Hepatitis E Virus in Manure and Its Removal by Psychrophilic anaerobic Biodigestion in Intensive Production Farms, Santa Catarina, Brazil, 2018–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Manure Sampling
2.2. Detection of HEV by Real-Time PCR
2.2.1. Sample Process Control Virus
2.2.2. Virus Concentration and Nucleic Acid Extraction from Manure
2.2.3. Virus Detection by RT-qPCR
2.2.4. Reporting and Interpretation of RT-qPCR Data
2.2.5. Extraction Efficiency
2.3. Statistical Analyses
3. Results
3.1. Efficiencies of Virus Concentration and RNA Extraction Form Manure Samples
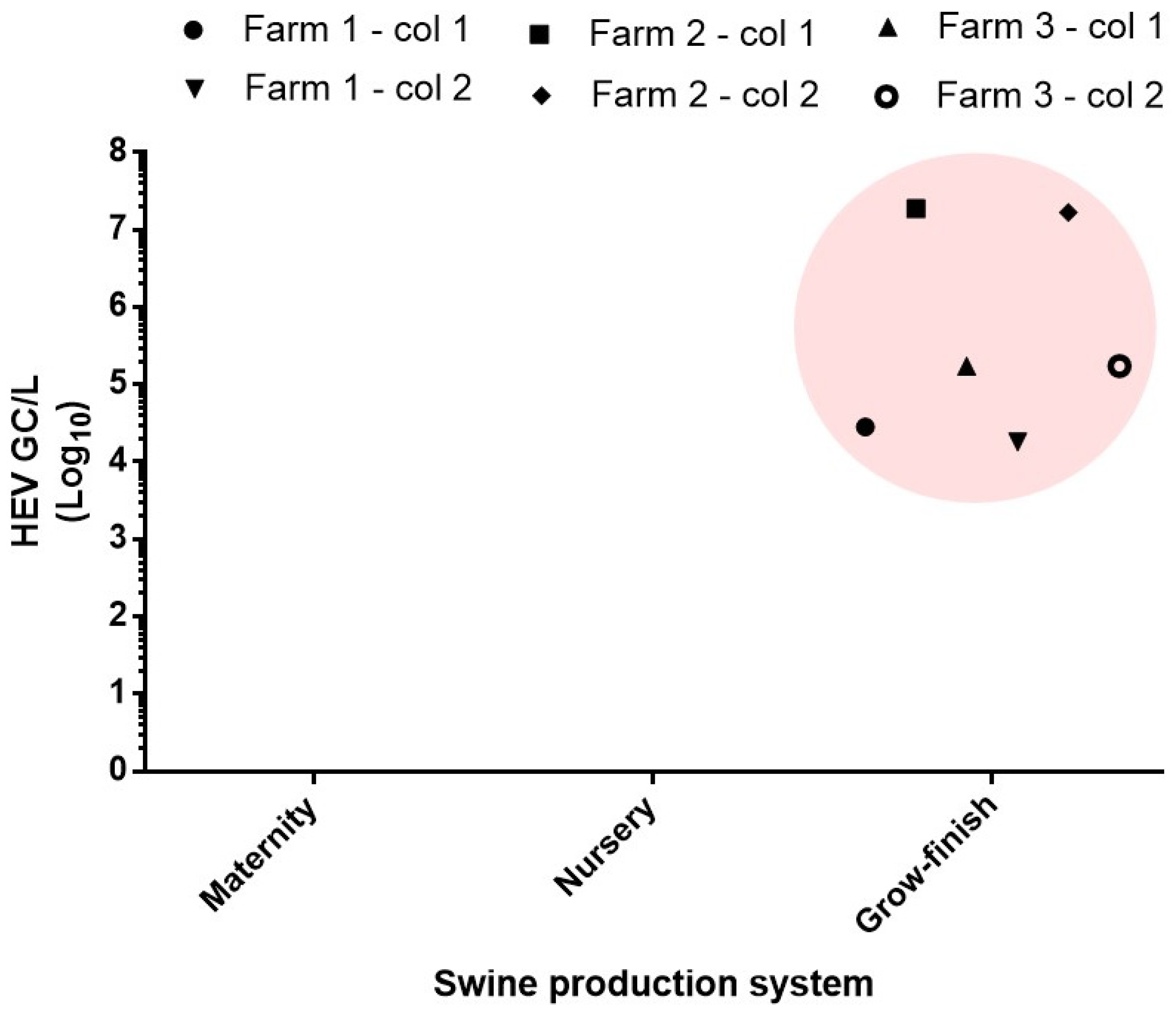
3.2. HEV Detection in Swine Farms
3.3. HEV Reduction in Swine Manure after PABs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Topp, E.; Scott, A.; Lapen, D.R.; Lyautey, E.; Duriez, P. Livestock waste treatment systems for reducing environmental exposure to hazardous enteric pathogens: Some considerations. Bioresour. Technol. 2009, 100, 5395–5398. [Google Scholar] [CrossRef] [PubMed]
- Viancelli, A.; Kunz, A.; Steinmetz, R.L.R.; Kich, J.D.; Souza, C.K.; Canal, C.W.; Coldebella, A.; Esteves, P.A.; Barardi, C.R.M. Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 2013, 90, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from anaerobically digested manure using gas-permeable membranes. Sci. Agric. 2016, 73, 434–438. [Google Scholar] [CrossRef]
- Kunz, A.; Miele, M.; Steinmetz, R.L.R. Advanced swine manure treatment and utilization in Brazil. Bioresour. Technol. 2009, 100, 5485–5489. [Google Scholar] [CrossRef]
- Bosshard, C.; Sørensen, P.; Frossard, E.; Dubois, D.; Mäder, P.; Nanzer, S.; Oberson, A. Nitrogen use efficiency of 15N-labelled sheep manure and mineral fertiliser applied to microplots in long-term organic and conventional cropping systems. Nutr. Cycl. Agroecosys. 2009, 83, 271–287. [Google Scholar] [CrossRef][Green Version]
- Burton, C.H.; Turner, C. Manure Management: Treatment Strategies for Sustainable Agriculture, 2nd ed.; Silsoe Research Institute: Silsoe, Bredford, UK, 2003. [Google Scholar]
- García, M.S.; Fernández-Barredo, S.; Pérez-Gracia, M.T. Detection of hepatitis E virus (HEV) through the different stages of pig manure composting plants. Microb. Biotechnol. 2014, 7, 26–31. [Google Scholar] [CrossRef]
- Estatísticas da Produção E Exportação de Suínos. Available online: www.embrapa.br (accessed on 4 August 2020).
- Pesquisa Trimestral do Abate de Animais. Available online: https://www.ibge.gov.br/ (accessed on 22 May 2020).
- Amorim, A.R.; Mendes, G.S.; Pena, G.P.A.; Santos, N. Hepatitis E virus infection of slaughtered healthy pigs in Brazil. Zoonoses Public Health 2018, 65, 501–504. [Google Scholar] [CrossRef]
- Ruhanya, V.; Diez-Valcarce, M.; D’Agostino, M.; Cook, N.; Hernández, M.; Rodríguez-Lázaro, D. Monitoring of extraction efficiency by a sample process control virus added immediately upon sample receipt. Food. Environ. Virol. 2015, 7, 413–416. [Google Scholar] [CrossRef]
- Diez-Valcarce, M.; Cook, N.; Hernandez, M.; Rodriguez-Lazaro, D. Analytical Application of a Sample Process Control in Detection of Foodborne Viruses. Food Anal. Meth. 2011, 4, 614–618. [Google Scholar] [CrossRef]
- Viancelli, A.; Garcia, L.A.T.; Kunz, A.; Steinmetz, R.; Esteves, P.A.; Barardi, C.R.M. Detection of circoviruses and porcine adenoviruses in water samples collected from swine manure treatment systems. Res. Vet. Sci 2011, 93, 538–543. [Google Scholar] [CrossRef]
- Diez-Valcarce, M.; Kovac, K.; Cook, N.; Rodriguez-Lazaro, D.; Hernandez, M. Construction and Analytical Application of Internal Amplification Controls (IAC) for Detection of Food Supply Chain-Relevant Viruses by Real-Time PCR-Based Assays. Food Anal. Meth. 2011, 4, 437–445. [Google Scholar] [CrossRef]
- Martinez-Martinez, M.; Diez-Valcarce, M.; Hernandez, M.; Rodriguez-Lazaro, D. Design and Application of Nucleic Acid Standards for Quantitative Detection of Enteric Viruses by Real-Time PCR. Food Environ. Virol. 2011, 3, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodrìguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Diez-Valcarce, M.; Kokkinos, P.; Soderberg, K.; Bouwknegt, M.; Willems, K.A.; De Roda-Husman, A.M.; Von Bonsdorff, C.-H.; Bellou, M.; Hernandez, M.; Maunula, L.; et al. Occurrence of human enteric viruses in commercial mussels at retail level in three European countries. Food Environ. Virol. 2012, 4, 73–80. [Google Scholar] [CrossRef]
- Rodriguez-Lazaro, D.; Diez-Valcarce, M.; Montes-Briones, R.; Gallego, D.; Hernandez, M.; Rovira, J. Presence of pathogenic enteric viruses in illegally imported meat and meat products to EU by international air travelers. Int. J. Food Microbiol. 2015, 209, 39–43. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- García, N.; Hernández, M.; Gutierrez-Boada, M.; Valero, A.; Navarro, A.; Muñoz-Chimeno, M.; Fernández-Manzano, A.; Escobar, F.M.; Martínez, I.; Bárcena, C.; et al. Occurrence of Hepatitis E Virus in Pigs and Pork Cuts and Organs at the Time of Slaughter, Spain, 2017. Front. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Leblanc, D.; Ward, P.; Gagné, M.-J.; Poitras, E.; Müller, P.; Trottier, Y.-L.; Simard, C.; Houde, A. Presence of hepatitis E virus in a naturally infected swine herd from nursery to slaughter. Int. J. Food Microbiol. 2007, 117, 160–166. [Google Scholar] [CrossRef]
- De Souza, A.J.S.; Gomes-Gouvêa, M.S.; Soares, M.D.C.P.; Pinho, J.R.R.; Malheiros, A.P.; Carneiro, L.A.; Dos Santos, D.R.L.; Pereira, W.L.A. HEV infection in swine from Eastern Brazilian Amazon: Evidence of co-infection by different subtypes. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 477–485. [Google Scholar] [CrossRef]
- Parashar, D.; Khalkar, P.; Arankalle, V.A. Survival of hepatitis A and E viruses in soil samples. Clin. Microbiol. Infect. 2011, 17, E1–E4. [Google Scholar] [CrossRef]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Vet. Res. 2013, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fongaro, G.; Viancelli, A.; Magri, M.; Elmahdy, E.M.; Biesus, L.; Kich, J.D.; Kunz, A.; Barardi, C. Utility of specific biomarkers to assess safety of swine manure for biofertilizing purposes. Sci. Total Environ. 2014, 479–480, 277–283. [Google Scholar] [CrossRef] [PubMed]
| Year | Farm 1 | Farm 2 | Farm 3 | Average | |
|---|---|---|---|---|---|
| HEV in Raw Manure (log GC L−1) | 2018 | 4.45 ± 0.9 | 7.27 ± 0.02 | 6.72 ± 0.10 | |
| 2019 | 4.26 ± 0.12 | 7.22 ± 0.03 | 6.51± 0.13 | ||
| Average | 4.36 ± 0.10 | 7.25 ± 0.02 | 6.63 ± 0.11 | ||
| HEV in Final Effluent (log GC L−1) | 2018 | 2.08 ± 0.01 | 4.27 ± 0.01 | 5.23 ± 0.02 | |
| 2019 | 2.05 ± 0.02 | 4.22 ± 0.03 | 5.24 ± 0.01 | ||
| Average | 2.06 ± 0.01 | 4.25 ± 0.02 | 5.23 ± 0.01 | ||
| Reduction (log GC L−1) (%) | 2018 | 2.37 ± 0.11 (99.57%) | 3.00 ± 0.01 (99.90%) | 1.46 ± 0.13 (96.79%) | 2.29 ± 0.53 (98.76%) |
| 2019 | 2.21 ± 0.09 (99.38%) | 3.00 ± 0.01 (99.90%) | 1.27 ± 0.08 (94.63%) | 2.16 ± 0.61 (97.97%) | |
| Average | 2.30 ± 0.08 (99.50%) | 3.00 ± 0.01 (99.90%) | 1.40 ± 0.11 (95.98%) | 2.23 ± 0.52 (99.41%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, D.S.M.; Tápparo, D.C.; Rogovski, P.; Cadamuro, R.D.; de Souza, E.B.; da Silva, R.; Degenhardt, R.; Lindner, J.D.D.; Viancelli, A.; Michelon, W.; et al. Hepatitis E Virus in Manure and Its Removal by Psychrophilic anaerobic Biodigestion in Intensive Production Farms, Santa Catarina, Brazil, 2018–2019. Microorganisms 2020, 8, 2045. https://doi.org/10.3390/microorganisms8122045
Souza DSM, Tápparo DC, Rogovski P, Cadamuro RD, de Souza EB, da Silva R, Degenhardt R, Lindner JDD, Viancelli A, Michelon W, et al. Hepatitis E Virus in Manure and Its Removal by Psychrophilic anaerobic Biodigestion in Intensive Production Farms, Santa Catarina, Brazil, 2018–2019. Microorganisms. 2020; 8(12):2045. https://doi.org/10.3390/microorganisms8122045
Chicago/Turabian StyleSouza, Doris Sobral Marques, Deisi Cristine Tápparo, Paula Rogovski, Rafael Dorighello Cadamuro, Estêvão Brasiliense de Souza, Raphael da Silva, Roberto Degenhardt, Juliano De Dea Lindner, Aline Viancelli, William Michelon, and et al. 2020. "Hepatitis E Virus in Manure and Its Removal by Psychrophilic anaerobic Biodigestion in Intensive Production Farms, Santa Catarina, Brazil, 2018–2019" Microorganisms 8, no. 12: 2045. https://doi.org/10.3390/microorganisms8122045
APA StyleSouza, D. S. M., Tápparo, D. C., Rogovski, P., Cadamuro, R. D., de Souza, E. B., da Silva, R., Degenhardt, R., Lindner, J. D. D., Viancelli, A., Michelon, W., Kunz, A., Treichel, H., Hernández, M., Rodríguez-Lázaro, D., & Fongaro, G. (2020). Hepatitis E Virus in Manure and Its Removal by Psychrophilic anaerobic Biodigestion in Intensive Production Farms, Santa Catarina, Brazil, 2018–2019. Microorganisms, 8(12), 2045. https://doi.org/10.3390/microorganisms8122045








