisms, 8(2), 285. https://doi.org/10.3390/microorganisms8020285





