Abstract
This trial tested the use of lactic acid bacteria (LAB) on pikeperch (Sander lucioperca) larvae during their first feeding. The trial included the use of two probiotic treatments and one control (no probiotics). Pikeperch larvae were exposed to LAB as follows: (1) the live feed (Treatment 1, live feed) or (2) via the live feed and the larval rearing water (Treatment 2, probiotic). Significant differences were found between the treatments in terms of total length (TL), myomere height (MH), overall survival, and the tolerance to a high salinity challenge. Larvae exposed to LAB via both the live feed and the rearing water had a significantly higher overall survival rate (85%) than the other two treatments at 21 dph. When both treatments were subjected to high salinity rates (18 parts per thousand (ppt)), both treatments exposed to LAB demonstrated higher survival rates than the control treatment (28% and 40% survival rate at 180 min for the live feed and probiotic treatments, respectively, as compared with a 100% mortality rate at 150 min for the control). At the same time, larvae exposed to the probiotic treatment had a significantly higher TL as compared to the control after 12 and 21 days post hatch (dph) (probiotic 7.13 ± 0.21 and 11.71 ± 1.1 mm, control 5.86 and 10.79 mm at 12 and 21 dph, respectively). The results suggest that the use of LAB in both the live feed and the rearing water has a positive effect on pikeperch larval quality by strengthening their resilience to stress conditions, as well as improving the growth and survival rates.
1. Introduction
Pikeperch (Sander lucioperca), a fresh and brackish water fish belonging to the Percidae family, is in high demand by recreational anglers and the gastronomic industry [1,2]. Because of the high demand, pikeperch is currently one of the targeted species included in the European Union’s plans to diversify the inland freshwater aquaculture. However, larviculture development in recirculating aquaculture systems (RAS) are encountering several obstacles such as low stress resistance, nutritional deficiencies, and cannibalism which result in low survival rates during the larval stage [3]. In order to supply the larvae with adequate nutrition, live feed is required.
Recently, rotifers were introduced to first feeding of pikeperch larvae with successful results [4,5,6]. Rotifers have the ability to absorb and retain the nutritional composition of any given diet that it is exposed, a quality that supplies pikeperch larvae with an adequate prey size and optimal nutrition [7]. These nutrients include highly polyunsaturated fatty acids that are essential for the survival of pikeperch [8,9,10]. However, the use of live feeds during a first feeding also introduces pathogenic bacteria into the closed system [11].
Probiotics are “live microbial feed supplements which beneficially affects the host animal by improving its intestinal microbial balance” [12]. Exposing fish larvae to selected probiotics has been proven to improve their health and increase their resilience to pathogens and disease due to the gastrointestinal microbiota dependency on the external environment [13]. Probiotics also compete with pathogens for nutrients and adhesion sites, which help to stimulate the immune system [13]. It is also important that defense mechanisms are present in the immune system before it is fully developed, so that non-pathogenic bacteria proliferate and inhibit colonization [14]. Probiotics play a role by occupying receptor sites and competing for food, thus, preventing detrimental bacteria in fish larvae from colonizing [14]. Additional benefits of probiotic bacteria supplementation through live feeds include improving the nutritional and growth performance of larval fish reared in RAS [15]. The bacteria contribute to the digestion of dietary macromolecules in the developing larval gut digestive system [16]. Moreover, recent studies with pikeperch [17,18] have also documented the beneficial effects of adding probiotics to the diet.
Most probiotic microorganisms belong to lactic acid bacteria (LAB) [19]. LAB are gram-positive, usually non-motile, nonsporulating bacteria that produce lactic acid as a major or sole product of fermentative metabolism. Nutritionally, LAB are fastidious, requiring carbohydrates, amino acids, peptides, nucleic acid derivate, and vitamins. The fish larvae’s gut is sterile until hatching, but soon after hatching, it comes in contact with the environment and live feeds lead to successive colonization by a variety of microbes [20,21]. The balance of this microbiota is influenced by a variety of factors including, but not limited to, feed type, animal physiology, and immunological factors. Such microbiota in endothermic animals are dominated by gram-positive bacteria such as LAB [22,23]. LAB are characterized by the following: (1) cell-surface properties for mediating adhesion, (2) survival within the gastrointestinal tract, (3) resilience to stress conditions, (4) ability to produce antioxidants, (5) antimicrobial effects, and (6) the positive influence on the immune system [24].
Objective
The aim of this study was to evaluate the influence of Pediococcus acidilactici MA 18/5M on pikeperch larval rearing during the first 21 days post hatching (dph).
2. Materials and Methods
The trial was run at the University of South Bohemia, Facility of Fisheries and Protection of Waters, Czech Republic (USB, FFPW). Spawning and fertilized egg production was from pond-cultured pikeperch broodstock [25,26] (TL = 517 ± 35 mm and W = 1215 ± 200 g) held at the same facility under controlled conditions [27] in RAS. Final oocyte and sperm maturation were performed under a 15 h:9 h light/darkness regime with a light intensity of 100 lux, and a water temperature of 15 ± 0.5 °C [27,28,29]. It was synchronized with an intramuscular hormonal injection of 500 IU·kg−1 of Human Chorionic Gonadotropin (hCG; Chorulon, Intervet International B.V. Ljubljana, Slovenia), as previously done by Křištan [30] and Blecha [28]. All broodstock were anesthetized with clove oil (Dr. Kulich Pharma Ltd., Hradec Králové, Czech Republic) at a concentration of 30 mgL−1 [31] before manipulation. After hormonal treatment, pairs of both sexes were separated and stocked in RAS tanks for nest spawning, as previously studied by Malinovskyi [25,26]. After spawning, egg fertilization, and laying, broodstock were removed and eggs on the nest were incubated in each tank under a water temperature of 16 ± 0.5 °C, for 8 days until hatching occurred [25]. Three-day old larvae were stocked at 100 larvae per liter into 2 L larval rearing tanks (n = 12). Water quality parameters, salinity (3 ± 0.5 ppt), dissolved oxygen (8.0 ± 1 mgl−l), temperature (17.1 ± 0.2 °C) in the RAS were monitored daily. Ammonia (NH3 = 0.20 ± 0.05 mgL−1), nitrite (NO2 = 0.02 ± 0.01 mgL−1) and nitrate (NO3 = 0.10 ± 0.03 mgL−1) levels were measured every 3 days.
Three treatments were tested in quadruplicate. The first was the control treatment, where larvae were offered rotifers fed with Nannochloropsis occulata for the first 11 days of exogenous feeding (15 dph) followed by unenriched artemia until the end of the trial (21 dph). No probiotics were used during this treatment. The second treatment (live feed) used the same live feed protocol with the addition of the probiotic (Bactocell Aqua 100, Pediococcus acidilactici, 1.1011 CFU/g of product, Lallemand SAS, Blagnac, France) at a daily dose of 1 g/m3 in the rotifer and artemia culture tanks, giving a probiotic concentration of 1.105 CFU/mL in the live feed culture water. In this treatment, the probiotic was only used on the larval feed (rotifer and artemia), therefore, larvae had no direct external contact with the probiotic. Their contact with the probiotics was limited to only when ingesting the prey. The third treatment (probiotic) followed the same live feed protocol as the control treatment with rotifers and artemia. They were exposed to a daily dose 1 g/m3 of Bactocell Aqua 100 during their culture. Additionally, the probiotic product was added daily to the larval rearing water at a dose of 0.1 g/m3 daily over the trial’s duration. In this treatment, larvae had direct external contact with probiotics (in the water), as well as when they were ingesting prey.
Rotifers were fed to the larvae three times per day (08:00, 11:30, and 15:30) starting at 4 days post hatching (dph) until 15 dph, with an initial concentration of 10 individuals per ml. Artemia were fed to each experimental group from day 12 post hatching. Feeding densities were steadily increased based on residual counts, performed prior to each feeding (Table 1). By 21 dph, rotifer density was 0 rotifers ml-1 and 8 artemia ml−1.

Table 1.
Experiment husbandry schedule. Amount of daily feed offered, and recirculation flow changes with time are shown.
Live feed culture for the trial was done onsite. Rotifers (average size of 280 µm) were produced following a batch culture protocol fed with N. occulata (Nanno 3600, Reed Mariculture, Campbell, USA) at a rate of 1 mL of paste per liter of culture twice a day. Artemia nauplii’s average size was 430 µm. Flow rates started at 100 mL.min−1 and increased with time (Table 1). Prior to each feeding, flow was stopped and re-started two hours after, in order to improve larval feeding efficiency.
Eight and 12 days after treatment initiation (12 and 16 dph), 40 larvae per treatment (10 per tank) were collected using a 300 micron diameter mesh, and their total length (TL), myomere height (MH), eye diameter (ED), stomach fullness (SF), and air bladder inflation were recorded according to Yanes-Roca [4]. Recordings were made using an Olympus BX41 microscope fitted with a Canon-72 digital camera (Tokyo, Japan) and the Olympus (Tokyo, Japan) cellSens imaging software (version 1.3).
Prior to the appearance of cannibalism and light photosensitivity, the trial was terminated at 21 dph. At the end of the trial 21 dph, final survival was assessed. One hundred larvae per treatment (25 per tank) were assessed for morphometric analysis (TL, MH, ED, SF), and 100 larvae per treatment were collected and used for a subsequent salinity stress challenge.
2.1. Salinity Stress Challenge
Twenty-one days after hatching, 100 larvae per treatment (25 per tank) were collected and transferred to a 2 L tank (n = 3), where they were exposed to a salinity of 18 ppt for three hours. Larval mortality was recorded in each tank every 10 min during the first hour, then, recordings were taken at 120, 130, 140, 150, and 180 min from the initial stocking. Water quality conditions were kept the same as the original trial tanks, with the exception of salinity (18 ppt).
Larvae during this trial were handled in accordance with national and international guidelines for the protection of animal welfare (EU-harmonized Animal Welfare Act of the Czech Republic). The experimental unit is licensed (no. 2293/2015-MZE-17214 and no. 55187/2016-MZE-17214 in project NAZV QK1820354) according to the Czech National Directive (Law against Animal Cruelty, no. 246/1992).
2.2. Statistical Analysis
Differences between the body measurements and stomach fullness in three different treatments of larvae (sampled at 12, 16, and 21 dph) were evaluated with linear mixed models (LMM, package lme4, version 1.1-7; [32]). The effect of the different probiotic treatment was tested on fish TL, MH, and ED (response variables). The tank was included as a random effect. Prior to LMM, the different response variables were transformed with the Box-Cox transformation, which gives the best power estimate for each variable (package car, version 2.1.2; Fox and Weisberg, 2011; [33]). Thereafter, multiple pairwise comparisons between treatments were obtained using Tukey’s all-pair comparisons, applying the Bonferroni correction to adjust the p-values (package multcomp, version 1.3-3; [34]).
Differences in stomach fullness (1 to 4, 1 being an empty gut and 4 a full gut) were evaluated with generalized linear mixed models (GLMM, package lme4), fitted with a binomial error structure. Stomach fullness was used as response variable and the tank as a random factor. These analyses were followed by multiple pairwise comparisons with Tukey’s all-pair comparisons.
The pikeperch fish survival rate was compared between treatments using a generalized linear mixed model (GLMM), with the survival fish (i.e., proportion of alive fish at 21 dph as a response variable) fitted with a binomial error structure, and with enrichment as a fixed effect and the tank as a random effect. After GLMM, pairwise comparisons were obtained with Tukey’s all-pair comparison test. A Bonferroni correction was applied to adjust the p-values of multiple comparisons.
To test the salinity stress tolerance response among the treatments, a non-parametric survival analysis (Kaplan–Meier method) was performed for all groups, using survival package (Therneau and Grambsch, 2000).
3. Results
3.1. Larval Growth
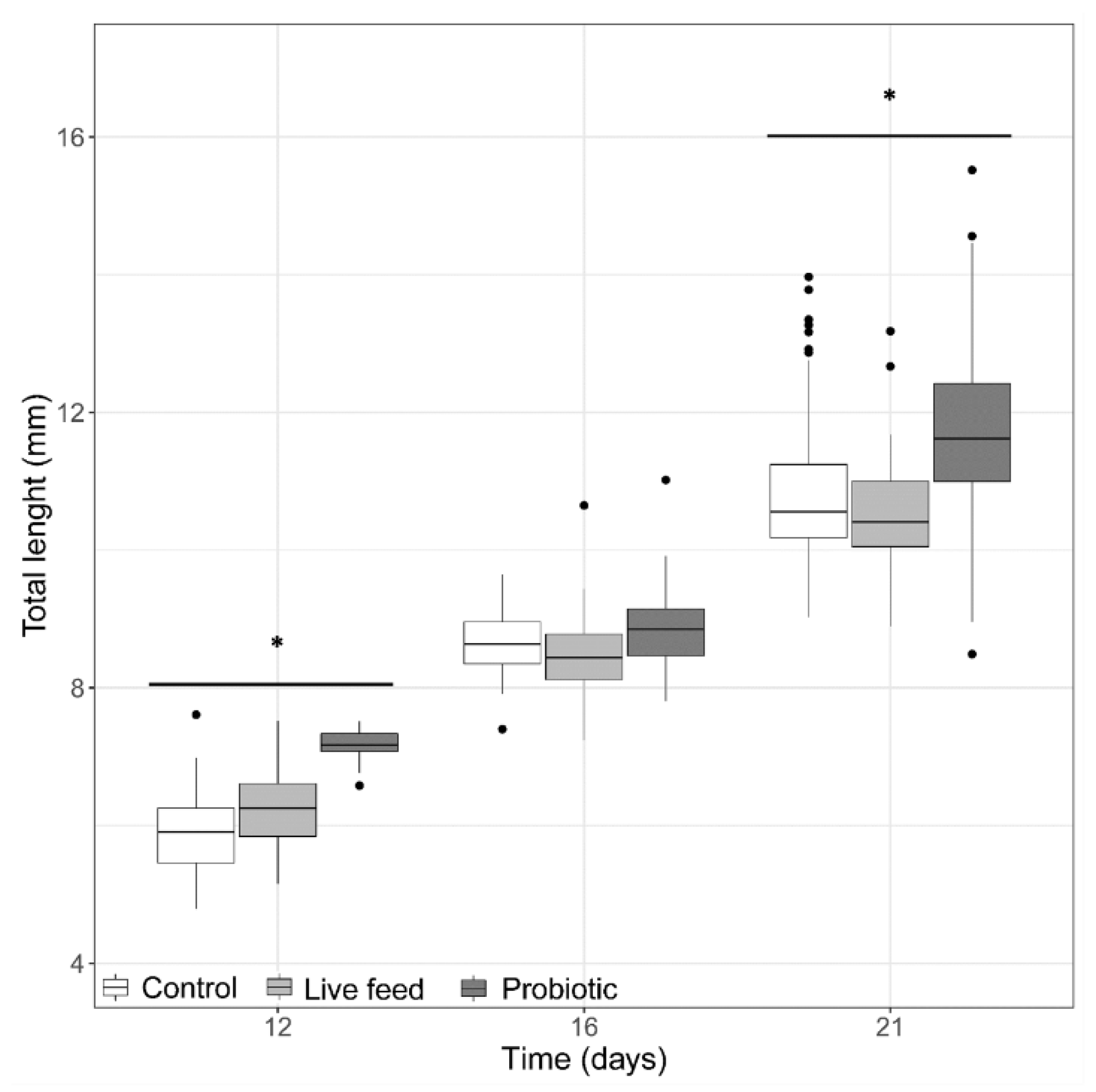
At the start of the trial (3 dph, prior first feeding), pikeperch larval TL and BW was 5.25 ± 0.5 mm. At 12 dph, the probiotic treatment group (Figure 1) had the larvae with the largest average total length (7.13 ± 0.21 mm). By the end of the trial (21 dph), the average total length was significantly greater (LMM, p-value <0.05) in the probiotic treatment (11.71 ± 1.15 mm) than in the control and live feed treatments (Figure 1), but no significant treatment differences (LMM, p-value > 0.05) were found in total length at 16 dph.
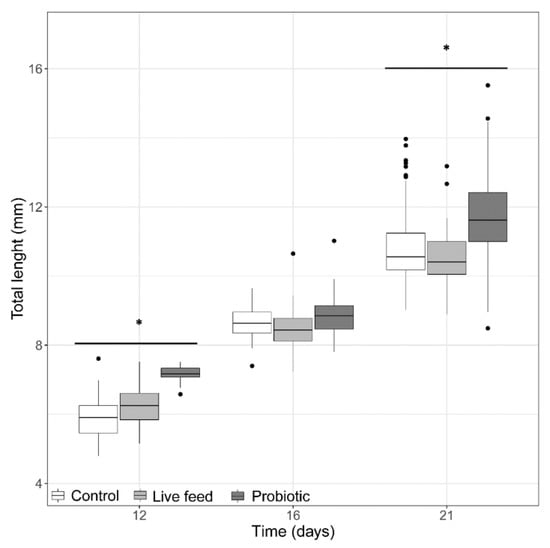
Figure 1.
Larval total length from three treatments at days 12 (n = 40), 16 (n = 40), and 21 dph (n = 100). Dots shown are the out layers, whiskers indicate the maximum and minimum values excluding out layers, the line in the middle of box is the median value and upper and lower quartiles are the ends of the box. Statistically significant differences between treatments are marked with an asterisk.
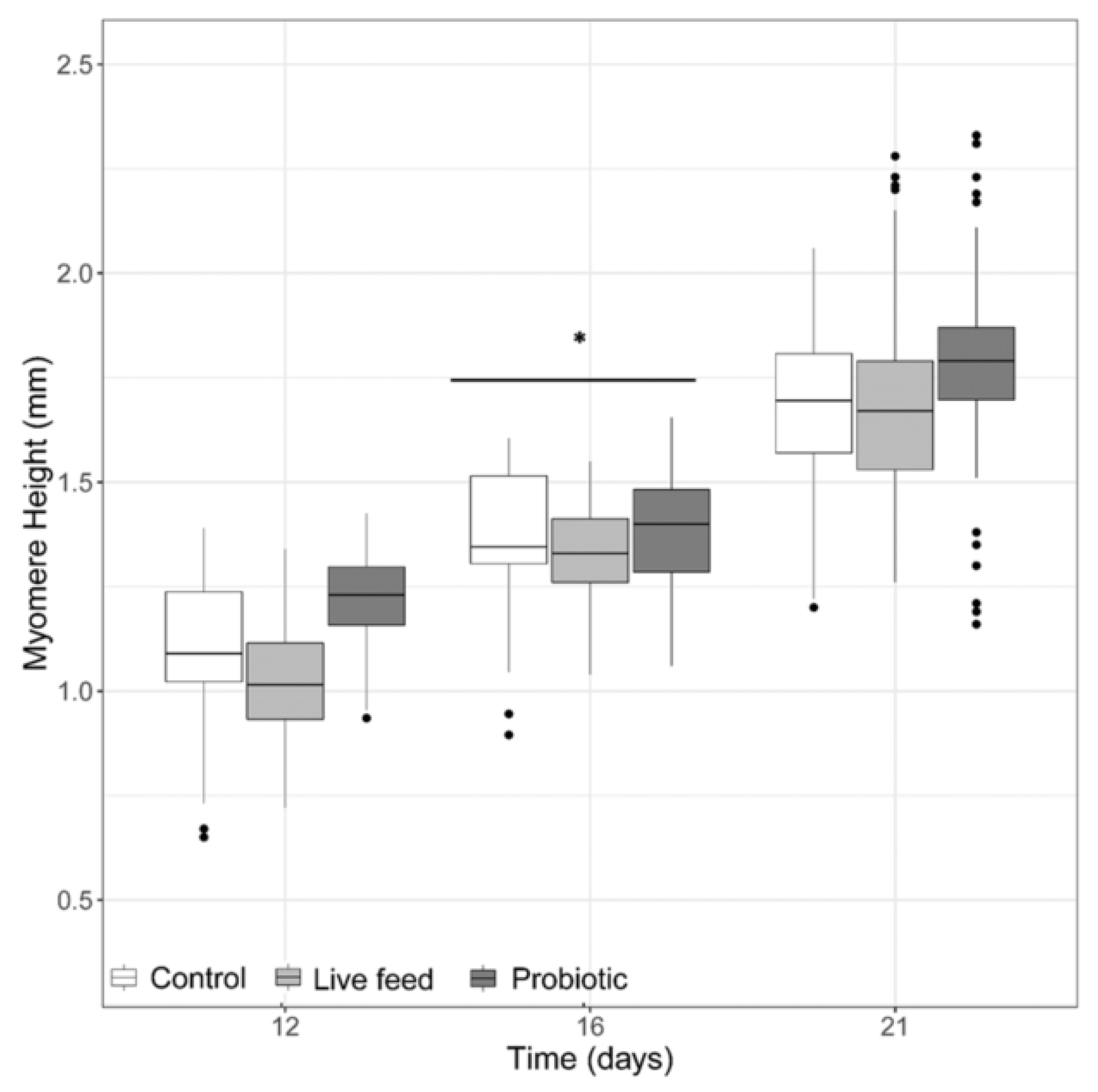
When looking at myomere height (Figure 2), no significant differences were detected (LMM, p-value >0.05), with an exception at 16 dph, where a treatment effect was found (LMM p-value <0.001). When looking at the eye diameter, no significant differences were found (LMM, p-value >0.05, data not shown).
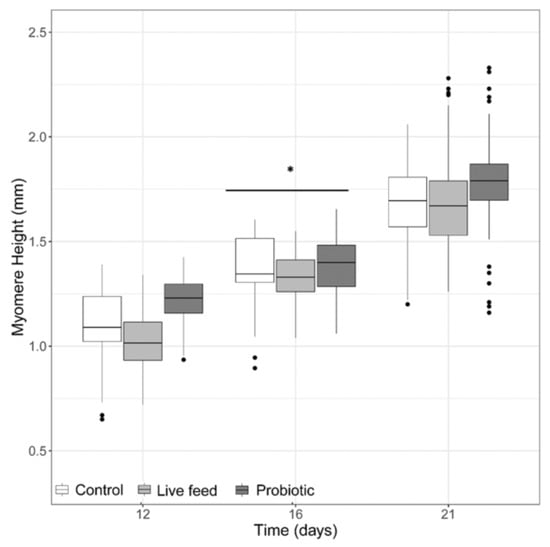
Figure 2.
Larval myomere height from three treatments at days 12 (n = 40), 16 (n = 40), and 21 dph (n = 100). Dots shown are the out layers, whiskers indicate the maximum and minimum values excluding out layers, the line in the middle of box is the median value and upper and lower quartiles are the ends of the box. Statistically significant differences between treatments are marked with an asterisk.
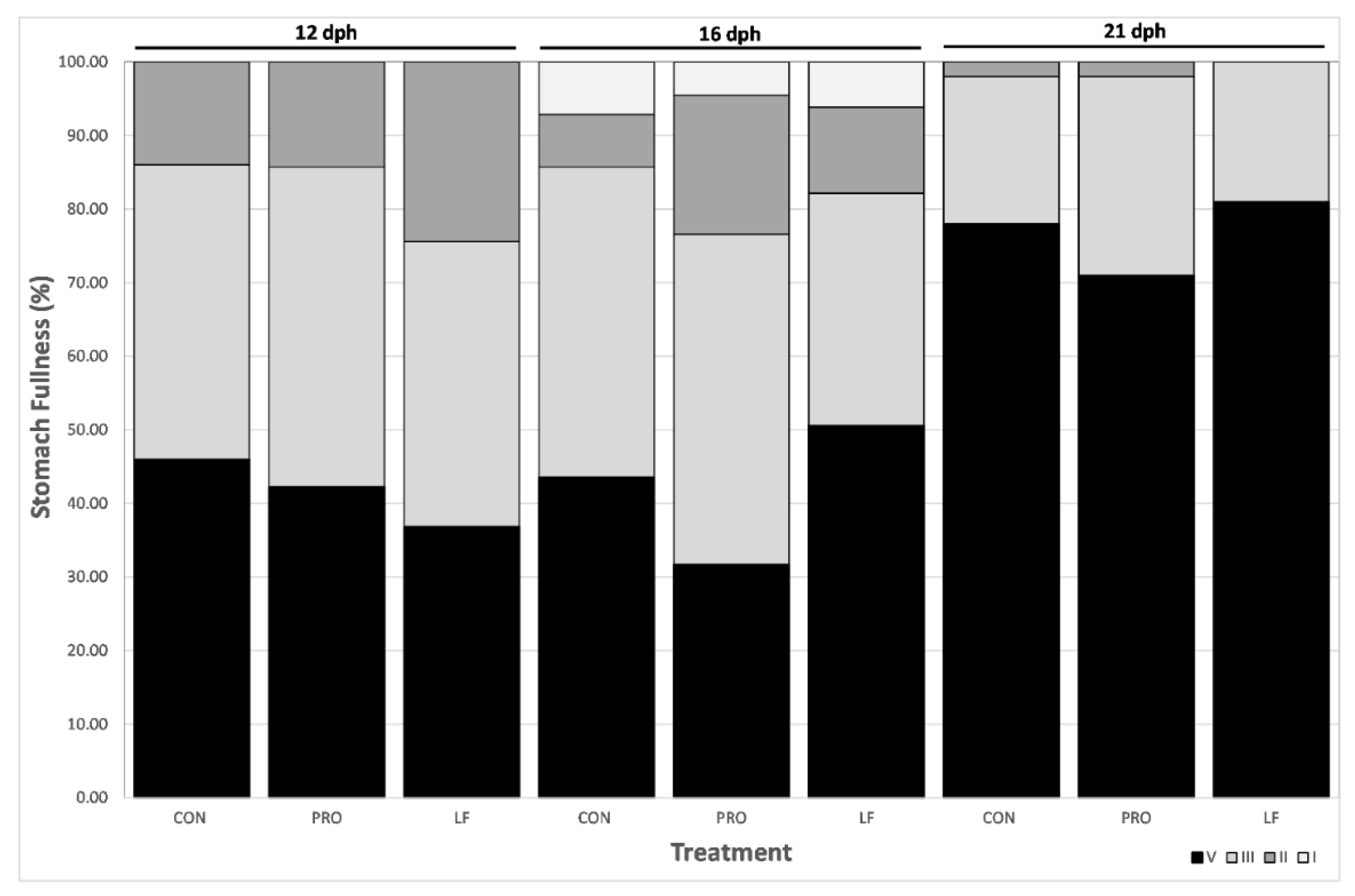
No significant differences in stomach fullness of larvae were found between treatments (GLMM p-value <0.05) and all prey were ingested by larvae, regardless of treatment (Figure 3).
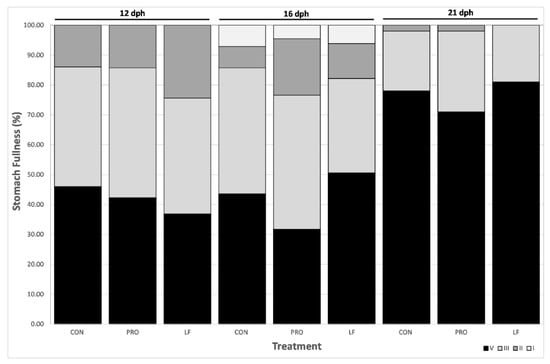
Figure 3.
Larval stomach fullness from three treatments at days 12 (n = 40), 16 (n = 40), and 21 dph (n = 100). Expressed in percentage (1 to 4, 4 being the maximum fullness, from darkest to lightest grey).
3.2. Survival
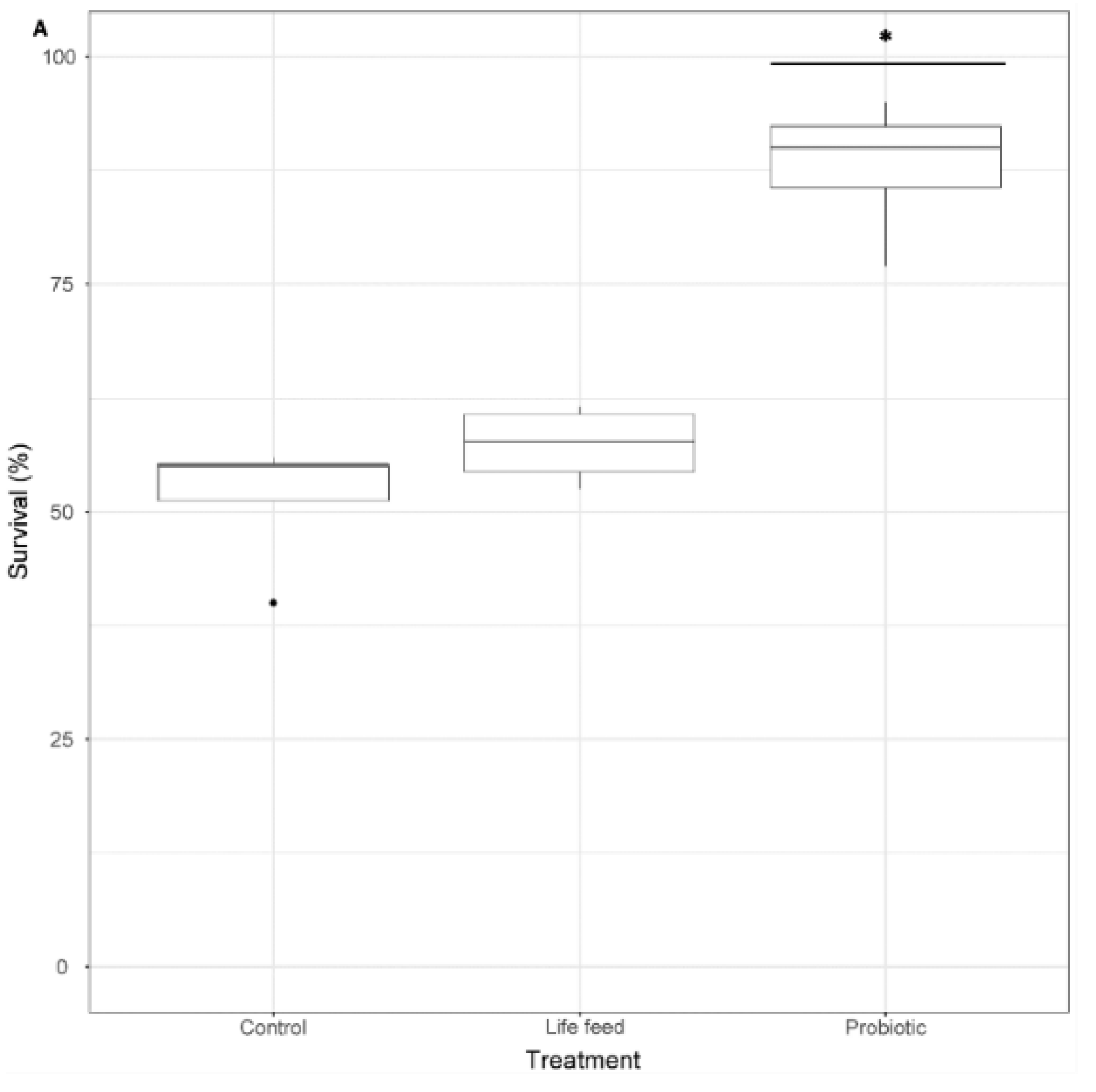
Survival rates at 21 dph were significantly different between treatments (GLMM and pairwise comparisons p < 0.001), showing that the survival of larvae exposed to the probiotic treatment was 1.7 times higher than larvae from the control treatment and 1.53 times higher than larvae exposed to the live feed treatment, whereas the survival of larvae from the live feed treatment was 1.1 times higher than of larvae from the control treatment (not significant p > 0.05) (Figure 4).
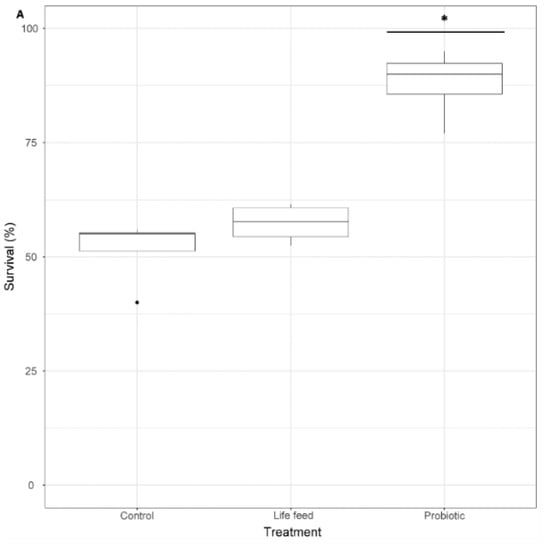
Figure 4.
A: Larval survival percentage (n = 100), after 21 dph. Dots shown are the out layers, whiskers indicate the maximum and minimum values excluding out layers, the line in the middle of box is the median value and upper and lower quartiles are the ends of the box. Statistically significant differences between treatments are marked with an asterisk. Statistically significant differences between samples are marked with an asterisk.
3.3. Salinity Stress Tolerance
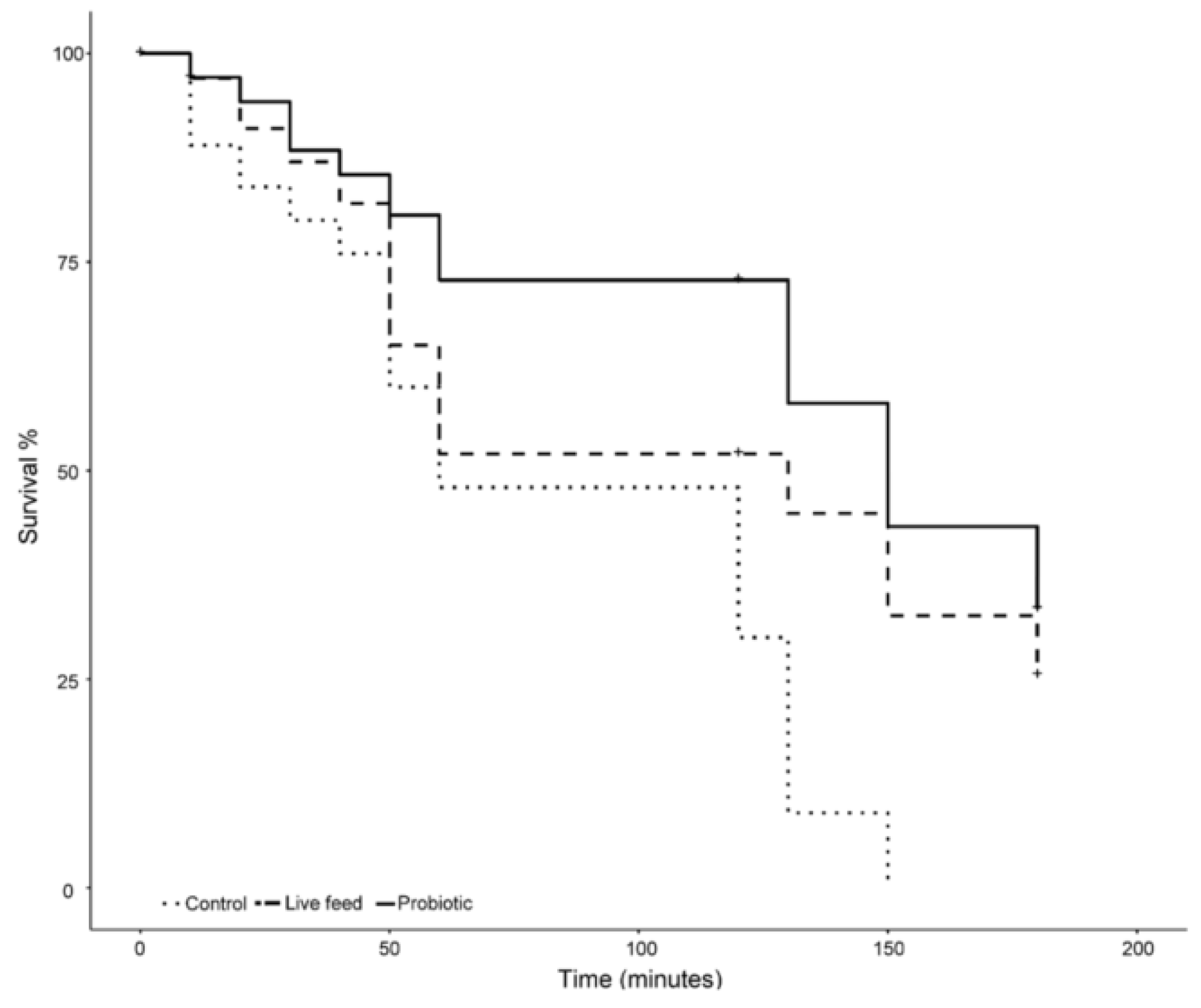
Larvae exposed to 18 ppt salinity from the different treatments reacted differently over time (Figure 5). On the one hand, larvae from the control treatment experienced mortality from the beginning of the exposure, having a 20% mortality after 30 min of exposure. On the other hand, larvae from both the probiotic and live feed treatment had a 13% mortality during the same period of time. After one hour of exposure, larvae from the control treatment had the highest mortality (52%), followed by the live feed treatment (48%), and the probiotic treatment (29%). Mortality in the control treatment slowed down during the following hour, resulting in an overall mortality rate of 70% (an 18% mortality rate increase). In contrast, tanks with larvae from the probiotic and live feed treatments experienced no mortality during the same period of time (Figure 5). During the following 60 min, a mortality increase was observed in all treatments. No larvae were alive after 150 min of exposure time in the control treatment, while, in comparison, tanks from the probiotic and live feed treatments had surviving larvae after 3 h of exposure. The probiotic treatment had the lowest final mortality rate (67%) as compared with the live feed treatment (75%); a significant difference (p-value <0.05) was found in the mortality rates between treatments after three hours.
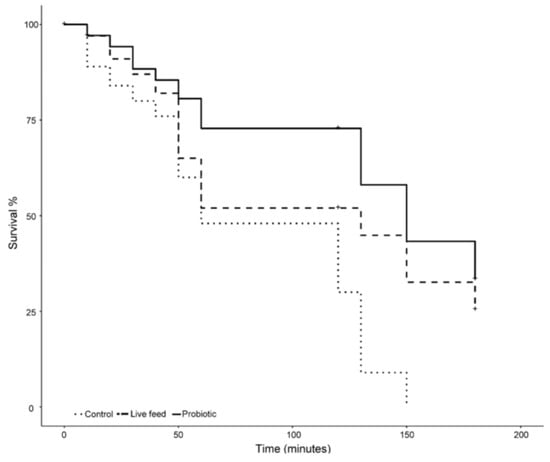
Figure 5.
Salinity stress test mortality over a 3 h time frame (n = 100), after 21 dph.
4. Discussion
As with other marine species of similar economic value, such as the grey mullet (Mugil cephalus) [35], sole (Solea solea) [36,37], gilthead seabream (Sparus aurata) [38,39], and sea bass (Dicentrarchus labrax) [40], the introduction of rotifers (Brachionus plicatilis) to pikeperch larval culture [4] has improved the survival rate and overall fitness.
However, with this new development in pikeperch larval culture, harmful pathogens and diseases can become a setback in larval hatcheries. Live food, such as rotifers, have a high bacterial load which becomes a factor for bacterial contamination, and therefore introduces diseases into larval cultures. Proliferation of harmful bacteria, such as Vibrio, are common in intensive aquaculture settings, therefore, several studies have stated the key importance of controlling the bacterial load in live food to reduce the negative effects [20,41,42]. The use of probiotics during this stage has been proven to control bacterial load in live food, as well as in the rearing environment [43]. The positive influence of probiotics during this experiment was observed when looking at other parameters such as total length and myomere height. Although it was not fully expected to be an outcome from this trial, growth was improved when using probiotics. Such results have also been observed in other species such as sea bass larvae, Nile tilapia (Oreochromis niloticus), swordtail (Xiphophorus Heller or X. maculatus), and guppy (Poecilia reticulate or P. sphenops) [44,45,46,47]. It has been speculated that such improvements in growth could be related to a positive effect on appetite stimulation or by just improving digestibility. Gastro-colonization is enhanced by probiotic microorganisms when administered over a long period of time, due to the higher proliferation rate as compared with the expulsion rate [16].
Carnevali [48] stated that probiotics positive effect on fish larvae is also due to the increase in levels of an insulin-like growth factor (IGF), which is responsible for muscle growth in fish. Such results, as well as a decrease in a growth antagonist such as myostatin (mstn), were observed in zebra fish (Danio rerio), sea bass, sea bream, and sole [49,50,51,52,53]. No metabolic analyses of such IGF receptors and binding proteins on pikeperch larvae were performed for this trial, but future work on this topic is recommended.
One of pikeperch culture’s main obstacles is their low tolerance to stress conditions, such as the handling and alteration of the fish’s physical conditions [1]. Such stress conditions are difficult to avoid when intensive culture methods of pikeperch are being developed to achieve mass production. The use of probiotics has been tested in several species, with the intent of increasing stress tolerance. Reducing cortisol levels [49,54], while increasing glycogen and triglycerides reserves in fish larval livers [55], are one of the benefits observed in other species, as well as increased antioxidant enzymes [56]. Such influences on the fish larvae metabolism could explained the results obtained with pikeperch, where stress tolerance was significantly enhanced with the use of Bactocell in both live feed and direct water application.
By the end of the trial, significant improvements of survival rates were observed, especially between the probiotic and the control treatment. As discussed earlier, such results can be attributed to the many benefits that probiotics have with fish larvae. Another benefit not discussed yet is the potential influence that some probiotic strains (gram positive) have over water quality by transforming organic matter to CO2 [57]. This could have had an influence in the survival differences between the live feed and probiotic treatments. Although no significant differences in ammonia, nitrate, and nitrite were found between treatments, a slight pattern was observed. The probiotic treatment had a slightly lower level of ammonia (NH3 = 0.18 ± 0.03 mgL−1) and nitrate (NO3 = 0.08 ± 0.04 mgL−1) than the live feed and control treatment. Such differences could be due to the fact that larvae in the probiotic treatment were exposed to the probiotic through the live feed and the culture water, potentially giving higher quality water conditions than the live feed treatment, whose water was not exposed to Bactocell.
5. Conclusions
There was a positive correlation between the survival rate and fitness of pikeperch larvae when Pediococcus acidilactici MA 18/5M was used during the first 21 days post hatching. The use of such commercial probiotics during live feed cultures, as well as in the larval rearing water (probiotic treatment), significantly increased the survival rates and stress tolerance. The growth parameters also improved, indicating this LAB strain contributed to support pikeperch larval quality. This warrants further research on the benefits of probiotics on larval metabolism, digestive enzyme activities, and on controlling harmful bacteria which are all key parameters for sensitive species, such a pikeperch, to achieve optimal rearing efficiency.
More in depth research, specifically addressing metabolic effects and digestive enzyme activity, is also needed to help identify how probiotics can improve overall pikeperch growth.
Funding
This research received no external funding.
Acknowledgements
This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic, projects CENAKVA (LM2018099), Biodiversity (CZ.02.1.01/0.0/0.0/16_025/0007370), and also by the Ministry of Agriculture of the Czech Republic, project NAZV QK1810296.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| dph | days post hatch |
| RAS | Recirculation aquaculture systems |
| TL | Total length |
| BW | Body weight |
| MH | Myomere height |
| ED | Eye diameter |
| SF | Stomach fullness |
| FFPW | Faculty of Fisheries and Protection of Waters |
| USB | University of South Bohemia |
| LMM | Linear mixed model |
| GLMM | Generalized linear mixed models |
| LAB | Lactic acid bacteria |
References
- Kestemont, P.; Dabrowski, K.; Summerfelt, R.C. Biology and Culture of Percid Fishes: Principles and Practices; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Policar, T.; Schaefer, F.J.; Panana, E.; Meyer, S.; Teerlinck, S.; Toner, D.; Żarski, D. Recent progress in European percid fish culture production technology—Tackling bottlenecks. In Aquaculture International; Springer International Publishing: New York, NY, USA, 2019; Volume 27, pp. 1151–1174. [Google Scholar]
- Steenfeldt, S.; Fontaine, P.; Overton, J.L.; Policar, T.; Toner, D.; Falahatkar, B.; Horváth, Á.; Khemis, I.B.; Hamza, N. Mohammed Mhetli Current Status of Eurasian Percid Fishes Aquaculture. In Biology and Culture of Percid Fishes; Springer: Dordrecht, The Netherlands, 2015; pp. 817–841. [Google Scholar]
- Yanes-Roca, C.; Mráz, J.; Born-Torrijos, A.; Holzer, A.S.; Imentai, A.; Policar, T. Introduction of rotifers (Brachionus plicatilis) during pikeperch first feeding. Aquaculture 2018, 497, 260–268. [Google Scholar] [CrossRef]
- Imentai, A.; Yanes-Roca, C.; Malinovskyi, O.; Policar, T. Effect of Brachionus plicatilis density on pikeperch (Sander lucioperca L.) larva performance at first feeding. J. Appl. Ichthyol. 2019, 35, 1292–1294. [Google Scholar] [CrossRef]
- Imentai, A.; Yanes-Roca, C.; Steinbach, C.; Policar, T. Optimized application of rotifers Brachionus plicatilis for rearing pikeperch Sander lucioperca L. larvae. Aquac. Int. 2019, 27, 1137–1149. [Google Scholar] [CrossRef]
- Lubzens, E.; Tandler, A.; Minkoff, G. Rotifers as food in aquaculture. Hydrobiologia 1989, 186–187, 387–400. [Google Scholar] [CrossRef]
- Kestemont, P.; Henrotte, E. Nutritional Requirements and Feeding of Broodstock and Early Life Stages of Eurasian Perch and Pikeperch. In Biology and Culture of Percid Fishes; Springer: Dordrecht, The Netherlands, 2015; pp. 539–564. [Google Scholar]
- Lund, I.; el Kertaoui, N.; Izquierdo, M.S.; Dominguez, D.; Hansen, B.W.; Kestemont, P. The importance of phospholipids combined with long-chain PUFA in formulated diets for pikeperch (Sander lucioperca) larvae. Br. J. Nutr. 2018, 120, 628–644. [Google Scholar] [CrossRef]
- Lund, I.; Vilhelm, P.; Winding, B. Comparative Biochemistry and Physiology, Part A Dietary supplementation of essential fatty acids in larval pikeperch (Sander lucioperca); short and long term effects on stress tolerance and metabolic physiology. Comp. Biochem. Physiol. Part A 2012, 162, 340–348. [Google Scholar] [CrossRef]
- Villamil, L.; Figueras, A.; Planas, M.; Novoa, B. Control of Vibrio alginolyticus in Artemia culture by treatment with bacterial probiotics. Aquaculture 2003, 219, 43–56. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Gatesoupe, F. The use of probiotics in aquaculture. Aquaculture 1999, 180, 147–165. [Google Scholar] [CrossRef]
- Vanbelle, M.; Teller, E.; Focant, M. Probiotics in animal nutrition: A review. Arch. für Tierernährung 1990, 40, 543–567. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Múzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ljubobratovic, U.; Kosanovic, D.; Vukotic, G.; Molnar, Z.; Stanisavljevic, N.; Ristovic, T.; Peter, G.; Lukic, J.; Jeney, G. Supplementation of lactobacilli improves growth, regulates microbiota composition and suppresses skeletal anomalies in juvenile pike-perch (Sander lucioperca) reared in recirculating aquaculture system (RAS): A pilot study. Res. Vet. Sci. 2017, 115, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Ljubobratovic, U.; Kosanovic, D.; Demény, F.Z.; Krajcsovics, A.; Vukotic, G.; Stanisavljevic, N.; Golic, N.; Jeney, G.; Lukic, J. The effect of live and inert feed treatment with lactobacilli on weaning success in intensively reared pike-perch larvae. Aquaculture 2019, 516, 734608. [Google Scholar] [CrossRef]
- Klein, G.; Pack, A.; Bonaparte, C.; Reuter, G. Taxonomy and physiology of probiotic lactic acid bacteria. Int. J. Food Microbiol. 1998, 41, 103–125. [Google Scholar] [CrossRef]
- Munro, P.O.; Barbour, A.; Blrkbeck, T.H. Comparison of the gut bacterial flora of start-feeding larval turbot reared under different conditions. J. Appl. Bacteriol. 1994, 77, 560–566. [Google Scholar] [CrossRef]
- Ringø, E.; Birkbeck, T.H.; Munro, P.O.; Vadstein, O.; Hjelmeland, K. The effect of early exposure to Vibrio pelagius on the aerobic bacterial flora of turbot, Scophthalmus maximus (L.) larvae. J. Appl. Bacteriol. 1996, 81, 207–211. [Google Scholar] [CrossRef]
- Ringø, E. Intestinal microflora of fish larvae and fry. Aquac. Res. 1999, 30, 73. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; van Doan, H.; Beck, B.R.; Song, S.K. Lactic Acid Bacteria in Finfish—An Update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Ljungh Åsa and Wadström Torkel. Lactic Acid Bacteria as Probiotics. Curr. Issues Intest. Microbiol. 2018, 7, 73–90. [Google Scholar]
- Malinovskyi, O.; Veselý, L.; Blecha, M.; Křišťan, J.; Policar, T. The substrate selection and spawning behaviour of pikeperch Sander lucioperca L. broodstock under pond conditions. Aquac. Res. 2018, 49, 3541–3547. [Google Scholar] [CrossRef]
- Malinovskyi, O.; Kolářová, J.; Blecha, M.; Stará, A.; Velíšek, J.; Křišťan, J.; Policar, T. Behavior and physiological status of pond-cultured pikeperch (Sander lucioperca) broodstock effected by sexual interactions throughout semi-artificial reproduction. Aquac. Int. 2019, 27, 1093–1107. [Google Scholar] [CrossRef]
- Blecha, M.; Kristan, J.; Samarin, A.M.; Rodina, M.; Policar, T. Quality and quantity of pikeperch (Sander lucioperca) spermatozoa after varying cold water treatments. J. Appl. Ichthyol. 2015, 31, 75–78. [Google Scholar] [CrossRef]
- Blecha, M.; Samarin, A.M.; Křišťan, J.; Policar, T. Benefits of hormone treatment of both sexes in semi-artificial reproduction of pikeperch (Sander lucioperca L.). Czech J. Anim. Sci. 2016, 61, 203–208. [Google Scholar] [CrossRef]
- Samarin, M.A.; Miroslav, D.B.; Bytyutskyy; Policar, T. Post-Ovulatory Oocyte Ageing in Pikeperch (Sander lucioperca L.) and its Effect on Egg Viability Rates and the Occurrence of Larval Malformations and Ploidy Anomalies. Turkish J. Fish. Aquat. Sci. 2015, 15, 429–435. [Google Scholar]
- Křištan, T.; Alavi, J.; Stejskal, S.M.H.; Policar, V. Hormonal induction of ovulation in pikeperch (Sander lucioperca L.) using human chorionic gonadotropin (hCG) and mammalian GnRH analogue. Aquac. Int. 2013, 21, 811–818. [Google Scholar] [CrossRef]
- Křištan, J.; Stara, J.; Polgesek, A.; Drasovean, M.; Kolarova, A.; Priborsky, J.; Blecha, J.; Svacina, M.; Policar, P.; Velisek, T. Efficacy of different anaesthetics for pikeperch (Sander lucioperca L.) in relation to water temperature. Neuro. Endocrinol. Lett. 2014, 35, 81–85. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4; Journal of Statistical Software: Innsbruck, Austria, 2015; Volume 67. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, Second; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Nash, C.E.; Kuo, C.-M. Hypotheses for problems impeding the mass propagation of grey mullet and other finfish. Aquaculture 1975, 5, 119–133. [Google Scholar] [CrossRef]
- Howell, B.R. A re-appraisal of the potential of the sole, Solea solea (L.), for commercial cultivation. Aquaculture 1997, 155, 355–365. [Google Scholar] [CrossRef]
- Fuchs, J. Influence de la photoperiode sur la croissance et la survie de la larve et du juvenile de sole (Solea solea) en elevage. Aquaculture 1978, 15, 63–74. [Google Scholar] [CrossRef]
- Person-Le^Ruyet, J.; Verillaud, P. Techniques d’elevage intensif de la daurade doree (sparus aurata (L.)) de la naissance a l’age de deux mois. Aquaculture 1980, 20, 351–370. [Google Scholar] [CrossRef]
- Tandler, A.; Helps, S. The effects of photoperiod and water exchange rate on growth and survival of gilthead sea bream (Sparus aurata, Linnaeus; Sparidae) from hatching to metamorphosis in mass rearing systems. Aquaculture 1985, 48, 71–82. [Google Scholar] [CrossRef]
- Girin, M. Marine fish culture in France: Recent developments. Aquaculture 1975, 5, 113. [Google Scholar] [CrossRef]
- Nicolas, J.L.; Robic, E.; Ansquer, D. Bacterial flora associated with a trophic chain consisting of microalgae, rotifers and turbot larvae: Influence of bacteria on larval survival. Aquaculture 1989, 83, 237–248. [Google Scholar] [CrossRef]
- Munro, P.; Henderson, R.; Barbour, A.; Birkbeck, T. Partial decontamination of rotifers with ultraviolet radiation: The effect of changes in the bacterial load and flora of rotifers on mortalities in start-feeding larval turbot. Aquaculture 1999, 170, 229–244. [Google Scholar] [CrossRef]
- Skjermo, J.; Vadstein, O. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 1999, 177, 333–343. [Google Scholar] [CrossRef]
- Hamza, A.; Fdhila, K.; Zouiten, D.; Masmoudi, A.S. Virgibacillus proomii and Bacillus mojavensis as probiotics in sea bass (Dicentrarchus labrax) larvae: Effects on growth performance and digestive enzyme activities. Fish Physiol. Biochem. 2016, 42, 495–507. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Dhevendaran, K. Evaluation of Streptomyces as a Probiotic Feed for the Growth of Ornamental Fish Xiphophorus helleri. Food Technol. Biotechnol. 2010, 48, 497–504. [Google Scholar]
- Ghosh, S.; SinhaI, A.; Sahu, C. Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac. Nutr. 2008, 14, 289–299. [Google Scholar]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzmán-Méndez, B.E.; López-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2003, 216, 193–201. [Google Scholar] [CrossRef]
- Carnevali, O.; Maradonna, F.; Gioacchini, G. Integrated control of fish metabolism, wellbeing and reproduction: The role of probiotic. Aquaculture 2017, 472, 144–155. [Google Scholar] [CrossRef]
- Carnevali, O.; Vivo, L.; Sulpizio, R.; Gioacchini, G.; Olivotto, I.; Silvi, S.; Cresci, A. Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture 2006, 258, 430–438. [Google Scholar] [CrossRef]
- Carnevali, O.; Claudia, M.; Sulpizio, Z.R.; Rollo, A.; Nardi, M.; Orpianesi, C.; Silvi, S.; Caggiano, M.; Polzonetti, A.M.; Cresci, A.; et al. Administration of Probiotic Strain to Improve Sea Bream Wellness during Development. Aquac. Int. 2004, 12, 377–386. [Google Scholar] [CrossRef]
- Avella, M.A.; Gioacchini, G.; Decamp, O.; Makridis, P.; Bracciatelli, C.; Carnevali, O. Application of multi-species of Bacillus in sea bream larviculture. Aquaculture 2010, 305, 12–19. [Google Scholar] [CrossRef]
- Avella, M.A.; Olivotto, I.; Silvi, S.; Ribecco, C.; Cresci, A.; Palermo, F.; Polzonetti, A.; Carnevali, O. Use of Enterococcus faecium to improve common sole (Solea solea) larviculture. Aquaculture 2011, 315, 384–393. [Google Scholar] [CrossRef]
- Carnevali, O.; Avella, M.A.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocrinol. 2013, 188, 297–302. [Google Scholar] [CrossRef]
- Vianello, S.; Brazzoduro, L.; Valle, L.D.; Belvedere, P.; Colombo, L. Myostatin expression during development and chronic stress in zebrafish (Danio rerio). J. Endocrinol. 2003, 176, 47–59. [Google Scholar] [CrossRef]
- Varela, J.L.; Ruiz-Jarabo, I.; Vargas-Chacoff, L.; Arijo, S.; León-Rubio, J.M.; García-Millán, I.; del Río, M.P.M.; Moriñigo, M.A.; Mancera, J.M. Dietary administration of probiotic Pdp11 promotes growth and improves stress tolerance to high stocking density in gilthead seabream Sparus auratus. Aquaculture 2010, 309, 265–271. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Díaz-Rosales, P.; León-Rubio, J.M.; de la Banda, I.G.; Lobo, C.; Alarcón, F.J.; Chabrillón, M.; Rosas-Ledesma, P.; Varela, J.L.; Ruiz-Jarabo, I.; et al. Use of the probiotic Shewanella putrefaciens Pdp11 on the culture of Senegalese sole (Solea senegalensis, Kaup 1858) and gilthead seabream (Sparus aurata L.). Aquac. Int. 2012, 20, 1025–1039. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).