H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice
Abstract
1. Introduction
2. Methods
2.1. Cell Line and C. albicans Culture
2.2. Expression of Pro-Inflammatory Cytokines in DSS-Challenged Caco-2 Cells and Macrophages
2.3. Migration of Macrophages to C. albicans through DSS-Challenged Caco-2 Cells
2.4. The Effect of H89 on C. albicans
2.5. Animals
2.6. Inoculum Preparation and Induction of Colitis
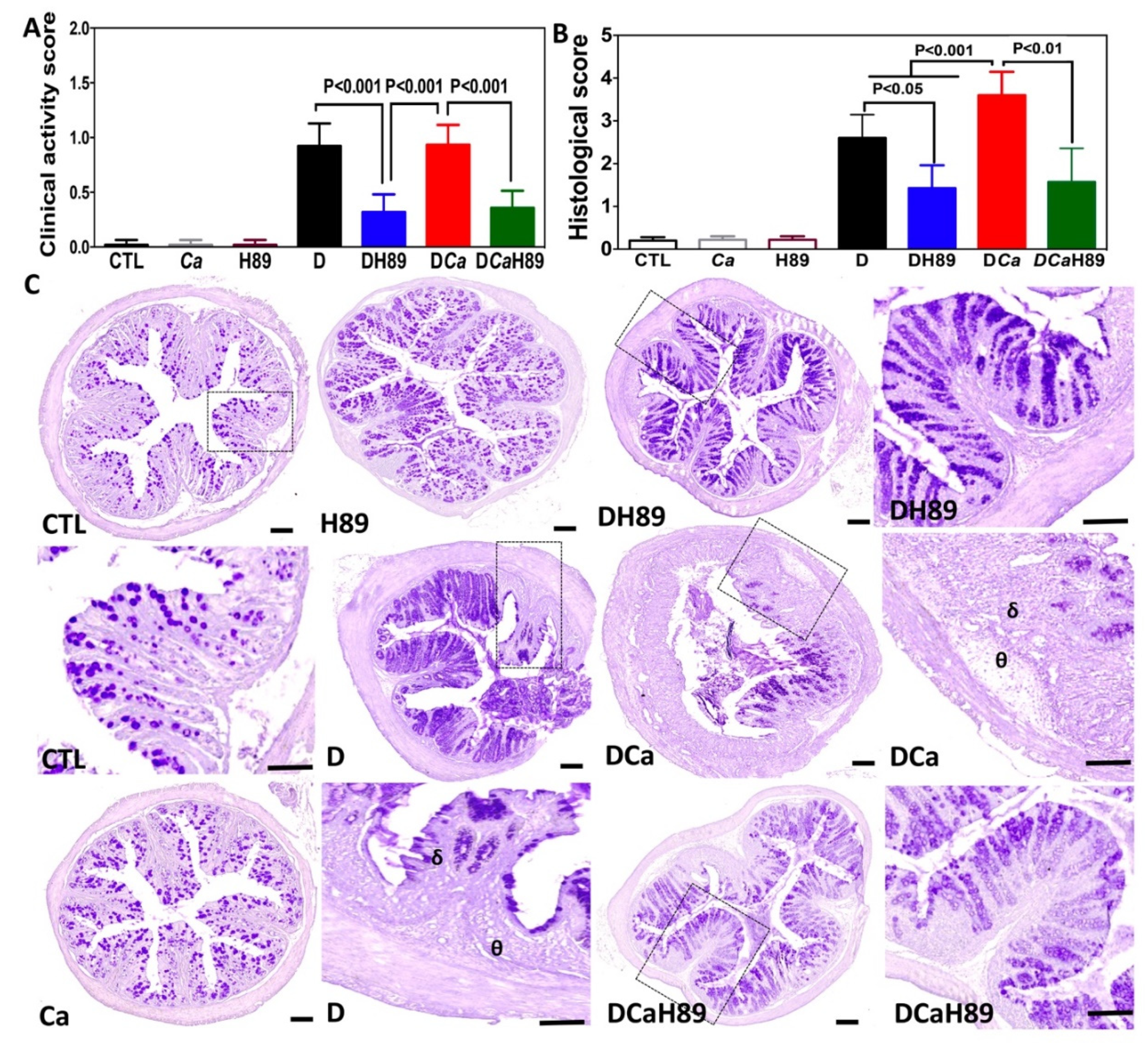
2.7. Clinical and Histological Scores for Inflammation
3. Statistical Analysis
4. Results
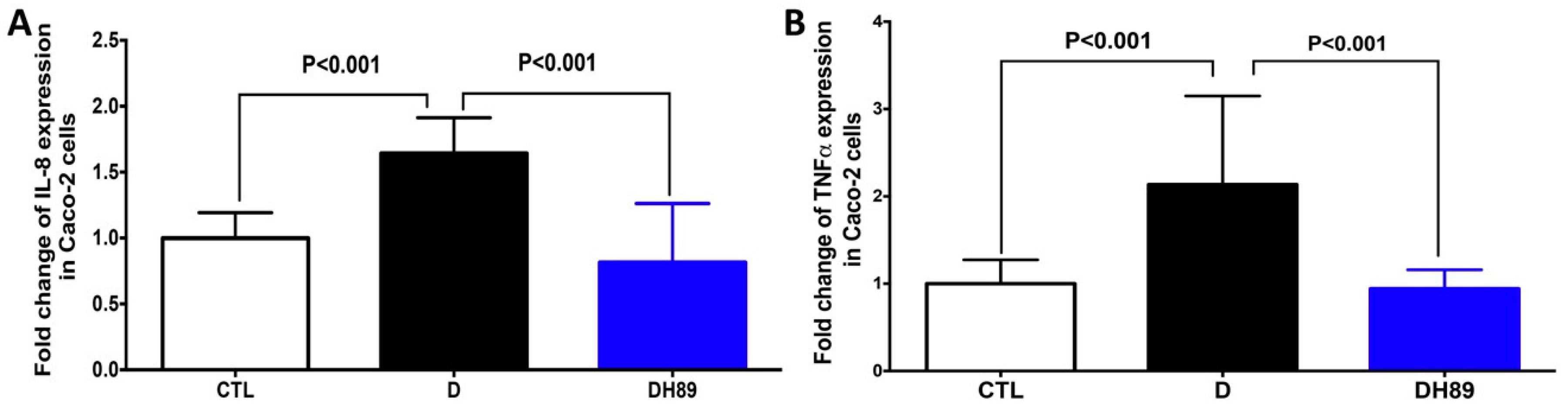
4.1. Pre-Treatment of DSS-Challenged Caco-2 Cells with H89 Reduces Pro-Inflammatory Cytokine Expression
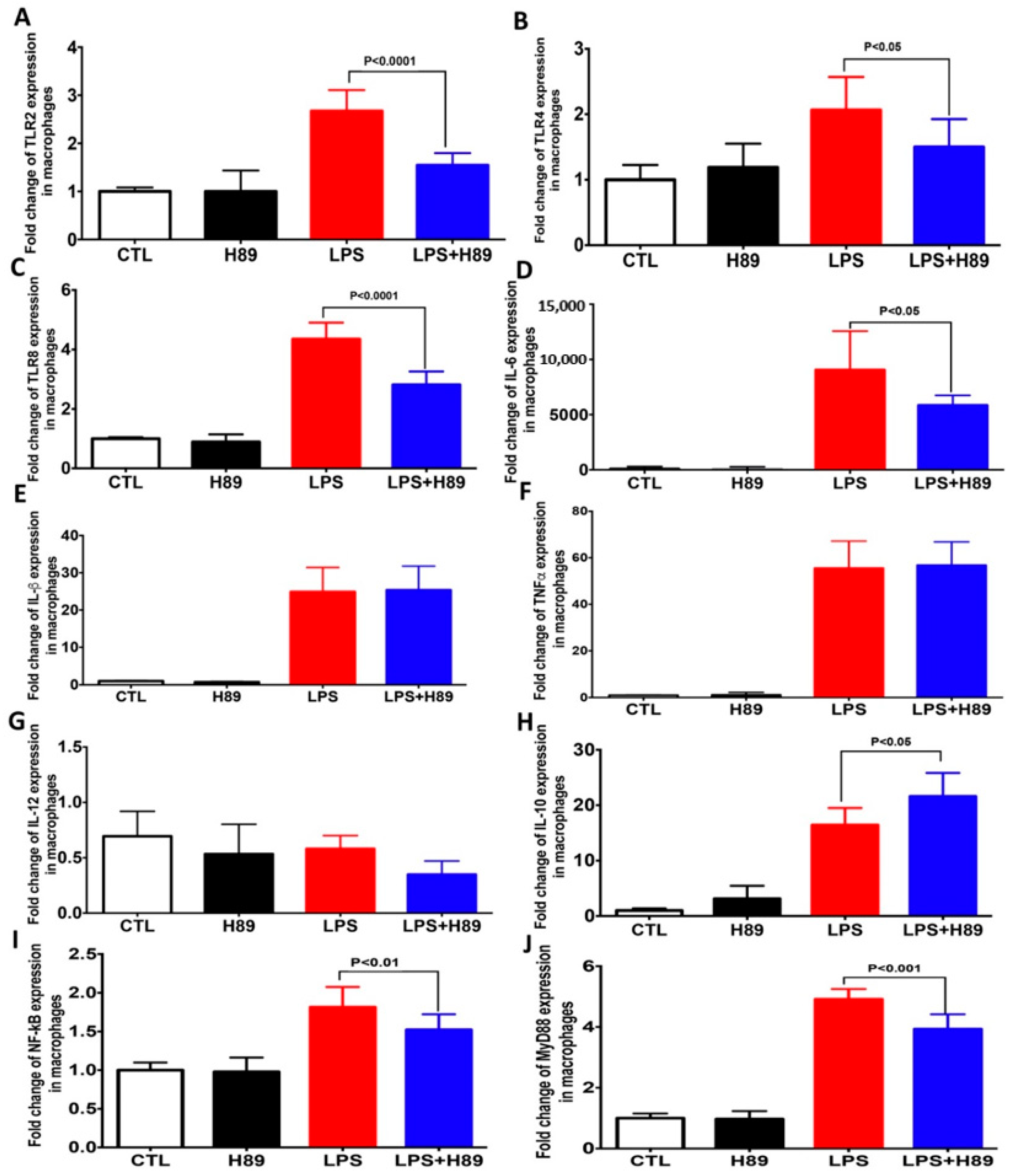
4.2. H89 Modulates the Expression of Pro-Inflammatory Cytokines and Toll-Like Receptors (TLRs) in Macrophages
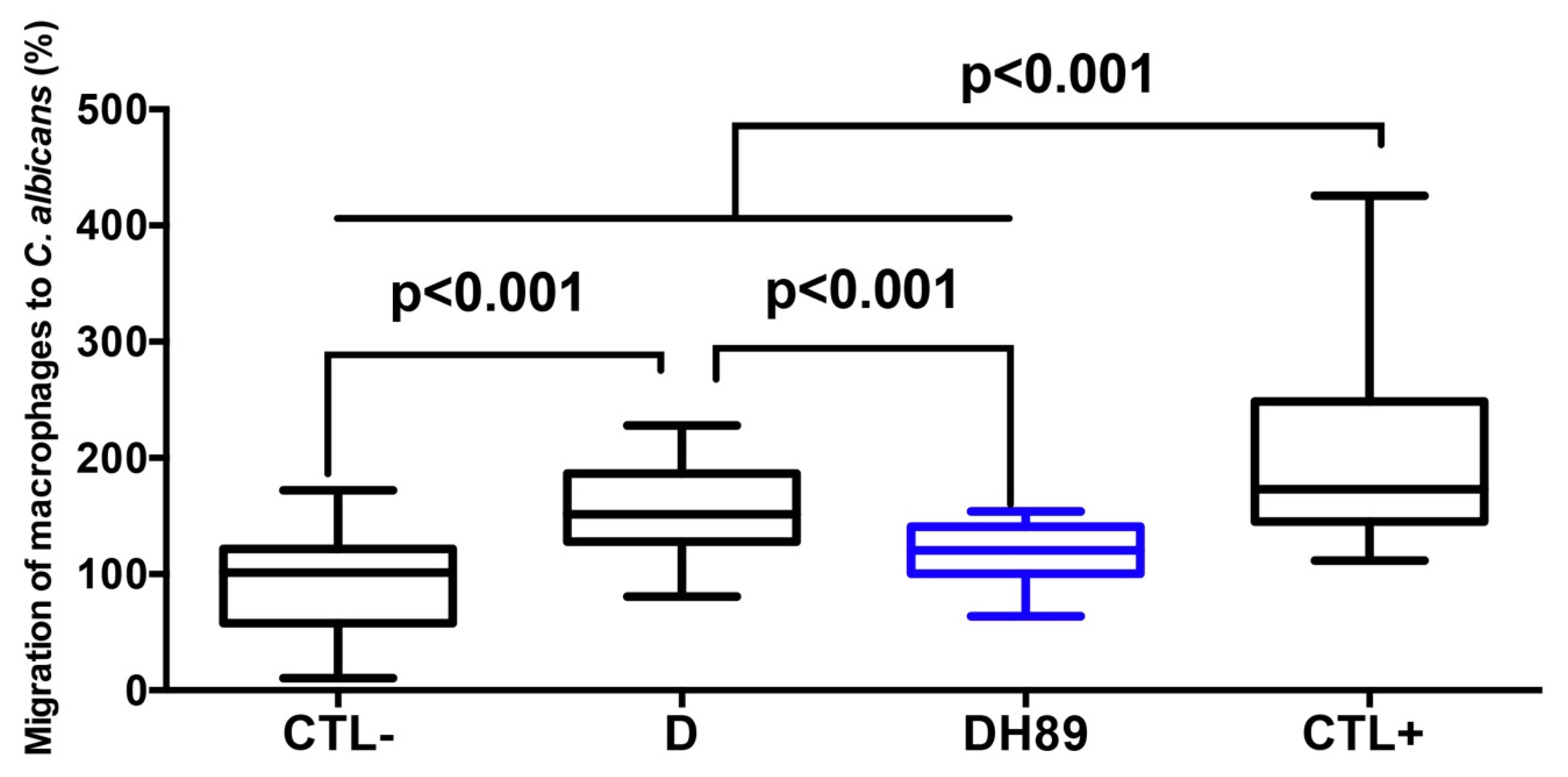
4.3. H89 Decreases Macrophage Migration to C. albicans through DSS-Challenged Caco-2 Cells
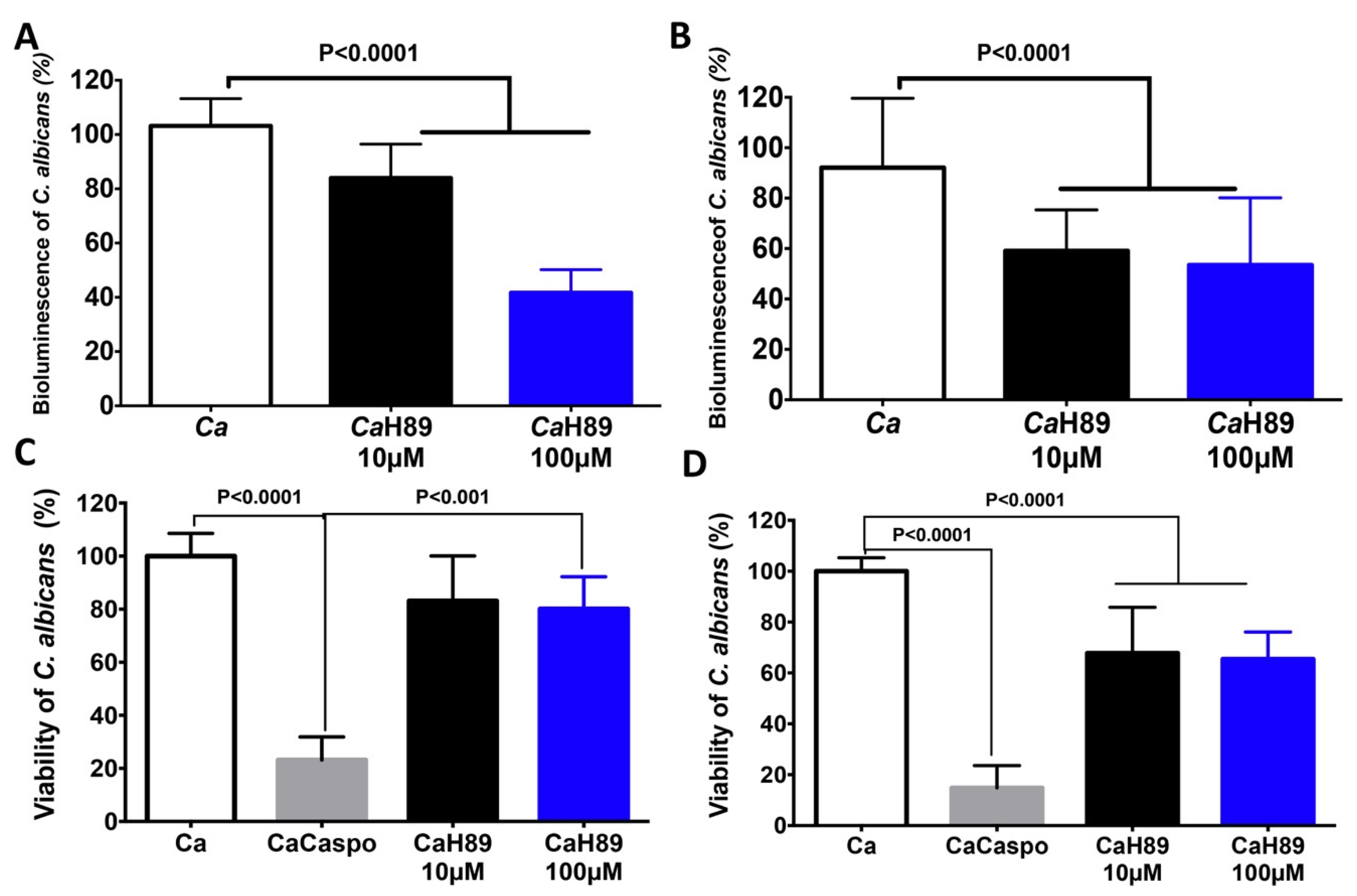
4.4. Antifungal Activity of H89 against C. albicans
4.5. H89 Decreased Inflammatory Parameters in DSS-Induced Colitis
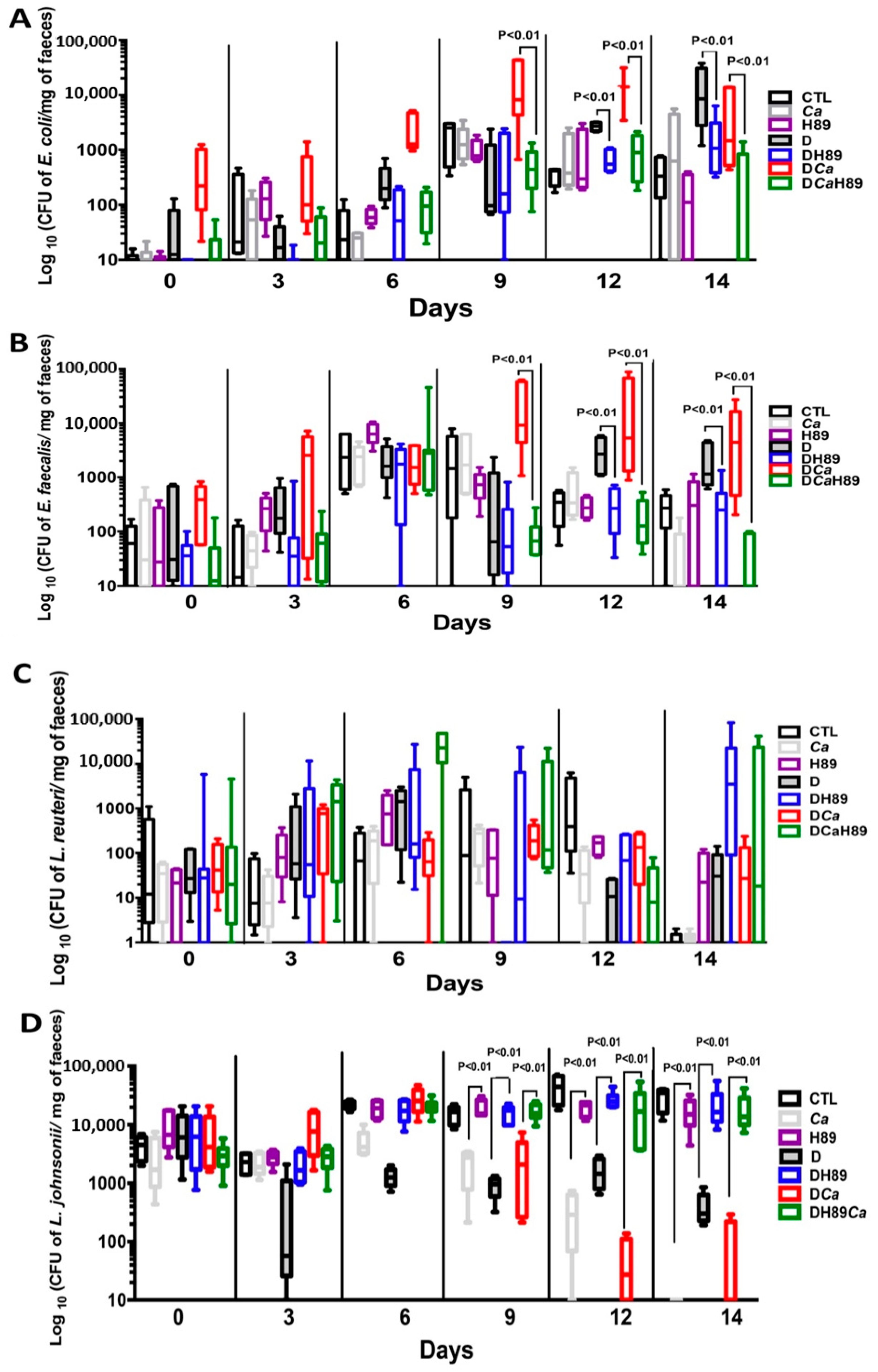
4.6. H89 Decreases the Amount of C. albicans and Restores Anaerobic Bacterial Populations
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef]
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Standaert-Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.M.; Colombel, J.F.; Poulain, D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Jawhara, S.; Mogensen, E.; Maggiotto, F.; Fradin, C.; Sarazin, A.; Dubuquoy, L.; Maes, E.; Guerardel, Y.; Janbon, G.; Poulain, D. Murine model of dextran sulfate sodium-induced colitis reveals Candida glabrata virulence and contribution of beta-mannosyltransferases. J. Biol. Chem. 2012, 287, 11313–11324. [Google Scholar] [CrossRef]
- Farrell, R.J.; Kelleher, D. Glucocorticoid resistance in inflammatory bowel disease. J. Endocrinol. 2003, 178, 339–346. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.P.; Kumar, S.N.; Turner, J.R.; Dwinell, M.B. Cyclic AMP dysregulates intestinal epithelial cell restitution through PKA and RhoA. Inflamm. Bowel Dis. 2012, 18, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Spadaccini, M.; D’Alessio, S.; Peyrin-Biroulet, L.; Danese, S. PDE4 Inhibition and Inflammatory Bowel Disease: A Novel Therapeutic Avenue. Int. J. Mol. Sci. 2017, 18, 1276. [Google Scholar] [CrossRef]
- Reber, L.L.; Daubeuf, F.; Nemska, S.; Frossard, N. The AGC kinase inhibitor H89 attenuates airway inflammation in mouse models of asthma. PLoS ONE 2012, 7, e49512. [Google Scholar] [CrossRef]
- Chijiwa, T.; Mishima, A.; Hagiwara, M.; Sano, M.; Hayashi, K.; Inoue, T.; Naito, K.; Toshioka, T.; Hidaka, H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990, 265, 5267–5272. [Google Scholar]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef]
- Choteau, L.; Parny, M.; Francois, N.; Bertin, B.; Fumery, M.; Dubuquoy, L.; Takahashi, K.; Colombel, J.F.; Jouault, T.; Poulain, D.; et al. Role of mannose-binding lectin in intestinal homeostasis and fungal elimination. Mucosal. Immunol. 2016, 9, 767–776. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Dufrenoy, P.; Charlet, R.; Hechelski, M.; Daich, A.; Waterlot, C.; Jawhara, S.; Ghinet, A. New Efficient Eco-Friendly Supported Catalysts for the Synthesis of Amides with Antioxidant and Anti-Inflammatory Properties. Chem. Med. Chem. 2020, 15, 459–467. [Google Scholar] [CrossRef]
- Soloviev, D.A.; Jawhara, S.; Fonzi, W.A. Regulation of innate immune response to Candida albicans infections by alphaMbeta2-Pra1p interaction. Infect. Immun. 2011, 79, 1546–1558. [Google Scholar] [CrossRef]
- Jawhara, S.; Pluskota, E.; Verbovetskiy, D.; Skomorovska-Prokvolit, O.; Plow, E.F.; Soloviev, D.A. Integrin alphaXbeta(2) is a leukocyte receptor for Candida albicans and is essential for protection against fungal infections. J. Immunol. 2012, 189, 2468–2477. [Google Scholar] [CrossRef]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Devel. Ther. 2013, 7, 1341–1357. [Google Scholar] [CrossRef]
- Nunes, N.S.; Chandran, P.; Sundby, M.; Visioli, F.; da Costa Goncalves, F.; Burks, S.R.; Paz, A.H.; Frank, J.A. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine 2019, 45, 495–510. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Wood, D.R.; Yuan, C.; Nicolas, J.; De Plaen, I.G.; Farrow, K.N.; Chou, P.; Turner, J.R.; Hunter, C.J. A Role for cAMP and Protein Kinase A in Experimental Necrotizing Enterocolitis. Am. J. Pathol 2017, 187, 401–417. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Yao, Y.; Hu, R.; Dai, F.F.; Zhang, H.; Mao, Z.F. Cyclic adenosine monophosphate-protein kinase A signal pathway may be involved in pulmonary aquaporin-5 expression in ischemia/reperfusion rats following deep hypothermia cardiac arrest. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Bortolus, C.; Billamboz, M.; Charlet, R.; Lecointe, K.; Sendid, B.; Ghinet, A.; Jawhara, S. A Small Aromatic Compound Has Antifungal Properties and Potential Anti-Inflammatory Effects against Intestinal Inflammation. Int J. Mol. Sci 2019, 20, 321. [Google Scholar] [CrossRef]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Dubuquoy, L.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of intestinal inflammation by yeasts and cell wall extracts: Strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Choteau, L.; Vancraeyneste, H.; Le Roy, D.; Dubuquoy, L.; Romani, L.; Jouault, T.; Poulain, D.; Sendid, B.; Calandra, T.; Roger, T.; et al. Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination. Gut Pathog. 2017, 9, 9. [Google Scholar] [CrossRef]
- Saegusa, S.; Totsuka, M.; Kaminogawa, S.; Hosoi, T. Cytokine responses of intestinal epithelial-like Caco-2 cells to non-pathogenic and opportunistic pathogenic yeasts in the presence of butyric acid. Biosci. Biotechnol. Biochem. 2007, 71, 2428–2434. [Google Scholar] [CrossRef]
- Rahabi, M.; Jacquemin, G.; Prat, M.; Meunier, E.; AlaEddine, M.; Bertrand, B.; Lefevre, L.; Benmoussa, K.; Batigne, P.; Aubouy, A.; et al. Divergent Roles for Macrophage C-type Lectin Receptors, Dectin-1 and Mannose Receptors, in the Intestinal Inflammatory Response. Cell Rep. 2020, 30, 4386–4398.e5. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Colombel, J.F.; Watson, A.J.; Neurath, M.F. The 10 remaining mysteries of inflammatory bowel disease. Gut 2008, 57, 429–433. [Google Scholar] [CrossRef]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome as a Target for IBD Treatment: Are We There Yet? Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef]
- Schafer, P.H.; Parton, A.; Gandhi, A.K.; Capone, L.; Adams, M.; Wu, L.; Bartlett, J.B.; Loveland, M.A.; Gilhar, A.; Cheung, Y.F.; et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br. J. Pharmacol. 2010, 159, 842–855. [Google Scholar] [CrossRef]
- Hidaka, H.; Inagaki, M.; Kawamoto, S.; Sasaki, Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 1984, 23, 5036–5041. [Google Scholar] [CrossRef]
- Engh, R.A.; Girod, A.; Kinzel, V.; Huber, R.; Bossemeyer, D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J. Biol. Chem. 1996, 271, 26157–26164. [Google Scholar] [CrossRef]
- Schenk, M.; Bouchon, A.; Seibold, F.; Mueller, C. TREM-1—Expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Investig. 2007, 117, 3097–3106. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef]
- Gao, N.; Hibi, Y.; Cueno, M.; Asamitsu, K.; Okamoto, T. A-kinase-interacting protein 1 (AKIP1) acts as a molecular determinant of PKA in NF-kappaB signaling. J. Biol. Chem. 2010, 285, 28097–28104. [Google Scholar] [CrossRef]
- Hildebrand, D.; Sahr, A.; Wolfle, S.J.; Heeg, K.; Kubatzky, K.F. Regulation of Toll-like receptor 4-mediated immune responses through Pasteurella multocida toxin-induced G protein signalling. Cell Commun. Signal. 2012, 10, 22. [Google Scholar] [CrossRef]
- Amador, P.; Garcia-Herrera, J.; Marca, M.C.; de la Osada, J.; Acin, S.; Navarro, M.A.; Salvador, M.T.; Lostao, M.P.; Rodriguez-Yoldi, M.J. Inhibitory effect of TNF-alpha on the intestinal absorption of galactose. J. Cell Biochem. 2007, 101, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Herrera, J.; Navarro, M.A.; Marca, M.C.; de la Osada, J.; Rodriguez-Yoldi, M.J. The effect of tumor necrosis factor-alpha on D-fructose intestinal transport in rabbits. Cytokine 2004, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cassola, A.; Parrot, M.; Silberstein, S.; Magee, B.B.; Passeron, S.; Giasson, L.; Cantore, M.L. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot. Cell 2004, 3, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bockmuhl, D.P.; Krishnamurthy, S.; Gerads, M.; Sonneborn, A.; Ernst, J.F. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 2001, 42, 1243–1257. [Google Scholar] [CrossRef]
- Inglis, D.O.; Sherlock, G. Ras signaling gets fine-tuned: Regulation of multiple pathogenic traits of Candida albicans. Eukaryot. Cell 2013, 12, 1316–1325. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, Y.L. Conserved and Divergent Functions of the cAMP/PKA Signaling Pathway in Candida albicans and Candida tropicalis. J. Fungi. 2018, 4, 68. [Google Scholar] [CrossRef]
- Cao, C.; Wu, M.; Bing, J.; Tao, L.; Ding, X.; Liu, X.; Huang, G. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans. Mol. Microbiol. 2017, 105, 46–64. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; He, H.; Du, Y.; Hu, J.; Li, Y.; Li, Y.; Zhou, Y.; Wang, H.; Chen, Y.; et al. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine 2016, 95, e5019. [Google Scholar] [CrossRef]
- Kang, S.; Denman, S.E.; Morrison, M.; Yu, Z.; Dore, J.; Leclerc, M.; McSweeney, C.S. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010, 16, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Balish, E.; Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 2002, 160, 2253–2257. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Sendid, B.; Jawhara, S. Bacteroides thetaiotaomicron and Lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 2020, 10, 11510. [Google Scholar] [CrossRef] [PubMed]

| Consistency of Faeces/Blood in Faeces | Score | Behavior | Score | Weight Loss | Score |
|---|---|---|---|---|---|
| Normal/No blood | 0 | Normal | 0 | None | 0 |
| 1–5% | 0.5 | ||||
| Wet/Bloody stools or blood around the anus | 1 | Hunched | 1 | 5–10% | 1 |
| 10–15% | 1.5 | ||||
| Soft/Severe bleeding | 2 | Frozen posture | 2 | >15% | 2 |
| Loss of Epithelium and Crypt Damage | Score | Infiltration of Inflammatory Cells | Score |
|---|---|---|---|
| None | 0 | None | 0 |
| 0–5% | 1 | Mild | 1 |
| 5–10% | 2 | Moderate | 2 |
| >10% loss | 3 | Severe | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumortier, C.; Charlet, R.; Bettaieb, A.; Jawhara, S. H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice. Microorganisms 2020, 8, 2039. https://doi.org/10.3390/microorganisms8122039
Dumortier C, Charlet R, Bettaieb A, Jawhara S. H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice. Microorganisms. 2020; 8(12):2039. https://doi.org/10.3390/microorganisms8122039
Chicago/Turabian StyleDumortier, Corentin, Rogatien Charlet, Ali Bettaieb, and Samir Jawhara. 2020. "H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice" Microorganisms 8, no. 12: 2039. https://doi.org/10.3390/microorganisms8122039
APA StyleDumortier, C., Charlet, R., Bettaieb, A., & Jawhara, S. (2020). H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice. Microorganisms, 8(12), 2039. https://doi.org/10.3390/microorganisms8122039







