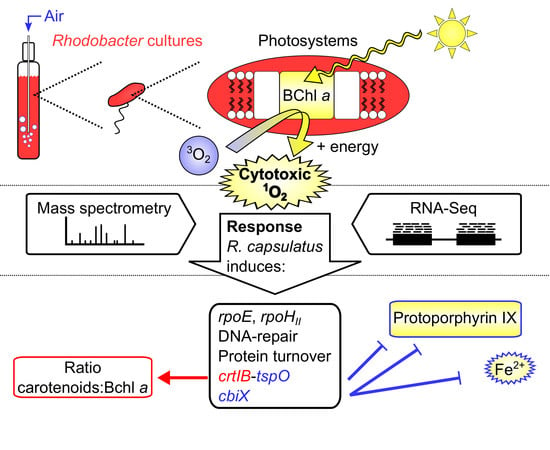
Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Photooxidative Stress Experiments
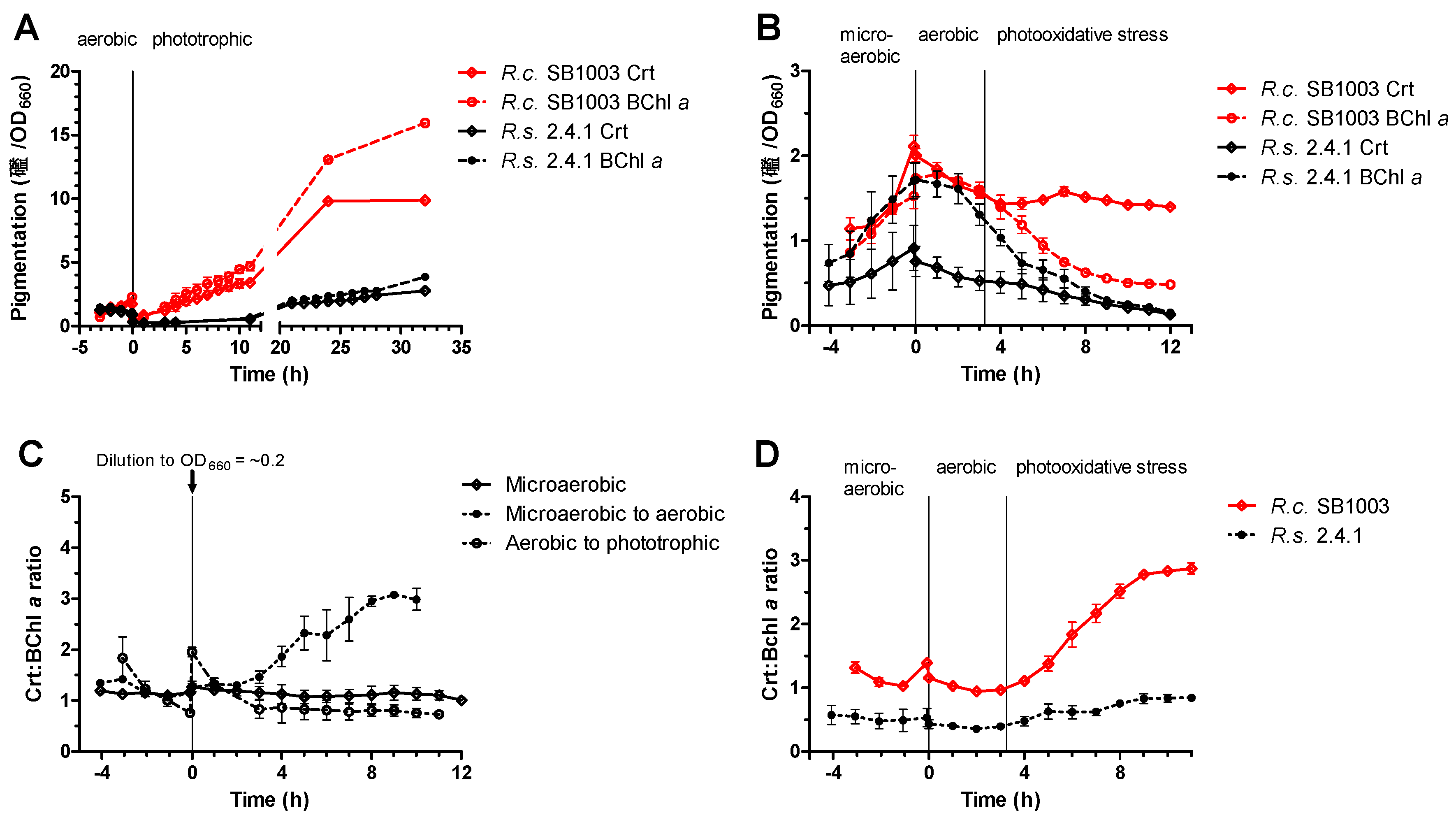
2.3. Analysis of Pigmentation
2.4. Measurement of Reactive Oxygen Species
2.5. Transcriptome Analysis by RNA-Sequencing
2.5.1. Sample Preparation for RNA-seq
2.5.2. Strand-Specific Library Preparation and Illumina Sequencing
2.5.3. Read Mapping, Bioinformatics and Statistics
2.5.4. ERCC Spike-in Control Analysis
2.5.5. RNA-seq Data Accessibility
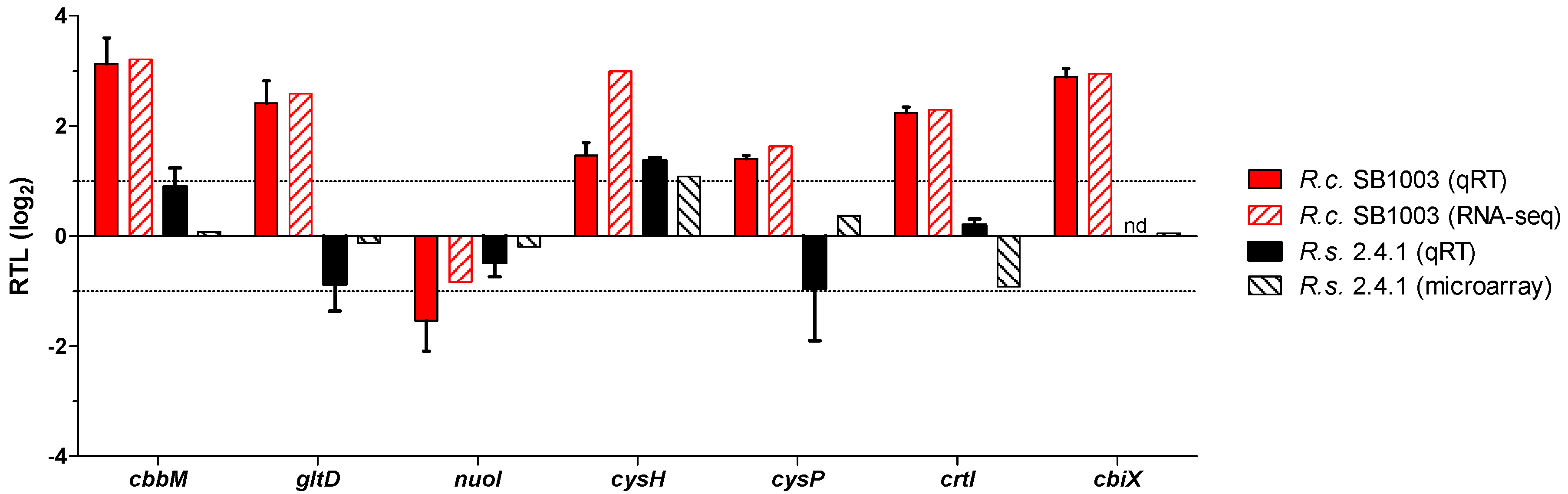
2.6. Quantitative RT-PCR
2.7. Protein Sample Preparation and Mass Spectrometry
2.8. Search for Orthologous Rhodobacter Genes and Synteny Analysis
2.9. Gene Ontology Enrichment Analysis
3. Results
3.1. Adaptation of R. sphaeroides and R. capsulatus to Different Growth Conditions
3.2. Generation of ROS in R. sphaeroides and R. capsulatus upon Photooxidative Stress
3.3. The rpoE-chrR Locus of R. capsulatus Shows a Unique Genetic Context in Comparison to Other Bacteria within the Rhodobacteraceae
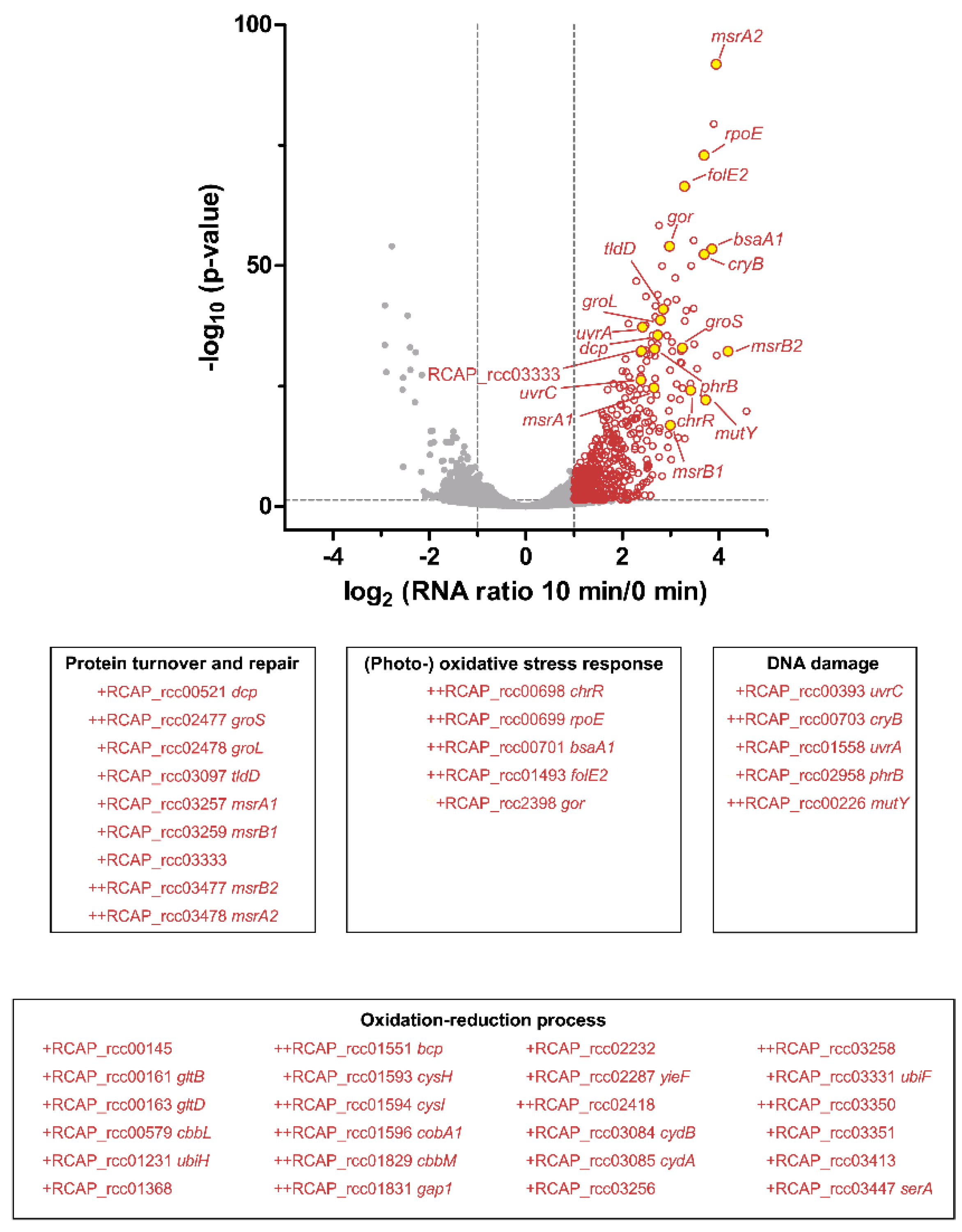
3.4. Transcriptome Analysis of the Response to Singlet Oxygen in R. capsulatus
3.5. Similar Proteins Fulfill Important Functions in Response to Singlet Oxygen in R. sphaeroides and R. capsulatus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glaeser, J.; Nuss, A.M.; Berghoff, B.A.; Klug, G. Singlet oxygen stress in microorganisms. In Advances in Microbial Physiology; Poole, R.K., Ed.; Elsevier Ltd. Academic Press: Amsterdam, The Netherlands, 2011; Volume 58, pp. 141–173. [Google Scholar]
- Braatsch, S.; Gomelsky, M.; Kuphal, S.; Klug, G. A single flavoprotein, AppA, integrates both redox and light signals in Rhodobacter sphaeroides. Mol. Microbiol. 2002, 45, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Meyer, M.H.F.; Keusgen, M.; Klug, G. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol. Microbiol. 2007, 64, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Mank, N.N.; Berghoff, B.A.; Hermanns, Y.N.; Klug, G. Regulation of bacterial photosynthesis genes by the small noncoding RNA PcrZ. Proc. Natl. Acad. Sci. USA 2012, 109, 16306–16311. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, K.M.H.; Reuscher, C.M.; Klug, G. PcrX, an sRNA derived from the 3’-UTR of the Rhodobacter sphaeroides puf operon modulates expression of puf genes encoding proteins of the bacterial photosynthetic apparatus. Mol. Microbiol. 2018, 110, 325–334. [Google Scholar] [CrossRef]
- Ryter, S.W.; Tyrrell, R.M. Singlet molecular oxygen (1O2): A possible effector of eukaryotic gene expression. Free Radic. Biol. Med. 1998, 24, 1520–1534. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Frimer, A.A. The reaction of singlet oxygen with olefins: The question of mechanism. Chem. Rev. 1979, 79, 359–387. [Google Scholar] [CrossRef]
- Cavalcante, A.K.D.; Martinez, G.R.; Di Mascio, P.; Menck, C.F.M.; Agnez-Lima, L.F. Cytotoxicity and mutagenesis induced by singlet oxygen in wild type and DNA repair deficient Escherichia coli strains. DNA Repair 2002, 1, 1051–1056. [Google Scholar] [CrossRef]
- Anthony, J.R.; Warczak, K.L.; Donohue, T.J. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 6502–6507. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Steenken, S.; Obendorf, M.S.W.; Schulz, W.A.; Sies, H. Formation of 8-hydroxy (deoxy) guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry 1991, 30, 6283–6289. [Google Scholar] [CrossRef]
- Floyd, R.A.; Schneider, J.E., Jr.; Dittmer, D.P. Methylene blue photoinactivation of RNA viruses. Antiviral Res. 2004, 61, 141–151. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Non-thermal dielectric-barrier discharge (DBD) plasma-induced inactivation involves oxidative-DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Riske, K.A.; Sudbrack, T.P.; Archilha, N.L.; Uchoa, A.F.; Schroder, A.P.; Marques, C.M.; Baptista, M.S.; Itri, R. Giant vesicles under oxidative stress induced by a membrane-anchored photosensitizer. Biophys. J. 2009, 97, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004, 3, 17–25. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef]
- Glaeser, J.; Zobawa, M.; Lottspeich, F.; Klug, G. Protein synthesis patterns reveal a complex regulatory response to singlet oxygen in Rhodobacter. J. Proteome Res. 2007, 6, 2460–2471. [Google Scholar] [CrossRef]
- Nuss, A.M.; Glaeser, J.; Klug, G. RpoHII activates oxidative-stress defense systems and is controlled by RpoE in the singlet oxygen-dependent response in Rhodobacter sphaeroides. J. Bacteriol. 2009, 191, 220–230. [Google Scholar] [CrossRef]
- Nuss, A.M.; Glaeser, J.; Berghoff, B.A.; Klug, G. Overlapping alternative sigma factor regulons in the response to singlet oxygen in Rhodobacter sphaeroides. J. Bacteriol. 2010, 192, 2613–2623. [Google Scholar] [CrossRef]
- Dufour, Y.S.; Imam, S.; Koo, B.-M.; Green, H.A.; Donohue, T.J. Convergence of the transcriptional responses to heat shock and singlet oxygen stresses. PLoS Genet. 2012, 8, e1002929. [Google Scholar] [CrossRef]
- Nuss, A.M.; Adnan, F.; Weber, L.; Berghoff, B.A.; Glaeser, J.; Klug, G. DegS and RseP homologous proteases are involved in singlet oxygen dependent activation of RpoE in Rhodobacter sphaeroides. PLoS ONE 2013, 8, e79520. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.-W.; Ziegelhoffer, E.C.; Lemke, R.A.S.; Donohue, T.J. Proteins needed to activate a transcriptional response to the reactive oxygen species singlet oxygen. MBio 2013, 4, e00541-12. [Google Scholar] [CrossRef] [PubMed]
- Hendrischk, A.-K.; Braatsch, S.; Glaeser, J.; Klug, G. The phrA gene of Rhodobacter sphaeroides encodes a photolyase and is regulated by singlet oxygen and peroxide in a σE-dependent manner. Microbiology 2007, 153, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Dufour, Y.S.; Landick, R.; Donohue, T.J. Organization and evolution of the biological response to singlet oxygen stress. J. Mol. Biol. 2008, 383, 713–730. [Google Scholar] [CrossRef]
- Lemke, R.A.S.; Peterson, A.C.; Zieglhoffer, E.C.; Westphall, M.S.; Tjellström, H.; Coon, J.J.; Donohue, T.J. Synthesis and scavenging role of furan fatty acids. Proc. Natl. Acad. Sci. USA 2014, 111, E3451. [Google Scholar] [CrossRef]
- Glaeser, J.; Klug, G. Photo-oxidative stress in Rhodobacter sphaeroides: Protective role of carotenoids and expression of selected genes. Microbiology 2005, 151, 1927–1938. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Konzer, A.; Mank, N.N.; Looso, M.; Rische, T.; Förstner, K.U.; Krüger, M.; Klug, G. Integrative “Omics”-approach discovers dynamic and regulatory features of bacterial stress responses. PLoS Genet. 2013, 9, e1003576. [Google Scholar] [CrossRef]
- Schmidt, K. Biosynthesis of carotenoids. In The Photosynthetic Bacteria; Clayton, R.K., Sistrom, W.R., Eds.; Prenum Press: New York, NY, USA, 1978; pp. 729–750. [Google Scholar]
- Scolnik, P.A.; Walker, M.A.; Marrs, B.L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem. 1980, 255, 2427–2432. [Google Scholar]
- Zhu, Y.S.; Cook, D.N.; Leach, F.; Armstrong, G.A.; Alberti, M.; Hearst, J.E. Oxygen-regulated mRNAs for light-harvesting and reaction center complexes and for bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus during the shift from anaerobic to aerobic growth. J. Bacteriol. 1986, 168, 1180–1188. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; Eraso, J.M.; Kaplan, S. Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides 2.4.1: Involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J. Bacteriol. 1996, 178, 5877–5883. [Google Scholar] [CrossRef]
- Li, Z.; Kong, L.; Hui, B.; Shang, X.; Gao, L.; Luan, N.; Zhuang, X.; Wang, D.; Bai, Z. Identification and antioxidant activity of carotenoids from superfine powder of Rhodobacter sphaeroides. Emirates J. Food Agric. 2017, 29, 833–845. [Google Scholar] [CrossRef]
- Šlouf, V.; Chábera, P.; Olsen, J.D.; Martin, E.C.; Qian, P.; Hunter, C.N.; Polívka, T. Photoprotection in a purple phototrophic bacterium mediated by oxygen-dependent alteration of carotenoid excited-state properties. Proc. Natl. Acad. Sci. USA 2012, 109, 8570–8575. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Glaeser, J.; Sharma, C.M.; Vogel, J.; Klug, G. Photooxidative stress-induced and abundant small RNAs in Rhodobacter sphaeroides. Mol. Microbiol. 2009, 74, 1497–1512. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Glaeser, J.; Sharma Cynthia, M.; Zobawa, M.; Lottspeich, F.; Vogel, J.; Klug, G. Contribution of Hfq to photooxidative stress resistance and global regulation in Rhodobacter sphaeroides. Mol. Microbiol. 2011, 80, 1479–1495. [Google Scholar] [CrossRef]
- Billenkamp, F.; Peng, T.; Berghoff, B.A.; Klug, G. A cluster of four homologous small RNAs modulates C-1 metabolism and the pyruvate dehydrogenase complex in Rhodobacter sphaeroides under various stress conditions. J. Bacteriol. 2015, 197, 1839–1852. [Google Scholar] [CrossRef]
- Müller, K.M.H.; Berghoff, B.A.; Eisenhardt, B.D.; Remes, B.; Klug, G. Characteristics of Pos19—A small coding RNA in the oxidative stress response of Rhodobacter sphaeroides. PLoS ONE 2016, 11, e0163425. [Google Scholar] [CrossRef]
- Adnan, F.; Weber, L.; Klug, G. The sRNA SorY confers resistance during photooxidative stress by affecting a metabolite transporter in Rhodobacter sphaeroides. RNA Biol. 2015, 12, 569–577. [Google Scholar] [CrossRef]
- Peng, T.; Berghoff, B.A.; Oh, J.-I.; Weber, L.; Schirmer, J.; Schwarz, J.; Glaeser, J.; Klug, G. Regulation of a polyamine transporter by the conserved 3’ UTR-derived sRNA SorX confers resistance to singlet oxygen and organic hydroperoxides in Rhodobacter’ sphaeroides. RNA Biol. 2016, 13, 988–999. [Google Scholar] [CrossRef]
- Klug, G.; Adams, C.W.; Belasco, J.; Doerge, B.; Cohen, S.N. Biological consequences of segmental alterations in mRNA stability: Effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 1987, 6, 3515–3520. [Google Scholar] [CrossRef]
- Gregor, J.; Klug, G. Regulation of bacterial photosynthesis genes by oxygen and light. FEMS Microbiol. Lett. 1999, 179, 1–9. [Google Scholar] [CrossRef]
- Remes, B.; Berghoff, B.A.; Foerstner, K.U.; Klug, G. Role of oxygen and the OxyR protein in the response to iron limitation in Rhodobacter sphaeroides. BMC Genom. 2014, 15, 794. [Google Scholar] [CrossRef]
- Shiozawa, J.A.; Welte, W.; Hodapp, N.; Drews, G. Studies on the size and composition of the isolated light-harvesting B800-850 pigment-protein complex of Rhodopseudomonas capsulata. Arch. Biochem. Biophys. 1982, 213, 473–485. [Google Scholar] [CrossRef]
- Clayton, R.K. The bacterial photosynthetic reaction center. In Proceedings of the Brookhaven Symposia in Biology, New York, NY, USA, 6–9 June 1966; Volume 19, pp. 62–70. [Google Scholar]
- Janzon, L.; Löfdahl, S.; Arvidson, S. Evidence for a coordinate transcriptional control of alpha-toxin and protein a synthesis in Staphylococcus aureus. FEMS Microbiol. Lett. 1986, 33, 193–198. [Google Scholar] [CrossRef]
- Nuss, A.M.; Heroven, A.K.; Waldmann, B.; Reinkensmeier, J.; Jarek, M.; Beckstette, M.; Dersch, P. Transcriptomic profiling of Yersinia pseudotuberculosis reveals reprogramming of the Crp regulon by temperature and uncovers Crp as a master regulator of small RNAs. PLoS Genet. 2015, 11, e1005087. [Google Scholar] [CrossRef]
- Aronesty, E. ea-Utils: Command-Line Tools for Processing Biological Sequencing Data. 2011. Available online: https://github.com/ExpressionAnalysis/ea-utils (accessed on 19 February 2020).
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bathke, J.; Konzer, A.; Remes, B.; McIntosh, M.; Klug, G. Comparative analyses of the variation of the transcriptome and proteome of Rhodobacter sphaeroides throughout growth. BMC Genom. 2019, 20, 358. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Chen, I.-M.A.; Chu, K.; Palaniappan, K.; Pillay, M.; Ratner, A.; Huang, J.; Huntemann, M.; Varghese, N.; White, J.R.; Seshadri, R.; et al. IMG/M v. 5.0: An integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 2019, 47, D666–D677. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Blom, J.; Kreis, J.; Spänig, S.; Juhre, T.; Bertelli, C.; Ernst, C.; Goesmann, A. EDGAR 2.0: An enhanced software platform for comparative gene content analyses. Nucleic Acids Res. 2016, 44, W22–W28. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; Cherry, J.M.; Ashburner, M.; Ball, C.A.; Blake, J.A.; Butler, H.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar]
- Carbon, S.; Douglass, E.; Dunn, N.; Good, B.; Harris, N.L.; Lewis, S.E.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; et al. The Gene Ontology Resource: 20 years and still going strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar]
- Prasad, A.; Sedlářová, M.; Pospíši, P. Singlet oxygen imaging using fluorescent probe Singlet Oxygen Sensor Green in photosynthetic organisms. Sci. Rep. 2018, 8, 13685. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Ziegelhoffer, E.C.; Donohue, T.J. Bacterial responses to photo-oxidative stress. Nat. Rev. Microbiol. 2009, 7, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Hendrischk, A.-K.; Frühwirth, S.W.; Moldt, J.; Pokorny, R.; Metz, S.; Kaiser, G.; Jäger, A.; Batschauer, A.; Klug, G. A cryptochrome-like protein is involved in the regulation of photosynthesis genes in Rhodobacter sphaeroides. Mol. Microbiol. 2009, 74, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, B.A.; Glaeser, J.; Nuss, A.M.; Zobawa, M.; Lottspeich, F.; Klug, G. Anoxygenic photosynthesis and photooxidative stress: A particular challenge for Roseobacter. Environ. Microbiol. 2011, 13, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Bali, S.; Rollauer, S.; Roversi, P.; Raux-Deery, E.; Lea, S.; Warren, M.J.; Ferguson, S.J. Identification and characterization of the “missing” terminal enzyme for siroheme biosynthesis in α-proteobacteria. Mol. Microbiol. 2014, 92, 153–163. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Klug, G. An omics view on the response to singlet oxygen. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 1, pp. 619–631. [Google Scholar]
- Brinkmann, H.; Göker, M.; Koblížek, M.; Wagner-Döbler, I.; Petersen, J. Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae. ISME J. 2018, 12, 1984–2010. [Google Scholar] [CrossRef]
- Schroeder, W.A.; Johnson, E.A. Carotenoids protect Phaffia rhodozyma against singlet oxygen damage. J. Ind. Microbiol. 1995, 14, 502–507. [Google Scholar] [CrossRef]
- Galbis-Martínez, M.; Padmanabhan, S.; Murillo, F.J.; Elías-Arnanz, M. CarF mediates signaling by singlet oxygen, generated via photoexcited protoporphyrin IX, in Myxococcus xanthus light-induced carotenogenesis. J. Bacteriol. 2012, 194, 1427–1436. [Google Scholar] [CrossRef]
- Zeng, X.; Kaplan, S. TspO as a modulator of the repressor/antirepressor (PpsR/AppA) regulatory system in Rhodobacter sphaeroides 2.4. 1. J. Bacteriol. 2001, 183, 6355–6364. [Google Scholar] [CrossRef]
- Leech, H.K.; Raux-Deery, E.; Heathcote, P.; Warren, M.J. Production of cobalamin and sirohaem in Bacillus megaterium: An investigation into the role of the branchpoint chelatases sirohydrochlorin ferrochelatase (SirB) and sirohydrochlorin cobalt chelatase (CbiX). Biochem. Soc. Trans. 2002, 30, 610–613. [Google Scholar] [CrossRef][Green Version]
- Gough, S.P.; Petersen, B.O.; Duus, J.Ø. Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor. Proc. Natl. Acad. Sci. USA 2000, 97, 6908–6913. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, K.; Hammad, L.A.; Karty, J.A.; Bauer, C.E. Vitamin B 12 regulates photosystem gene expression via the CrtJ antirepressor AerR in Rhodobacter capsulatus. Mol. Microbiol. 2014, 91, 649–664. [Google Scholar] [CrossRef]
- Klug, G. Beyond catalysis: Vitamin B12 as a cofactor in gene regulation. Mol. Microbiol. 2014, 91, 635–640. [Google Scholar] [CrossRef]
- Kim, J.-G.; Back, K.; Lee, H.Y.; Lee, H.-J.; Phung, T.-H.; Grimm, B.; Jung, S. Increased expression of Fe-chelatase leads to increased metabolic flux into heme and confers protection against photodynamically induced oxidative stress. Plant Mol. Biol. 2014, 86, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wei, H.; Tian, C.; Damron, F.H.; Zhou, J.; Qiu, D. An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis. BMC Microbiol. 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Xie, M.; Dai, J.; An, W.; Wei, H.; Tian, C.; Kempher, M.L.; Zhou, A.; He, Z.; Gu, B.; et al. Differential regulation of the two ferrochelatase paralogues in Shewanella loihica PV-4 in response to environmental stresses. Appl. Environ. Microbiol. 2016, 82, 5077–5088. [Google Scholar] [CrossRef][Green Version]





| R. sphaeroides Gene | Description | Log2 FC 1 (7 min) | Log2 FC 1 (45 min) | R. capsulatus Gene 2 | Log2 FC 1 (10 min) |
|---|---|---|---|---|---|
| RSP_1092 rpoE | RNA polymerase sigma-70 factor | 2.2 ** | 2.0 *** | RCAP_rcc00699 rpoE | 3.7 *** |
| RSP_1093 chrR | Antisigma factor ChrR | 2.0 ** | 2.2 *** | RCAP_rcc00698 chrR | 3.4 *** |
| RSP_2144 cfaS | Cyclopropane-fatty-acyl-phospholipid synthase CfaS | 1.4 * | 1.0 *** | RCAP_rcc00273 rsmB1 | 0.3 |
| RSP_2143 phrA | DNA photolyase | 1.6 ** | 1.5 *** | RCAP_rcc02958 phrB | 2.7 *** |
| RSP_1091 | Putative cyclopropane or cyclopropene fatty acid synthesis protein | 2.2 *** | 2.0 *** | No homolog | |
| RSP_1090 | Putative cyclopropane/cyclopropene fatty acid synthesis protein | 2.4 | 1.9 *** | No homolog | |
| RSP_1089 | Sugar/cation symporter, GPH family | 1.9 * | 1.8 *** | No homolog | |
| RSP_1088 | Hypothetical protein | 1.1 * | 0.8 ** | No homolog | |
| RSP_1087 | Short-chain dehydrogenase/reductase family member | 0.9 | 0.7 ** | No homolog | |
| RSP_0601 rpoHII | RNA polymerase sigma factor | 2.0 * | 2.1 * | RCAP_rcc00458 rpoHII 3 | 2.1 *** |
| RSP_1409 | Beta-Ig-H3/fasciclin | 2.8 ** | 4.5 * | No homolog | |
| RSP_1852 folE2 | Hypothetical protein | 2.2 *** | 2.3 *** | RCAP_rcc01493 folE2 | 3.3 *** |
| RSP_0296 cycA | Cytochrome c2 | −0.3 | 0.5 ** | RCAP_rcc01240 cycA1 | 0.2 |
| RSP_3336 | ABC spermidine/putrescine transporter, inner membrane subunit | 0.0 | 0.2 * | RCAP_rcc01895 potH1 | −0.6 * |
| RSP_6222 | Hypothetical protein | 0.1 | 0.3 | No homolog |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licht, M.K.; Nuss, A.M.; Volk, M.; Konzer, A.; Beckstette, M.; Berghoff, B.A.; Klug, G. Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus. Microorganisms 2020, 8, 283. https://doi.org/10.3390/microorganisms8020283
Licht MK, Nuss AM, Volk M, Konzer A, Beckstette M, Berghoff BA, Klug G. Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus. Microorganisms. 2020; 8(2):283. https://doi.org/10.3390/microorganisms8020283
Chicago/Turabian StyleLicht, Mathieu K., Aaron M. Nuss, Marcel Volk, Anne Konzer, Michael Beckstette, Bork A. Berghoff, and Gabriele Klug. 2020. "Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus" Microorganisms 8, no. 2: 283. https://doi.org/10.3390/microorganisms8020283
APA StyleLicht, M. K., Nuss, A. M., Volk, M., Konzer, A., Beckstette, M., Berghoff, B. A., & Klug, G. (2020). Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus. Microorganisms, 8(2), 283. https://doi.org/10.3390/microorganisms8020283